Evaluation of Clinical Results regarding Peristomal Skin Health Associated with the Adjustment and Formulation of the New Moderma Flex One-Piece Ostomy Devices
Abstract
1. Introduction
2. Material and Methods
2.1. Design and Participants
2.2. Procedure for the Collection of Information
2.3. Information Sources and Study Variables
2.4. Statistical Analysis Used
3. Results
3.1. Description of the Device Used in the Initial Visit
3.2. Description of the Moderma Flex Device Used
3.3. Evaluation of Improvement, Adverse Events, Evaluation of the Barrier, Viewing Window, and Urostomy after the Use of the New Device
3.4. Subanalysis of Peristomal Skin Enhancement Depending on Device Characteristics and Ostomized Time
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doughty, D. Principles of ostomy management in the oncology patient. J. Support. Oncol. 2005, 3, 59–69. Available online: https://pubmed.ncbi.nlm.nih.gov/15724947/ (accessed on 28 June 2022). [PubMed]
- Charúa-Guindic, L.; Benavides-León, C.; Villanueva-Herrero, J.; Jiménez-Bobadilla, B.; Abdo-Francis, J.; Hernández-Labra, E. Quality of life in ostomized patients. Cir. Cir. 2011, 59, 149–155. Available online: https://pubmed.ncbi.nlm.nih.gov/21631976/ (accessed on 28 June 2022).
- Neuberger, L.; Braude, P.; Weeks, K.; Braude, P.; Halliday, R.; McCarthy, K.; Carter, B. A new stoma for an older person—An association with quality of life and physical function: A systematic review. J. Am. Geriatr. Soc. 2022, 70, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.; Thyø, A.; Christensen, P. Systematic review of the impact of demographic and socioeconomic factors on quality of life in ostomized colorectal cancer survivors. Acta Oncol. 2019, 58, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, J.A.; Tenfelde, S.; Hayden, D.M. Sexual Dysfunction and Intimacy for Ostomates. Clin. Colon Rectal Surg. 2017, 30, 201–206. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28684938 (accessed on 30 June 2022). [PubMed]
- Danielsen, A.K.; Burcharth, J.; Rosenberg, J. Spouses of patients with a stoma lack information and support and are restricted in their social and sexual life: A systematic review. Int. J. Colorectal Dis. 2013, 28, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Tripaldi, C. Sexual function after stoma formation in women with colorectal cancer. Br. J. Nurs. 2019, 28, S4–S15. [Google Scholar] [CrossRef] [PubMed]
- Ayaz-Alkaya, S. Overview of psychosocial problems in individuals with stoma: A review of literature. Int. Wound J. 2019, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Maglio, A.; Malvone, A.P.; Scaduto, V.; Brambilla, D.; Denti, F.C. The frequency of early stomal, peristomal and skin complications. Br. J. Nurs. 2021, 30, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, E.; Persson, E.; Carlsson, E.; Hallén, A.M.; Fingren, J.; Berndtsson, I. Ostomy-related complications after emergent abdominal surgery a 2-year follow-up study. J. Wound Ostomy Cont. Nurs. 2013, 40, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.A.M.; Lee, M.J.; Harikrishnan, A.B. The incidence of stoma related morbidity—A systematic review of randomised controlled trials. Ann. R. Coll. Surg. Engl. R. Coll. Surg. Engl. 2018, 100, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, E.; Colwell, J.; Cannon, L.M. Intestinal Stomas-Postoperative Stoma Care and Peristomal Skin Complications. Clin. Colon Rectal Surg. 2017, 30, 184–192. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28684936 (accessed on 30 June 2022). [CrossRef] [PubMed]
- Berg, K.; Seidler, H. Randomized crossover comparison of adhesively coupled colostomy pouching systems. Ostomy Wound Manag. 2005, 51, 36. Available online: https://pubmed.ncbi.nlm.nih.gov/15984397/ (accessed on 28 June 2022).
- Marquis, P.; Marrel, A.; Jambon, B. Quality of life in patients with stomas: The Montreux Study. Ostomy Wound Manag. 2003, 49, 48–55. [Google Scholar]
- Malbos, N.; Ruelle, M.O.; Bodenez, C. Les stomies digestives et leur appareillage. Actual. Pharm. 2022, 61, 18–25. [Google Scholar] [CrossRef]
- Palmer, S.J. Overview of stoma care for community nurses. Br. J. Commun. Nurs. 2020, 25, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Mora Rivas, C. Estudio Avance de los dispositivos de ostomía de una y dos piezas: Moderma flex y Conform 2 de Hollister. Metas Enferm. 2012, 14, 22–26. [Google Scholar]
- Hayles, K. Getting it right the first time: How to select stoma accessories. Gastrointest. Nurs. 2014, 12, 36–46. [Google Scholar] [CrossRef]
- Gachabayov, M.; Dyatlov, A.; Bergamaschi, R. Hawthorne Effect Should Be Controlled for in Quality Control Studies. JAMA Surg. Am. Med. Assoc. 2019, 154, 977. [Google Scholar] [CrossRef] [PubMed]
| Clinical and Sociodemographic Characteristics | % (n) |
|---|---|
| Gender | |
| Man | 54.6 (167) |
| Woman | 45.4 (139) |
| Age (Years) | 64.5 (12.43) |
| Time from completion of ostomy | |
| <1 week | 8.8 (27) |
| 1 week–1 month | 22.5 (69) |
| 1 month–3 months | 26.5 (81) |
| 3 months–6 months | 19.3 (59) |
| 6 months–12 months | 22.9 (70) |
| Type of ostomy (depending on surgery) | |
| Urostomy | 17.6 (54) |
| Ileostmy | 25.2 (77) |
| Colostomy | 57.2 (175) |
| Type of ostomy according to duration | |
| Temporary | 44.1 (135) |
| Permanent | 55.9 (171) |
| Type of ostomy according to technique | |
| Loop ostomy | 20.6 (63) |
| Termina | 69.9 (214) |
| Other Type | 9.5 (29) |
| Morphology of the stoma | |
| Round | 52.6 (161) |
| Protruding | 32.0 (98) |
| Oval | 27.1 (83) |
| Flat | 29.1 (89) |
| Irregular | 6.9 (21) |
| Invaginate | 8.2 (25) |
| Edematous | 0.7 (2) |
| Stenosed | 1.3 (4) |
| Type of abdomen | |
| Smooth | 48.4 (148) |
| Globulous | 42.2 (129) |
| Folds | 32.0 (98) |
| Flaccid | 40.8 (125) |
| Scars | 26.1 (80) |
| Skin Type | |
| Dry | 40.5 (124) |
| Mixed | 54.9 (168) |
| Oily | 4.6 (14) |
| Skin type according to integrity | |
| Intact | 65.0 (199) |
| Irritated | 27.8 (85) |
| Damaged | 7.2 (22) |
| Complications observed in the skin | |
| None | 64.1 (196) |
| Irritative contact dermatitis | 21.9 (67) |
| Mechanical dermatitis | 6.9 (21) |
| Granuloma | 3.3 (10) |
| Pressure ulcer | 0.3 (1) |
| Dehiscence mucocutaneous suture | 2.3 (7) |
| Dermatitis Incrustation | 0.3 (1) |
| Incrustation | 0.3 (1) |
| Neoplasia | 0.3 (1) |
| You have a condition that can put peristomal health at risk | |
| No | 90.8 (278) |
| Yes | 9.2 (28) |
| Oncological treatment | 8 |
| Crohn desease | 2 |
| Paraestomal Hernia | 2 |
| Ulcerative colitis | 1 |
| Atopy/eczema/psoriasis | 3 |
| Characteristics of the Device Used | % (n) |
|---|---|
| Type of device you currently use (opening) | |
| Closed | 36.3 (111) |
| Drainable | 25.2 (77) |
| With valve | 14.7 (45) |
| Does not apply | 23.8 (73) |
| Device currently in use (by pieces) | |
| 1 piece | 25.2 (77) |
| 2 pieces | 51.0 (156) |
| 3 pieces | 1.6 (5) |
| Others | 0.7 (2) |
| Does not apply | 21.6 (66) |
| Device currently used according to foil type | |
| Flat barrier | 52.6 (161) |
| Convex barrier | 23.2 (71) |
| Does not apply | 24.2 (74) |
| Accessories used with the device | |
| Hydrocolloid rings | 17.0 (52) |
| Hydrocolloid powders | 17.6 (54) |
| Protective strips | 2.0 (6) |
| Hydrocolloid paste | 18.3 (56) |
| Barrier cream | 0.0 (0) |
| Convex ring | 3.6 (11) |
| Belt | 22.9 (70) |
| Skin barrier spray | 17.0 (52) |
| Adhesive remover spray | 12.4 (38) |
| Other accessories | 17.0 (52) |
| Does not apply | 26.1 (80) |
| Number of accessories used | |
| None | 42.8 (131) |
| One | 22.2 (68) |
| Two | 20.9 (64) |
| Three | 11.1 (34) |
| Four | 1.6 (5) |
| Five | 1.0 (3) |
| Six | 0.3 (1) |
| Frequency of the device change | |
| More than once a day | 19.0 (58) |
| Every day | 20.9 (64) |
| Every two days | 21.9 (67) |
| Every three days | 16.0 (49) |
| Every four days | 2.6 (8) |
| Does not apply | 19.6 (60) |
| Reason for the device change | |
| Rutine | 50.3 (154) |
| Leaks | 24.2 (74) |
| Irritation | 9.8 (30) |
| Itching | 5.2 (16) |
| Peeling Sheet | 9.2 (28) |
| Erosion Sheet | 2.0 (6) |
| Characteristics of the Device Used | % (n) |
|---|---|
| Type of device you currently use (opening) | |
| Closed | 45.1 (138) |
| Drainable | 34.0 (104) |
| With valve | 17.0 (52) |
| Missing | 12 |
| Device currently used (per sheet) | |
| Flat barrier | 47.7 (146) |
| Soft convex barrier | 38.9 (119) |
| Moderate convex barrier | 9.5 (29) |
| Missing | 12 |
| Adhesive border | |
| No | 59.9 (176) |
| Yes | 40.1 (118) |
| Missing | 12 |
| Viewing window | |
| No | 44.6 (131) |
| Yes | 55.4 (163) |
| Missing | 12 |
| Accessories used with the device | |
| Hydrocolloid rings | 15.6 (46) |
| Hydrocolloid powders | 19.0 (56) |
| Protective strips | 1.7 (5) |
| Hydrocolloid paste | 11.2 (33) |
| Barrier cream | 1.0 (3) |
| Convex rings | 4.1 (12) |
| Belt | 25.2 (74) |
| Skin barrier spray | 19.0 (56) |
| Adhesive remover spray | 17.0 (50) |
| Other accessories | 37.4 (110) |
| Number of accessories used | |
| None | 38.1 (112) |
| One | 28.6 (84) |
| Two | 20.4 (60) |
| Three | 8.2 (24) |
| Four | 4.1 (12) |
| Five | 0.3 (1) |
| Six | 0.3 (1) |
| Frequency of the device change | |
| More than once a day | 40.1 (118) |
| Every day | 41.2 (121) |
| Every two days | 16.7 (49) |
| Every three days | 1.7 (5) |
| Every four days | 0.3 (1) |
| Missing | 12 |
| Degree of Agreement (Scale 1 Nothing According to 5 Very Agree) | ||||||
|---|---|---|---|---|---|---|
| Nothing 1 n (%) | Little 2 n (%) | Indifferent 3 n (%) | Fairly 4 n (%) | Very 5 n (%) | Average (DE) | |
| Improvement of the periostomal skin | ||||||
| All (n = 294) | 18 (6.1) | 4 (1.4) | 43 (14.6) | 88 (29.9) | 141 (48.8) | 4.12 (1.11) |
| With intact skin (n = 190) | 15 (7.9) | 2 (1.1) | 28 (14.7) | 55 (28.9) | 90 (47.4) | 4.07 (1.17) |
| With damaged or irritated skin (n = 104) | 3 (2.9) | 2 (1.9) | 15 (14.4) | 33 (31.7) | 51 (49.0) | 4.22 (0.97) |
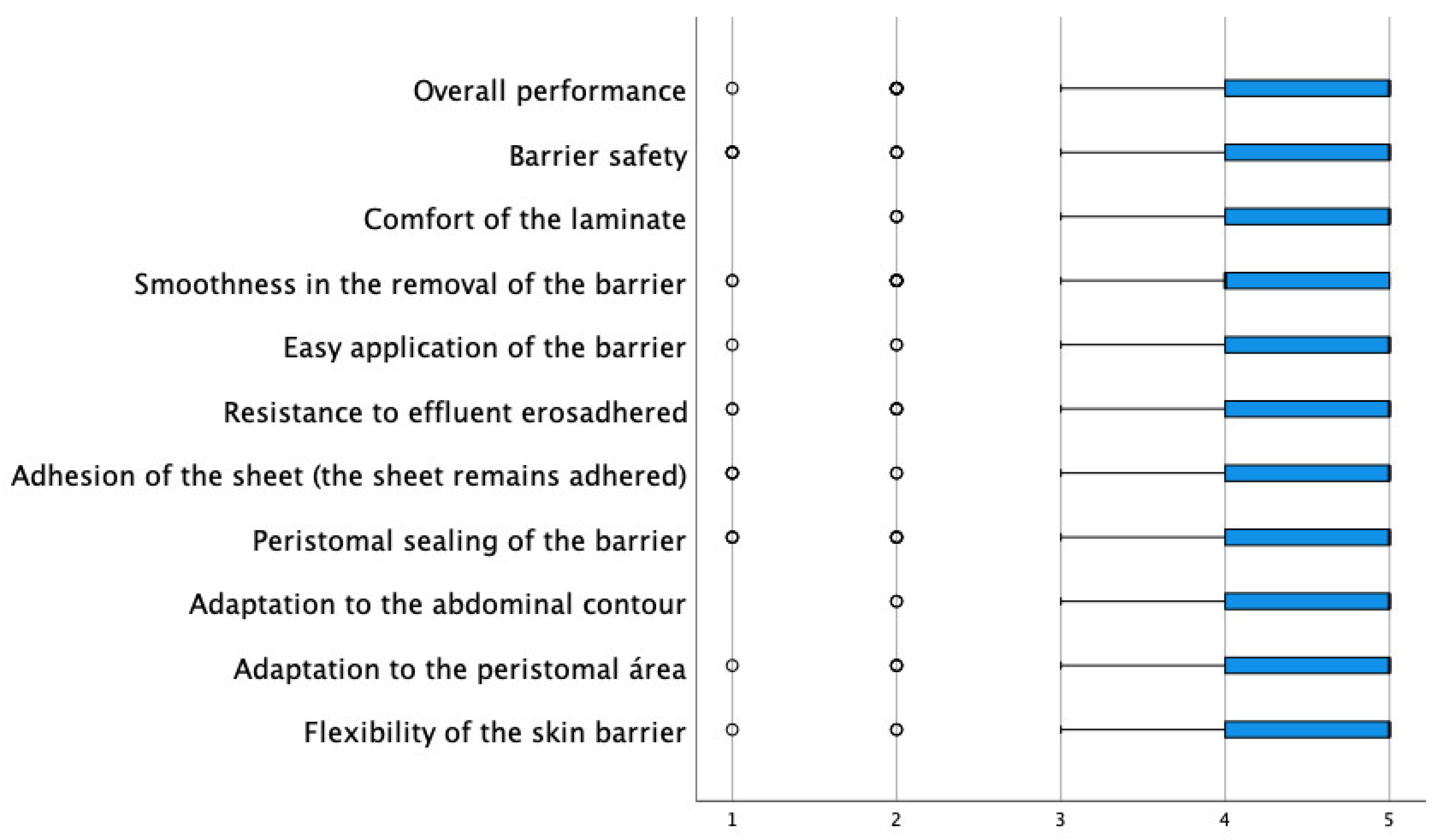
| Cutaneous lamina (n = 294) | ||||||
| Flexibility of the skin barrier | 1 (0.3) | 2 (0.7) | 17 (5.8) | 109 (37.1) | 165 (56.1) | 4.48 (0.67) |
| Adaptation to the peristomal area | 1 (0.3) | 3 (1.0) | 16 (5.4) | 94 (32.0) | 180 (61.2) | 4.45 (0.68) |
| Adaptation to the abdominal contour | 0 (0.0) | 2 (0.7) | 15 (4.9) | 84 (27.5) | 191 (62.4) | 4.59 (0.62) |
| Peristomal sealing of the barrier | 3 (1.0) | 4 (1.4) | 26 (8.5) | 103 (35.3) | 156 (53.4) | 4.39 (0.79) |
| Adhesion of the blade | 4 (1.4) | 2 (0.7) | 15 (5.1) | 89 (30.3) | 184 (62.6) | 4.52 (0.75) |
| Resistance to effluent erosion | 4 (1.4) | 4 (1.4) | 27 (9.2) | 107 (36.4) | 154 (52.4) | 4.38 (0.77) |
| Easy application of the barrier | 1 (0.3) | 2 (0.7) | 19 (6.5) | 88 (30.1) | 182 (62.3) | 4.53 (0.68) |
| Smoothness in the removal of the barrier | 2 (0.7) | 8 (2.7) | 31 (10.6) | 109 (37.2) | 143 (48.8) | 4.31 (0.82) |
| Blade comfort | 0 (0.0) | 2 (1.0) | 11 (3.7) | 91 (31.0) | 189 (64.3) | 4.59 (0.62) |
| Barrier safety | 6 (2.0) | 4 (1.4) | 21 (7.1) | 89 (30.3) | 174 (59.2) | 4.43 (0.85) |
| Overall performance of the sheet | 1 (0.3) | 10 (3.4) | 12 (4.1) | 106 (34.6) | 164 (56.0) | 4.44 (0.76) |
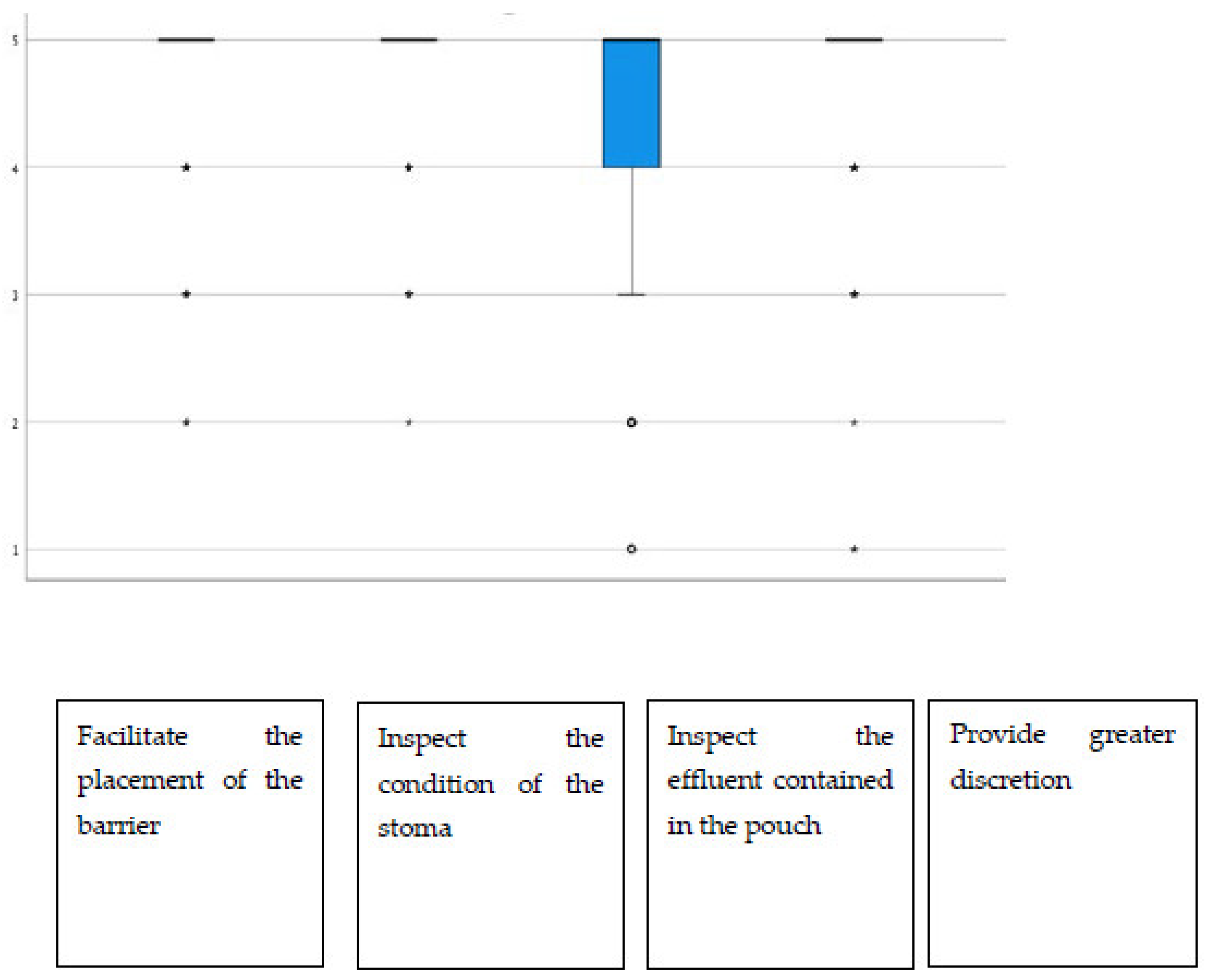
| Viewing option (n = 186) | ||||||
| Facilitate the placement of the barrier | 0 (0.0) | 3 (1.6) | 10 (5.4) | 32 (17.2) | 141 (75.8) | 4.67 (0.65) |
| Inspect the condition of the stoma | 0 (0.0) | 1 (0.5) | 9 (4.8) | 32 (17.2) | 146 (77.7) | 4.72 (0.58) |
| Inspect the effluent contained in the pouch | 2 (1.1) | 6 (3.2) | 15 (8.0) | 45 (24.1) | 119 (63.6) | 4.46 (0.86) |
| Provide greater discretion | 3 (1.6) | 1 (0.5) | 11 (5.9) | 29 (15.5) | 143 (76.5) | 4.65 (0.76) |
| Facilitate the placement of the barrier | 0 (0.0) | 3 (1.6) | 10 (5.4) | 32 (17.2) | 141 (75.8) | 4.67 (0.65) |
| Inspect the condition of the stoma | 0 (0.0) | 1 (0.5) | 9 (4.8) | 32 (17.2) | 146 (77.7) | 4.72 (0.58) |
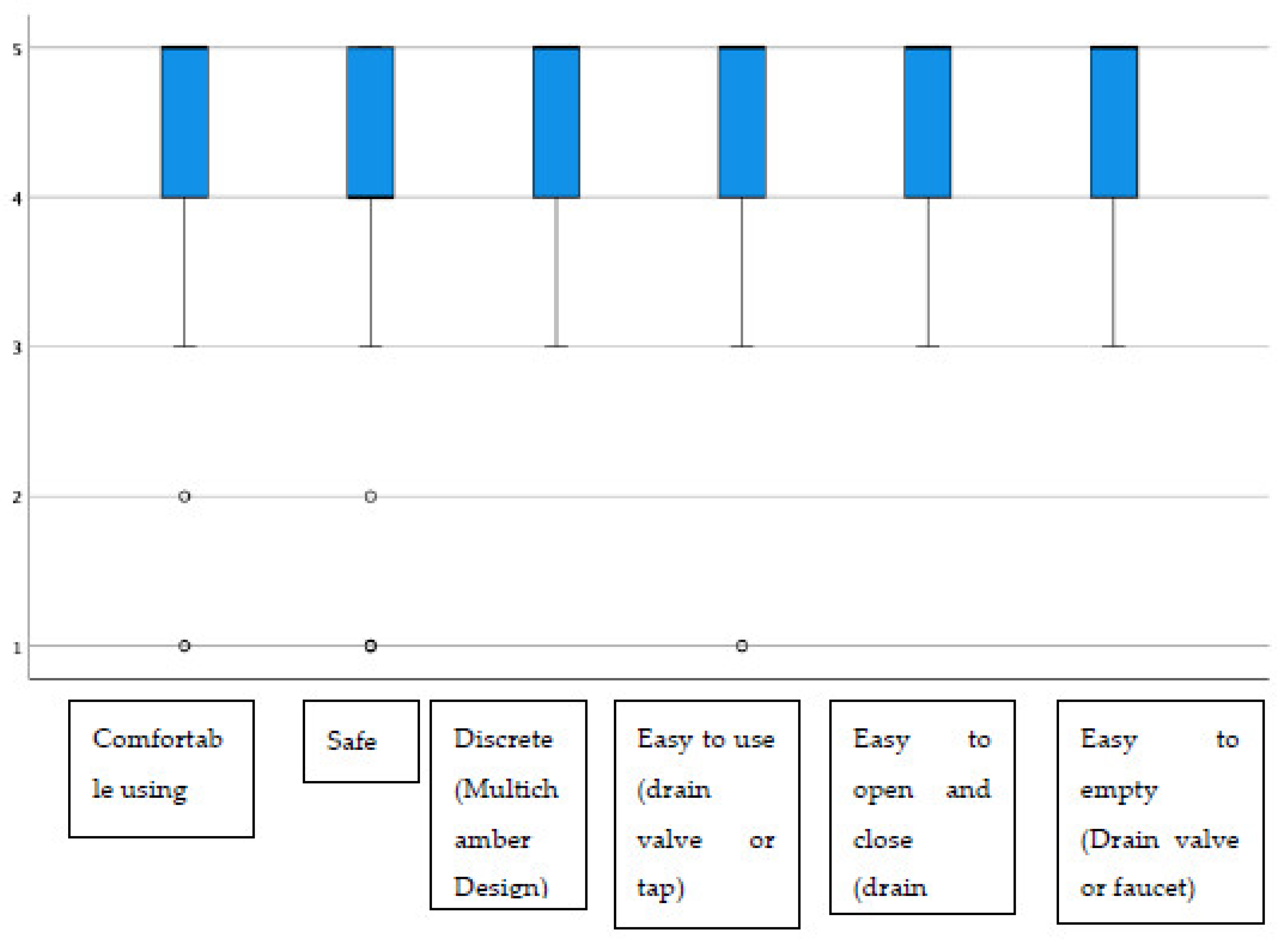
| Urostomy device (N = 58) | ||||||
| Comfortable using | 1 (1.7) | 1 (1.7) | 4 (6.9) | 18 (31.0) | 34 (58.6) | 4.43 (0.84) |
| Safe | 3 (5.2) | 1 (1.7) | 2 (3.4) | 24 (41.4) | 28 (48.3) | 4.26 (1.00) |
| Discrete (multichamber design) | 0 (0.0) | 0 (0.0) | 10 (17.5) | 13 (22.8) | 34 (59.6) | 4.42 (0.78) |
| Easy to use (drain valve or tap) | 1 (1.8) | 0 (0.0) | 3 (5.3) | 21 (36.8) | 32 (56.1) | 4.46 (0.76) |
| Easy to open and close (drain valve or tap) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 22 (39.3) | 32 (57.1) | 4.54 (0.57) |
| Easy to empty (drain valve or tap) | 0 (0.0) | 0 (0.0) | 1 (1.8) | 19 (33.3) | 37 (64.9) | 4.63 (0.52) |
| Adverse Event | Not Recovered Yet% (n) | Recovered % (n) | Total N = 278 % (n) |
|---|---|---|---|
| None | 92.4 (257) | ||
| Erythema | 12.5 (1) | 87.5 (7) | 2.7 (8) |
| Erosion | 100 (2) | 0.0 (0) | 0.7 (2) |
| Hypergranulation | 100 (2) | 0.0 (0) | 0.7 (2) |
| Necrotic tissue | 50.0 (1) | 50.0 (1) | 0.7 (2) |
| Leakage | 33.3 (1) | 66.6 (2) | 1.02 (3) |
| Prolapse | 100 (1) | 0.0 (0) | 0.34 (1) |
| Rod | 0.0 (0) | 100 (1) | 0.34 (1) |
| Irritation | 0.0 (0) | 100 (1) | 0.34 (1) |
| Pruritus | 0.0 (0) | 100 (1) | 0.34 (1) |
| Has Peristomal Skin Improved? (Scale 1 Nothing According to 5 Very Agree) (N = 294) | Value p | ||||||
|---|---|---|---|---|---|---|---|
| Nothing 1 n (%) | Little 2 n (%) | Indifferent 3 n (%) | Fairly 4 n (%) | Very 5 n (%) | Average (DE) | ||
| Type of device you currently use (opening) | 0.232 | ||||||
| Close | 10 (7.2) | 1 (0.7) | 15 (10.9) | 32 (23.2) | 80 (58.0) | 4.24 (1.15) | |
| Open | 5 (4.8) | 1 81.0) | 20 (19.2) | 40 (38.5) | 38 (36.5) | 4.01 (1.02) | |
| Drain valve | 3 (5.8) | 2 (3.8) | 8 (15.4) | 16 (30.8) | 23 (44.2) | 4.04 (1.14) | |
| Device currently used according to the type of sheet | 0.244 | ||||||
| Flat | 7 (4.8) | 1 (0.7) | 23 (15.8) | 39 (26.7) | 76 (52.1) | 4.21 (1.05) | |
| Soft Convexity | 10 (8.4) | 3 (2.5) | 16 (13.4) | 39 (32.8) | 51 (42.9) | 3.99 (1.20) | |
| Moderate Convexity | 1 (3.4) | 0 (0.0) | 4 (13.8) | 10 (34.5) | 14 (48.3) | 4.24 (0.95) | |
| Adhesive Border | 0.008 | ||||||
| No | 10 (5.7) | 2 (1.1) | 21 (11.9) | 42 (23.9) | 101 (57.4) | 4.26 (1.09) | |
| Yes | 8 (6.8) | 1 (1.7) | 22 (18.6) | 46 (39.0) | 40 (33.9) | 3.92 (1.09) | |
| Viewing option | 0.593 | ||||||
| No | 7 (5.3) | 2 (1.5) | 22 (16.8) | 42 (32.1) | 58 (44.3) | 4.08 (1.07) | |
| Yes | 11 (6.7) | 2 (1.2) | 21 (12.9) | 46 (28.2) | 83 (50.9) | 4.15 (1.31) | |
| Ostomized time | 0.236 | ||||||
| <1 week | 2 (7.4) | 0 (0.0) | 0 (0.0) | 7 (25.9) | 18 (66.7) | 4.44 (1.09) | |
| 1 week–1 month | 4 (5.9) | 1 (1.5) | 14 (20.6) | 27 (39.7) | 22 (32.4) | 3.91 (1.06) | |
| 1 month–3 months | 4 (5.9) | 1 (1.3) | 11 (14.3) | 24 (31.2) | 37 (48.1) | 4.16 (1.07) | |
| 3 months–6 months | 3 (5.5) | 1 (1.8) | 6 (10.9) | 15 (27.3) | 30 (54.5) | 4.24 (1.09) | |
| 6 months–12 months | 5 (7.5) | 1 (1.5) | 12 (17.9) | 15 (22.4) | 34 (50.7) | 4.07 (1.20) | |
| Has the Peristomal Skin Improved? (Scale 1 Nothing According to 5 Very Agree) (N = 104) | Value p | ||||||
|---|---|---|---|---|---|---|---|
| Nothing 1 n (%) | Little 2 n (%) | Indifferent 3 n (%) | Fairly 4 n (%) | Very 5 n (%) | Average (DE) | ||
| Type of device you currently use (opening) | 0.230 | ||||||
| Closed | 1 (2.8) | 0 (0.0) | 2 (5.6) | 1 (25.6) | 23 (53.5) | 4.44 (0.84) | |
| Drainable | 1 (2.0) | 1 (2.0) | 8 (15.7) | 18 (35.3) | 23 (45.2) | 4.11 (0.92) | |
| With valve | 0 (0.0) | 0 (0.0) | 1 (10.0) | 4 (40.0) | 5 (50.0) | 4.09 (1.19) | |
| Device currently used (per sheet) | 0.828 | ||||||
| Flat | 2 (4.7) | 1 (2.3) | 6 (14.0) | 11 (25.6) | 23 (53.5) | 4.21 (1.08) | |
| Soft convexity | 1 (2.0) | 1 (2.0) | 8 (15.7) | 18 (35.3) | 23 (45.1) | 4.20 (0.92) | |
| Moderate convexity | 0 (0.0) | 0 (0.0) | 1 (10.0) | 4 (40.0) | 5 (50.0) | 4.40 (0.70) | |
| Adhesive border | 0.011 | ||||||
| No | 1 (1.8) | 1 (1.8) | 4 (7.0) | 17 (29.8) | 34 (59.6) | 4.44 (0.85) | |
| Yes | 2 (4.3) | 1 (2.1) | 11 (23.4) | 16 (34.0) | 17 (36.2) | 3.96 (1.04) | |
| Viewing option | 0.437 | ||||||
| No | 1 (2.0) | 1 (2.0) | 10 (20.4) | 15 (30.6) | 22 (44.9) | 4.14 (0.96) | |
| Yes | 2 (3.6) | 1 (1.8) | 5 (9.1) | 18 (32.7) | 29 (52.7) | 4.29 (0.98) | |
| Ostomized time | 0.350 | ||||||
| <1 week | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 4 (80.0) | 4.80 (0.45) | |
| 1 week–1 month | 0 (0.0) | 0 (0.0) | 4 (25.0) | 7 (43.8) | 5 (31.3) | 4.07 (0.77) | |
| 1 month–3 months | 0 (0.0) | 1 (3.0) | 7 (21.2) | 10 (30.3) | 15 (45.5) | 4.18 (0.88) | |
| 3 motnhs–6 months | 1 (4.8) | 0 (0.0) | 0 (0.0) | 7 (33.3) | 13 (61.9) | 4.48 (0.93) | |
| 6 months–12 months | 2 (6.9) | 1 (3.4) | 4 (13.8) | 8 (27.6) | 14 (48.3) | 4.07 (1.20) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera García, S.; Espejo Lunar, E.-M.; Rodríguez-Almagro, J.; Louzao Méndez, S. Evaluation of Clinical Results regarding Peristomal Skin Health Associated with the Adjustment and Formulation of the New Moderma Flex One-Piece Ostomy Devices. J. Pers. Med. 2023, 13, 219. https://doi.org/10.3390/jpm13020219
Rivera García S, Espejo Lunar E-M, Rodríguez-Almagro J, Louzao Méndez S. Evaluation of Clinical Results regarding Peristomal Skin Health Associated with the Adjustment and Formulation of the New Moderma Flex One-Piece Ostomy Devices. Journal of Personalized Medicine. 2023; 13(2):219. https://doi.org/10.3390/jpm13020219
Chicago/Turabian StyleRivera García, Sebastian, Esperanza-Macarena Espejo Lunar, Julian Rodríguez-Almagro, and Silvia Louzao Méndez. 2023. "Evaluation of Clinical Results regarding Peristomal Skin Health Associated with the Adjustment and Formulation of the New Moderma Flex One-Piece Ostomy Devices" Journal of Personalized Medicine 13, no. 2: 219. https://doi.org/10.3390/jpm13020219
APA StyleRivera García, S., Espejo Lunar, E.-M., Rodríguez-Almagro, J., & Louzao Méndez, S. (2023). Evaluation of Clinical Results regarding Peristomal Skin Health Associated with the Adjustment and Formulation of the New Moderma Flex One-Piece Ostomy Devices. Journal of Personalized Medicine, 13(2), 219. https://doi.org/10.3390/jpm13020219






