Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Statistical Analysis
3. Results
3.1. Selected Studies
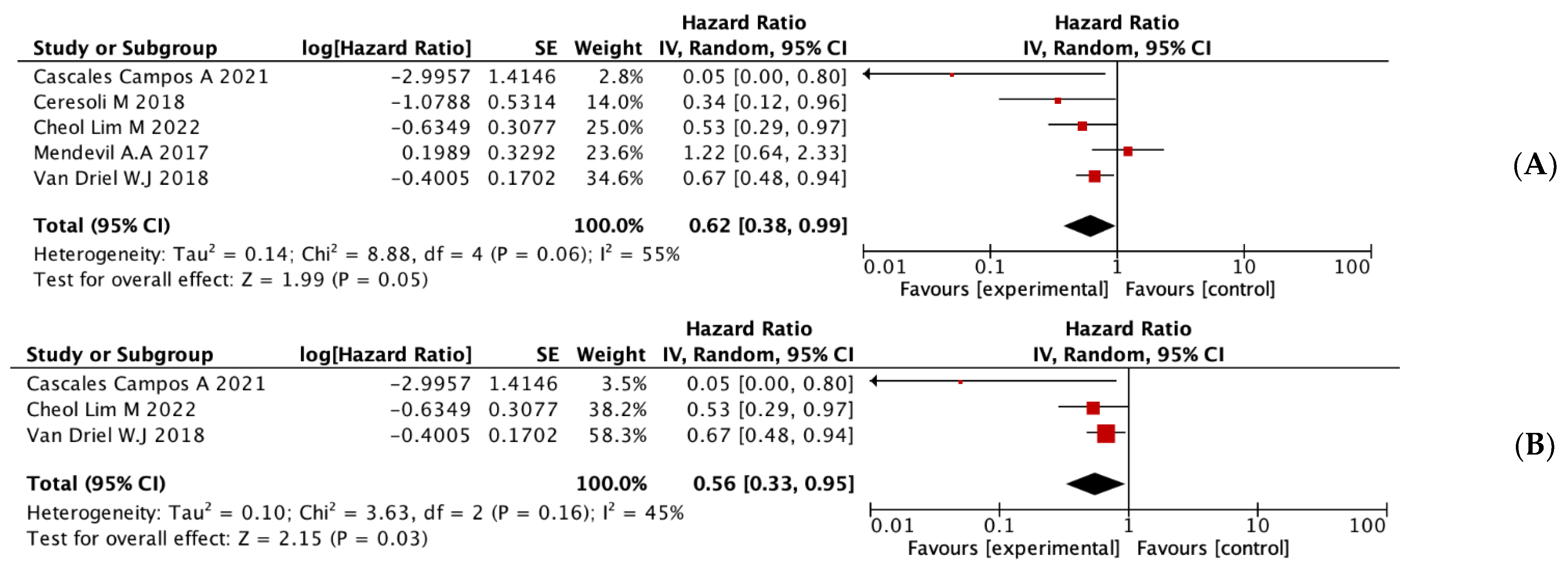
3.2. Overall Meta-Analyses of OS and DFS
3.3. Subgroup Analyses
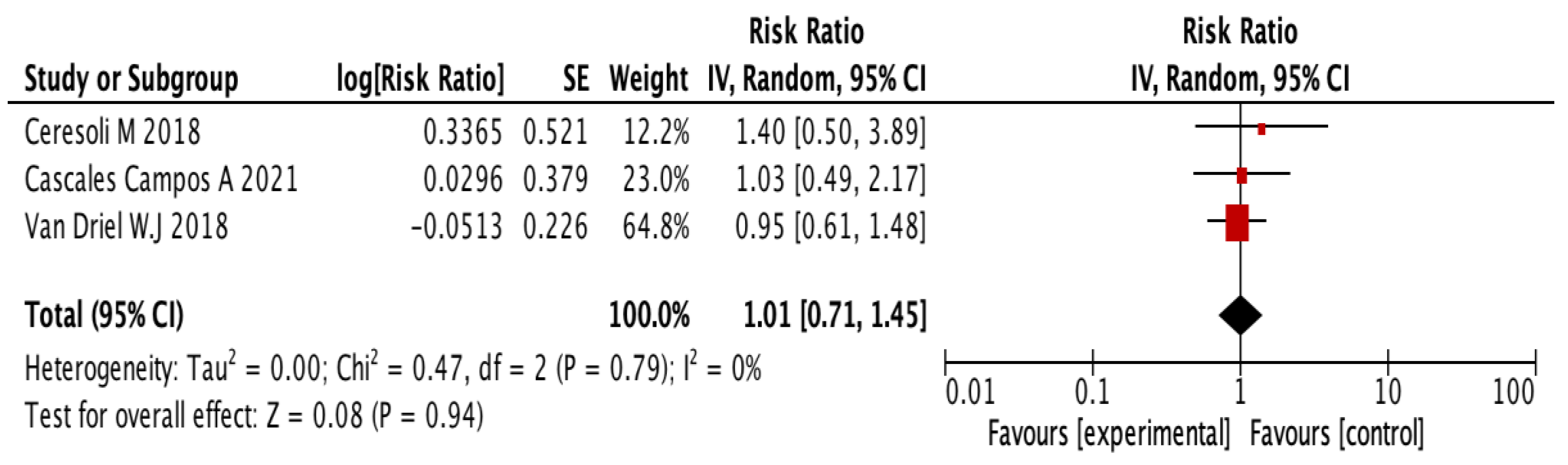
3.4. Systematic Review of Adverse Events, Morbidity, and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. Available online: https://pubmed.ncbi.nlm.nih.gov/21296855/ (accessed on 1 June 2022).
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. Available online: https://pubmed.ncbi.nlm.nih.gov/8849943/ (accessed on 1 June 2022).
- Flessner, M.F. Endothelial glycocalyx and the peritoneal barrier. Perit. Dial. Int. 2008, 28, 6–12. Available online: https://pubmed.ncbi.nlm.nih.gov/18178940/ (accessed on 1 June 2022). [CrossRef]
- Speeten, K.; Stuart, O.; Sugarbaker, P. Using pharmacologic data to plan clinical treatments for patients with peritoneal surface malignancy. Curr. Drug Discov. Technol. 2009, 6, 72–81. Available online: https://pubmed.ncbi.nlm.nih.gov/19275544/ (accessed on 1 June 2022). [CrossRef]
- Sugarbaker, P.H.; van der Speeten, K.; Anthony Stuart, O.; Chang, D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2011, 37, 719–726. Available online: https://pubmed.ncbi.nlm.nih.gov/21621952/ (accessed on 1 June 2022). [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.; Walker, J.; Burger, R.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. Available online: https://pubmed.ncbi.nlm.nih.gov/16394300/ (accessed on 1 June 2022). [CrossRef]
- Barlin, J.N.; Dao, F.; Zgheib, N.B.; Ferguson, S.E.; Sabbatini, P.J.; Hensley, M.L.; Bell-McGuinn, K.; Konner, J.; Tew, W.; Aghajanian, C.; et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2012, 125, 621–624. Available online: https://pubmed.ncbi.nlm.nih.gov/22446622/ (accessed on 1 June 2022). [CrossRef]
- Rothenberg, M.L.; Liu, P.Y.; Braly, P.S.; Wilczynski, S.P.; Hannigan, E.V.; Wadler, S.; Stuart, G.; Jiang, C.; Markman, M.; Alberts, D. Combined intraperitoneal and intravenous chemotherapy for women with optimally debulked ovarian cancer: Results from an intergroup phase II trial. J. Clin. Oncol. 2003, 21, 1313–1319. Available online: https://pubmed.ncbi.nlm.nih.gov/12663720/ (accessed on 1 June 2022). [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. Available online: https://pubmed.ncbi.nlm.nih.gov/18789678/ (accessed on 1 June 2022). [CrossRef]
- Elias, D.; Gilly, F.; Quenet, F.; Bereder, J.M.; Sidéris, L.; Mansvelt, B.; Lorimier, G.; Glehen, O.; Association Française de Chirurgie. Pseudomyxoma peritonei: A French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2010, 36, 456–462. Available online: https://pubmed.ncbi.nlm.nih.gov/20227231/ (accessed on 1 June 2022). [CrossRef]
- Elias, D.; Benizri, E.; di Pietrantonio, D.; Menegon, P.; Malka, D.; Raynard, B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2007, 14, 509–514. Available online: https://pubmed.ncbi.nlm.nih.gov/17096054/ (accessed on 1 June 2022). [CrossRef]
- Ansaloni, L.; Agnoletti, V.; Amadori, A.; Catena, F.; Cavaliere, D.; Coccolini, F.; De Iaco, P.; Di Battista, M.; Framarini, M.; Gazzotti, F.; et al. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 778–785. Available online: https://pubmed.ncbi.nlm.nih.gov/22572845/ (accessed on 1 June 2022). [CrossRef]
- Cavaliere, D.; Cirocchi, R.; Coccolini, F.; Fagotti, A.; Fambrini, M.; Federici, O.; Lorusso, D.; Vaira, M.; Ceresoli, M.; Delrio, P.; et al. 1st Evidence-based Italian consensus conference on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinosis from ovarian cancer. Tumori 2017, 103, 525–536. Available online: https://pubmed.ncbi.nlm.nih.gov/28430350/ (accessed on 1 June 2022). [CrossRef]
- Hotouras, A.; Desai, D.; Bhan, C.; Murphy, J.; Lampe, B.; Sugarbaker, P.H. Heated IntraPEritoneal Chemotherapy (HIPEC) for Patients With Recurrent Ovarian Cancer: A Systematic Literature Review. Int. J. Gynecol. Cancer 2016, 26, 661–670. Available online: https://pubmed.ncbi.nlm.nih.gov/26844612/ (accessed on 1 June 2022). [CrossRef]
- Coccolini, F.; Campanati, L.; Catena, F.; Ceni, V.; Ceresoli, M.; Cruz, J.J.; Lotti, M.; Magnone, S.; Napoli, J.; Rossetti, D.; et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: A multicenter prospective observational study. J. Gynecol. Oncol. 2015, 26, 54–61. Available online: https://pubmed.ncbi.nlm.nih.gov/25376916/ (accessed on 1 June 2022). [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.; van der Velden, J.; Arts, H.J.; Massuger, L.F. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1708618 (accessed on 1 June 2022). [CrossRef]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.-Y.; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. Available online: https://jamanetwork.com/journals/jamasurgery/fullarticle/2789724 (accessed on 1 June 2022). [CrossRef]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. Available online: https://pubmed.ncbi.nlm.nih.gov/34812982/ (accessed on 1 June 2022). [CrossRef]
- Baiocchi, G.; Ferreira, F.O.; Mantoan, H.; da Costa, A.A.B.A.; Faloppa, C.C.; Kumagai, L.Y.; de Mello, C.A.L.; Takahashi, R.M.; Nakagawa, W.T.; Jr, S.A.; et al. Hyperthermic Intraperitoneal Chemotherapy after Secondary Cytoreduction in Epithelial Ovarian Cancer: A Single-center Comparative Analysis. Ann. Surg. Oncol. 2016, 23, 1294–1301. Available online: https://link.springer.com/article/10.1245/s10434-015-4991-4 (accessed on 1 June 2022). [CrossRef]
- Chiva, L.M.; Gonzalez-Martin, A. A critical appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of advanced and recurrent ovarian cancer. Gynecol. Oncol. 2015, 136, 130–135. Available online: http://www.gynecologiconcology-online.net/article/S0090825814015376/fulltext (accessed on 1 June 2022). [CrossRef]
- Bhatt, A.; Glehen, O. The role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer: A Review. Indian J. Surg. Oncol. 2016, 7, 188–197. Available online: https://pubmed.ncbi.nlm.nih.gov/27065709/ (accessed on 1 June 2022). [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 148–160. Available online: https://pubmed.ncbi.nlm.nih.gov/25554246/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Ceresoli, M.; Verrengia, A.; Montori, G.; Busci, L.; Coccolini, F.; Ansaloni, L.; Frigerio, L. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: A propensity score based case-control study. J. Gynecol. Oncol. 2018, 29, e53. Available online: https://pubmed.ncbi.nlm.nih.gov/29533028/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Mendivil, A.A.; Rettenmaier, M.A.; Abaid, L.N.; Brown, J.V.; Mori, K.M.; Lopez, K.L.; Goldstein, B. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: A 3 year experience. Cancer Chemother. Pharmacol. 2017, 80, 405–410. Available online: https://pubmed.ncbi.nlm.nih.gov/28669065/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Cascales-Campos, P.A.; Gil, J.; Gil, E.; Feliciangeli, E.; González-Gil, A.; Parrilla, J.J.; Parrilla, P. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann. Surg. Oncol. 2014, 21, 2383–2389. Available online: https://pubmed.ncbi.nlm.nih.gov/24599409/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Wright, A.A.; Cronin, A.; Milne, D.E.; Bookman, M.A.; Burger, R.A.; Cohn, D.E.; Cristea, M.; Griggs, J.; Keating, N.; Levenback, C. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Oncol. 2015, 33, 2841–2847. Available online: https://pubmed.ncbi.nlm.nih.gov/26240233/ (accessed on 1 June 2022). [CrossRef]
- Dudar, T.E.; Jain, R.K. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984, 44, 605–612. Available online: https://pubmed.ncbi.nlm.nih.gov/6692365/ (accessed on 1 June 2022).
- Brown, S.L.; Hunt, J.W.; Hill, R.P. Differential thermal sensitivity of tumour and normal tissue microvascular response during hyperthermia. Int. J. Hyperth. 1992, 8, 501–514. Available online: https://pubmed.ncbi.nlm.nih.gov/1402130/ (accessed on 1 June 2022). [CrossRef]
- Salle, B.; Gilly, F.N.; Carry, P.Y.; Sayag, A.; Brachet, A.; Braillon, G. Intraperitoneal chemo-hyperthermia in the treatment of peritoneal carcinomatosis of ovarian origin. Initial cases, physiopathologic data. J. Gynecol. Obstet. Biol. Reprod. 1993, 22, 369–371. Available online: https://pubmed.ncbi.nlm.nih.gov/8360435/ (accessed on 1 June 2022).
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. Available online: https://pubmed.ncbi.nlm.nih.gov/25391263/ (accessed on 1 June 2022). [CrossRef]
- Koole, S.; van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.-E. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer 2020, 30, 888–892. Available online: https://pubmed.ncbi.nlm.nih.gov/32205449/ (accessed on 1 June 2022). [CrossRef]
- Llueca, A.; Serra, A.; Climent, M.T.; Segarra, B.; Maazouzi, Y.; Soriano, M.; Escrig, J.; on behalf MUAPOS Working Group. Outcome quality standards in advanced ovarian cancer surgery. World J. Surg. Oncol. 2020, 18, 1–9. Available online: https://wjso.biomedcentral.com/articles/10.1186/s12957-020-02064-7 (accessed on 1 June 2022). [CrossRef] [PubMed]
- Llueca, A.; Climent, M.T.; Escrig, J.; Carrasco, P.; Serra, A.; Gomez-Quiles, L. Validation of three predictive models for suboptimal cytoreductive surgery in advanced ovarian cancer. Sci. Rep. 2021, 11, 1–8. Available online: https://www.nature.com/articles/s41598-021-86928-2 (accessed on 1 June 2022). [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Herraiz, J.L.; Rivadulla, I.; Gomez-Quiles, L.; Gilabert-Estelles, J.; Escrig, J. Peritoneal carcinomatosis index as a predictor of diaphragmatic involvement in stage III and IV ovarian cancer. Onco. Targets Ther. 2018, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Cotte, E.; Colomban, O.; Guitton, J.; Tranchand, B.; Bakrin, N.; Gilly, F.N.; Glehen, O.; Tod, M. Population pharmacokinetics and pharmacodynamics of cisplatinum during hyperthermic intraperitoneal chemotherapy using a closed abdominal procedure. J. Clin. Pharmacol. 2011, 51, 9–18. Available online: https://pubmed.ncbi.nlm.nih.gov/20173087/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Hettinga, J.V.E.; Lemstra, W.; Meijer, C.; Dam, W.A.; Uges, D.R.A.; Konings, A.W.T.; Kanaar, R.; Krawczyk, P.M. Mechanism of hyperthermic potentiation of cisplatin action in cisplatin-sensitive and -resistant tumour cells. Br. J. Cancer 1997, 75, 1735–1743. Available online: https://pubmed.ncbi.nlm.nih.gov/9192975/ (accessed on 1 June 2022). [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Chen, P.; Liu, S.; Song, Z.; Ma, X. Effects of CytoReductive surgery plus hyperthermic IntraPEritoneal chemotherapy (HIPEC) versus CytoReductive surgery for ovarian cancer patients: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 301–309. Available online: https://pubmed.ncbi.nlm.nih.gov/30786961/ (accessed on 1 June 2022). [CrossRef]


| Population | Women with ovarian epithelial carcinoma in FIGO stages III–IVa. |
| Intervention | Neoadjuvant chemotherapy + Cytoreductive surgery + HIPEC ± Adjuvant chemotherapy |
| Comparison | Neoadjuvant chemotherapy + Cytoreductive surgery ± Adjuvant chemotherapy |
| Outcome | Overall survival (OS) |
| Disease free survival (DFS) | |
| Complications | |
| Study design | Clinical trials |
| Observational studies |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Publication Date | Type of Study | Number of Patients | Control Arm | Experimental Arm | NACT Scheme | HIPEC | |||
|---|---|---|---|---|---|---|---|---|---|
| HIPEC Scheme | Temperature | Duration | |||||||
| Cheol Lim M. et al. [17] | 2022 | RCT | 77 | N = 43 NACT + CRS+ ASC | N = 34 NACT + CRS + HIPEC + ASC | Carboplatin (5 mg/mL/min) + Paclitaxel (175 mg/m2) | Cisplatin (75 mg/m2) | 41.5 °C | 90 min |
| Cascales Campos P. et al. [18] | 2021 | RCT | 71 | N = 36 NACT+ CRS+ ASC | N = 35 NACT + CRS + HIPEC + ASC | Carboplatin (AUC 5) + Paclitaxel (175 mg/m2) | Cisplatin (75 mg/m2) | 42–43 °C | 60 min |
| Van Driel W.J. et al. [16] | 2018 | RCT | 245 | N = 123 NACT + CRS + ASC | N = 122 NACT + CRS + HIPEC + ASC | Carboplatin (5 mg/m2) + Paclitaxel (175 mg/m2) | Cisplatin (100 mg/m2) | 40 °C | 90 min |
| Mendevil A. et al. [24] | 2017 | CC | 138 | N = 69 NACT + CRS + ASC | N = 69 NACT + CRS + HIPEC | NE | Carboplatin (6 AUC) + Paclitaxel (80 mg/m2) | 41.5 °C | 90 min |
| Ceresoli M. et al. [23] | 2018 | CC | 56 | N = 28 NACT + CRS + ASC | N = 28 NACT + CRS + HIPEC + ASC | Carboplatin + Paclitaxel | Cisplatin (100 mg/m2) + Paclitaxel (175 mg/m2) | 41.5 °C | 90 min |
| Cascales Campos P. et al. [25] | 2014 | CC | 87 | N = 35 NACT + CRS + ASC | N = 52 NACT + CRS + HIPEC + ASC | NE | Paclitaxel 60 mg/m2 | 42 °C | 60 min |
| Arm | Patients | Age Median (Range) | FIGO n (%) | PCI Median (Range) | Median Intraoperative Time Min (Range) | Cytoreductive Surgery n (%) | |
|---|---|---|---|---|---|---|---|
| Cheol Lim M. et al. [17] | Control | n = 43 | 54 (48–61) | III n = 17 (36.5) IV n = 26 (60.5) | >6 (6–10) | 384 (328–437) | CC-0 37 (86) CC-1 6 (14) |
| Experimental | n = 34 | 55 (47–64) | III n = 15 (44.1) IV n = 19 (55.9) | >6 (6–10) | 506.5 (449–570) | CC-0 27 (79.4) CC-1 7 (20.6) | |
| Cascales Campos P. et al. [18] | Control | n = 36 | 65.5 (40–75) | III n = 30 (83.5) IV n = 6 (16.7) | 7 (2–29) | 220 (140–345) | CC-0 32 (88.9) CC-1 4 (11.1) |
| Experimental | n = 35 | 56 (29–75) | III n = 33 (94.3) IV n = 2 (5.7) | 10 (2–22) | 300 (220–490) | CC-0 33 (94.3) CC-1 2 (5.7) | |
| Van Driel W.J. et al. [16] | Control | n = 123 | 63 (56–66) | III n = 123 | NE | 192 (153–251) | CC-0 82 (67) CC-1 24 (20) |
| Experimental | n = 122 | 61 (55–66) | III n = 122 | NE | 338 (299–426) | CC-0 84 (69) CC-1 22 (18) | |
| Ceresoli M. et al. [23] | Control | n = 28 | 61.55 | III n = 20 (71.4) IV n = 8 (28.6) | 6,36 | 194 | CC-0 n = 23 (92.9) CC-1 n = 1 (36.6) |
| Experimental | n = 28 | 58.99 | III n = 22 (78.6) IV n = 6 (21.4) | 8,25 | 533 | CC-0 n = 23 (92.9) CC-1 n = 1 (3.6) | |
| Mendevil A.A et al. [24] | Control | n = 69 | 62.9 | III n = 61 (88.4) IV n = 8 (11.6) | NE | NE | CC-0 n = 64 (92.7) CC-1 n = 5 (7.3) |
| Experimental | n = 69 | 59.8 | III n = 62 (89.9) IV n = 7 (10.1) | NE | NE | CC-0 n = 69 (100) | |
| Cascales Campos P. et al. [25] | Control | n = 35 | 57 (29–73) | III n = 29 (83) IV n = 6 (17) | 6 (3–16) | 148,8 | CC-0 n = 35 (100) |
| Experimental | n = 52 | 57 (34–79) | III n = 47 (90) IV n = 5 (10) | 9 (3–26) | 360,8 | CC-0 n = 52 (100) |
| FEATURES | OVERALL SURVIVAL (OS) | DISEASE FREE SURVIVAL (DFS) | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Studies | HR (95% IC) | p Value | Heterogeneity | Number of Studies | HR (95% IC) | p Value | Heterogeneity | |
| TYPE OF STUDY | ||||||||
| RCTS | 3 | 0.56 (0.33–0.95) | 0.03 | 45% | 3 | 0.61 (0.43, 0.86) | <0.01 | 28% |
| OBSERVATIONAL | 3 | 0.69 (0.20–2.39) | 0.56 | 79% | 3 | 1.02 (0.30–3.43) | 0.97 | 91% |
| YEAR OF PUBLICATION | ||||||||
| ≤2018 | 3 | 0.71 (0.41–1.25) | 0.24 | 58% | 4 | 0.90 (0.43–1.89) | 0.77 | 88% |
| >2018 | 2 | 0.24 (0.03–2.15) | 0.2 | 75% | 2 | 0.34 (0.08–1.54) | 0.16 | 78% |
| NUMBER OF PATIENTS | ||||||||
| <100 | 4 | 0.44 (0.26–0.77) | <0.01 | 33% | 4 | 0.52 (0.27–1.00) | 0.05 | 63% |
| >100 | 2 | 0.85 (0.45–1.50) | 0.57 | 62% | 2 | 1.16 (0.37–3.60) | 0.80 | 88% |
| HIPEC REGIME | ||||||||
| CISPLATIN | 3 | 0.56 (0.33–0.95) | 0.03 | 45% | 3 | 0.61 (0.43–0.86) | <0.01 | 28% |
| CARBOPLATIN/CISPLATIN + PACLITAXEL | 2 | 0.69 (0.2–2.39) | 0.56 | 76% | 2 | 1.95 (1.26–3.01) | <0.01 | 0% |
| PACLITAXEL | 1 | NR | 1 | 0.36 (0.21–0.68) | <0.01 | NA | ||
| HIPEC TEMPERATURE | ||||||||
| <42 | 4 | 0.68 (0.52–0.88) | <0.01 | 46% | 4 | 1.00 (0.53–1.86) | 1.00 | 85% |
| ≥42 | 2 | 0.05 (0.00–0.8) | 0.03 | NA | 2 | 0.32 (0.16–0.66) | <0.01 | 11% |
| HIPEC DURATION | ||||||||
| >60 MIN | 4 | 0.68 (0.52–0.88) | <0.01 | 46% | 4 | 1.00 (0.53–1.86) | 1.00 | 85% |
| ≤60 MIN | 2 | 0.05 (0.00–0.8) | 0.03 | NA | 2 | 0.32 (0.16–0.66) | <0.01 | 11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llueca, M.; Ibañez, M.V.; Climent, M.T.; Serra, A.; Llueca, A., on behalf of MUAPOS and OSRG Working Group. Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 258. https://doi.org/10.3390/jpm13020258
Llueca M, Ibañez MV, Climent MT, Serra A, Llueca A on behalf of MUAPOS and OSRG Working Group. Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2023; 13(2):258. https://doi.org/10.3390/jpm13020258
Chicago/Turabian StyleLlueca, Maria, Maria Victoria Ibañez, Maria Teresa Climent, Anna Serra, and Antoni Llueca on behalf of MUAPOS and OSRG Working Group. 2023. "Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis" Journal of Personalized Medicine 13, no. 2: 258. https://doi.org/10.3390/jpm13020258
APA StyleLlueca, M., Ibañez, M. V., Climent, M. T., Serra, A., & Llueca, A., on behalf of MUAPOS and OSRG Working Group. (2023). Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 13(2), 258. https://doi.org/10.3390/jpm13020258






