Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
- Chest deformity other than funnel-shaped;
- Advanced chronic respiratory or circulatory failure;
- Emergency surgery or reoperation;
- History of thoracotomy or thoracic surgery;
- Mental impairment precluding communication with the patient or lack of consent for cryoanalgesia or regional analgesia;
- History of allergy to local anaesthetics;
- History of use of medication for chronic pain [22].
2.2. Preparation for Surgery
2.3. Anaesthesia
2.4. Surgery
2.5. Postoperative Course
2.6. Outcomes
- The acute pain intensity (maximum) on the first day after surgery—measured every 1 h for 24 h using the NRS numerical scale (0–10 points);
- The duration of the requirement for intravenous opioid use (days after surgery);
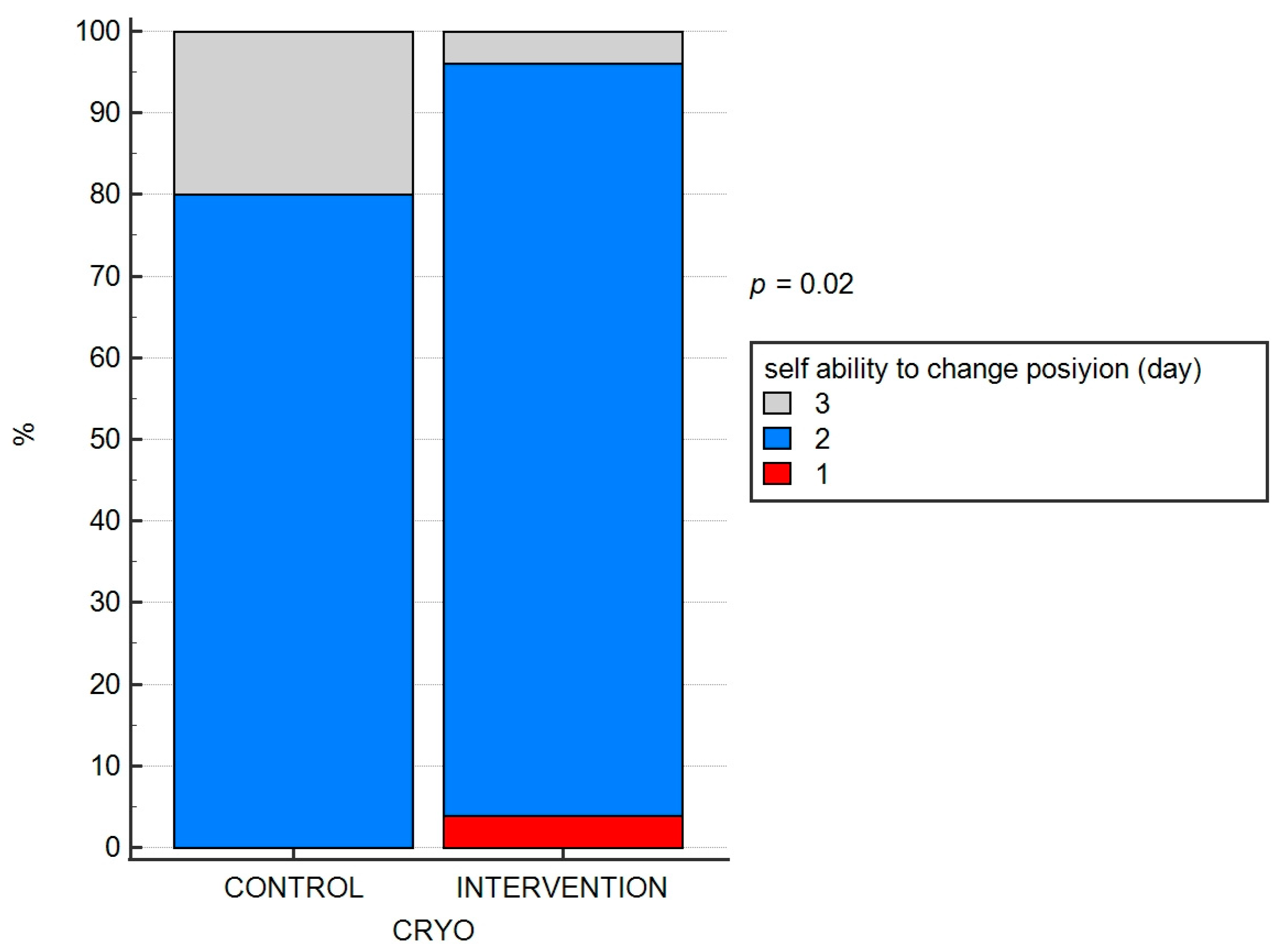
- The quality of postoperative rehabilitation in terms of correctness of exercises and achievement of independence of movement in daily activities (days after surgery) based on a self-assessment;
- The duration of surgery from skin incision to skin suture (minutes);
- The length of stay (LOS) in a hospital (days);
- The occurrence of adverse reactions to pharmacotherapy and anaesthesia;
- The occurrence of complications after cryoablation and Nuss surgery [22].
2.7. Statistics
3. Results
3.1. Patients Characteristics
3.2. Treatment Efficacy
3.3. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashcraft, K.W.; Holcomb, G.W.; Murphy, J.P.; Ostlie, D.J. Ashcraft’s Pediatric Surgery, 6th ed.; Saunders/Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Linton, S.C.; Ghomrawi, H.M.; Tian, Y.; Many, B.T.; Vacek, J.; Bouchard, M.E.; De Boer, C.; Goldstein, S.D.; Abdullah, F. Association of Operative Volume and Odds of Surgical Complication for Patients Undergoing Repair of Pectus Excavatum at Children’s Hospitals. J. Pediatr. 2021, 244, 154–160.e3. [Google Scholar] [CrossRef] [PubMed]
- Delande, S.; Lavand’homme, P. Acute pain management and long term outcomes. Curr. Opin. Anaesthesiol. 2023, 36, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, R.L.M.; Vissers, K.C.P.; van der Sande, R.; Bronkhorst, E.; Lerou, J.G.C.; Steegers, M.A.H. Moving beyond pain scores: Multidimensional pain assessment is essential for adequate pain management after surgery. PLoS ONE 2017, 12, e0177345. [Google Scholar] [CrossRef]
- De Loos, E.; Pennings, A.; van Roozendaal, L.; Daemen, J.; van Gool, M.; Lenderink, T.; van Horck, M.; Hulsewe, K.; Vissers, Y. Nuss Procedure for Pectus Excavatum: A Comparison of Complications Between Young and Adult Patients. Ann. Thorac. Surg. 2021, 112, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Cettler, M.; Zielińska, M.; Rosada-Kurasińska, J.; Kubica-Cielińska, A.; Jarosz, K.; Bartkowska-Śniatkowska, A. Guidelines for treatment of acute pain in children—The consensus statement of the Section of Paediatric Anaesthesiology and Intensive Therapy of the Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2022, 54, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Dekonenko, C.; Dorman, R.M.; Duran, Y.; Juang, D.; Aguayo, P.; Fraser, J.D.; Oyetunji, T.A.; Snyder, C.L.; Holcomb, G.W.; Millspaugh, D.L.; et al. Postoperative pain control modalities for pectus excavatum repair: A prospective observational study of cryoablation compared to results of a randomized trial of epidural vs patient-controlled analgesia. J. Pediatr. Surg. 2020, 55, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.R.; Jones, J.; Semenova, J.; Williamson, A.; McCollum, K.; Tong, D.; Jerman, J.; Notrica, D.M.; Nguyen, H. Multimodal anesthesia with the addition of methadone is superior to epidural analgesia: A retrospective comparison of intraoperative anesthetic techniques and pain management for 124 pediatric patients undergoing the Nuss procedure. J. Pediatr. Surg. 2016, 51, 612–616. [Google Scholar] [CrossRef]
- Singhal, N.R.; Jerman, J.D. A review of anesthetic considerations and postoperative pain control after the Nuss procedure. Semin. Pediatr. Surg. 2018, 27, 156–160. [Google Scholar] [CrossRef]
- Kukreja, P.; Herberg, T.J.; Johnson, B.M.; Kofskey, A.M.; Short, R.T.; MacBeth, L.; Paul, C.; Kalagara, H. Retrospective Case Series Comparing the Efficacy of Thoracic Epidural with Continuous Paravertebral and Erector Spinae Plane Blocks for Postoperative Analgesia After Thoracic Surgery. Hari Cureus 2021, 13, e18533. [Google Scholar] [CrossRef]
- Chin, K.J.; Versyck, B.; Pawa, A. Ultrasound-guided fascial plane blocks of the chest wall: A state-of-the-art review. Anaesthesia 2021, 76, 110–126. [Google Scholar] [CrossRef]
- Chin, J.K.; Pawa, A.; Forero, M.; Adhikary, S. Ultrasound-Guided Fascial Plane Blocks of the Thorax: Pectoral I and II, Serratus Anterior Plane, and Erector Spinae Plane Blocks. Adv. Anesth. 2019, 37, 187–205. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Pawa, A. The erector spinae plane block: Plane and simple. Anaesthesia 2017, 72, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kambin, P.; Casey, K.F.; Bonner, F.J.; O’Brien, E.; Shao, Z.; Ou, S. Mechanism research of cryoanalgesia. Neurol. Res. 1995, 17, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of Action and Devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. S8), S187–S191. [Google Scholar] [CrossRef] [PubMed]
- Zeineddin, S.; Goldstein, S.D.; Linton, S.; DeBoer, C.; Alayleh, A.; Ortiz, I.; Sands, L.; Kujawa, S.; Suresh, S.; Ghomrawi, H.; et al. Effectiveness of one minute per level intercostal nerve cryoablation for postoperative analgesia after surgical correction of pectus excavatum. J. Pediatr. Surg. 2023, 58, 34–40. [Google Scholar] [CrossRef]
- Kim, S.; Idowu, O.; Palmer, B.; Lee, S.H. Use of transthoracic cryoanalgesia during the Nuss procedure. J. Thorac. Cardiovasc. Surg. 2016, 151, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Stevenson, F.F. Wallerian degeneration and recovery of motor nerves after multiple focused cold therapies. Muscle Nerve 2015, 51, 268–275. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Gabriel, R.A.; Trescot, A.M. Ultrasound-guided percutaneous cryoneurolysis for treatment of acute pain: Could cryo-analgesia replace continuous peripheral nerve blocks? Br. J. Anaesth. 2017, 119, 703–706. [Google Scholar] [CrossRef]
- Velayos, M.; Alonso, M.; Delgado-Miguel, C.; Estefanía-Fernández, K.; Muñoz-Serrano, A.J.; Santamaría, M.V.L.; Reinoso-Barbero, F.; De La Torre, C.A. Percutaneous Cryoanalgesia: A New Strategy for Pain Management in Pectus Excavatum Surgery. Eur. J. Pediatr. Surg. 2022, 32, 73–79. [Google Scholar] [CrossRef]
- Torre, M.; Mameli, L.; Bonfiglio, R.; Guerriero, V.; Derosas, L.; Palomba, L.; Disma, N. A New Device for Thoracoscopic Cryoanalgesia in Pectus Excavatum Repair: Preliminary Single Center Experience. Front. Pediatr. 2021, 8, 614097. [Google Scholar] [CrossRef]
- Zacha, S.; Andrzejewska, A.; Jastrzębska-Ligocka, B.; Szwed, A.; Modrzejewska, E.; Zacha, W.; Skonieczna-Żydecka, K.; Miegoń, J.; Jarosz, K.; Biernawska, J. Intercostal nerve cryoanalgesia in the treatment of pain in patients operated on by the modified Nuss method with the BackOnFeet application—A new strategy to improve outcomes. Front. Pediatr. 2023, 10, 1069805. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.R.; Farina, J.M.; Botros, M.M.; Jaroszewski, D.E. Minimally invasive repair of pectus excavatum in adults: A review article of presentation, workup, and surgical treatment. J. Thorac. Dis. 2023, 15, 5150–5173. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Notrica, D.M.; McMahon, L.E.; Kang, P.; Molitor, M.S.; Egan, J.C.; Bae, J.-O.; Hunteman, Z.M.; Ostlie, D.J.; Lee, J.H.; et al. Cryoablation in 350 Nuss Procedures: Evolution of Hospital Length of Stay and Opioid Use. J. Pediatr. Surg. 2023, 58, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, H.C.; Hrachovec, J.; Schnuck, J.; Nchinda, N.; Meehan, J. Implementation of a Cryo-ablation-based Pain Management Protocol for Pectus Excavatum. J. Pediatr. Surg. 2023, 58, 1239–1245. [Google Scholar] [CrossRef]
- Arshad, S.A.; Garcia, E.I.; Bell, C.; Avritscher, E.B.; Kumar, M.; Brahmamdam, P.; Fraser, J.A.; Peter, S.D.S.; Aranda, A.; Hill, M.; et al. Multicenter Assessment of Cryoanalgesia Use in Minimally Invasive Repair of Pectus Excavatum: A 20-center Retrospective Cohort Study. Ann. Surg. 2023, 277, E1373–E1379. [Google Scholar] [CrossRef]
- Breglio, A.M.; Fitzgerald, T.N.; Moore, C.B.; Einhorn, L.M. Evaluation of Analgesic Practice Changes Following the Nuss Procedure in Pediatric Patients. J. Surg. Res. 2023, 291, 289–295. [Google Scholar] [CrossRef]
- Holguin, R.A.P.; DeAngelo, N.; Sinha, A.; Shen, C.; Tsai, A.Y. Cost and outcomes of intercostal nerve cryoablation versus thoracic epidural following the nuss procedure. J. Pediatr. Surg. 2023, 58, 608–612. [Google Scholar] [CrossRef]
- Fraser, J.A.; Briggs, K.B.; Svetanoff, W.J.; Aguayo, P.; Juang, D.; Fraser, J.D.; Snyder, C.L.; Oyetunji, T.A.; Peter, S.D.S. Short and long term outcomes of using cryoablation for postoperative pain control in patients after pectus excavatum repair. J. Pediatr. Surg. 2022, 57, 1050–1055. [Google Scholar] [CrossRef]
- Graves, C.E.; Moyer, J.; Zobel, M.J.; Mora, R.; Smith, D.; O’Day, M.; Padilla, B.E. Intraoperative intercostal nerve cryoablation During the Nuss procedure reduces length of stay and opioid requirement: A randomized clinical trial. J. Pediatr. Surg. 2019, 54, 2250–2256. [Google Scholar] [CrossRef]
- Scalise, N.; Demehri, F. The management of pectus excavatum in pediatric patients: A narrative review. Transl. Pediatr. 2023, 12, 208–220. [Google Scholar] [CrossRef]
- Zobel, M.J.; Ewbank, C.; Mora, R.; Idowu, O.; Kim, S.; Padilla, B.E. The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr. Surg. Int. 2020, 36, 317–324. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Pawa, A.; Chin, K.J. Local anesthetic systemic toxicity: Current perspectives. Local Reg. Anesth. 2018, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D.P., Jr.; Strandness, T.B.; Derderian, S.C.; Kaizer, A.M.; Partrick, D.A. Ultrasound-guided erector spinae plane block versus thoracic epidural analgesia: Postoperative pain management after Nuss repair for pectus excavatum. J. Pediatr. Surg. 2022, 57, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C. Efficacy of Methods of Intercostal Nerve Blockade for Pain Relief After Thoracotomy. Ann. Thorac. Surg. 2005, 80, 1550–1559. [Google Scholar] [CrossRef]
- Muhly, W.T.; Maxwell, L.G.; Cravero, J.P. Pain management following the Nuss procedure: A survey of practice and review. Acta Anaesthesiol. Scand. 2014, 58, 1134–1139. [Google Scholar] [CrossRef]
- Hall Burton, D.M.; Boretsky, K.R. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for post-operative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr. Anaesth. 2014, 24, 516–520. [Google Scholar] [CrossRef]


| Control | Intervention |
|---|---|
| Preparation: pre-emptive analgesia | |
|
|
| Induction of general anaesthesia under standard monitoring | |
|
|
| Maintenance of general anaesthesia | |
|
|
|
|
|
|
| At the end of surgery | |
|
|
| Postoperative course: standard monitoring and regular pain assessment | |
|
|
| Parameter | Control (n = 10) | Intervention (n = 26) | p |
|---|---|---|---|
| Age | 15 (12–17) | 15 (11–17) | 0.77 |
| BMI | 18 (14–20) | 18 (14–20) | 0.46 |
| ASA 1 | 9 (90) | 25 (96) | 0.47 |
| Haller index | 3.35 (3.2–3.9) | 3.4 (3.2–3.9) | 0.87 |
| Parameter | Control (n = 10) | Intervention (n = 26) | p |
|---|---|---|---|
| Surgery time, median (IQR) | 80 (60.0–92.5) | 72.5 (65.0–90.0) | 0.863 |
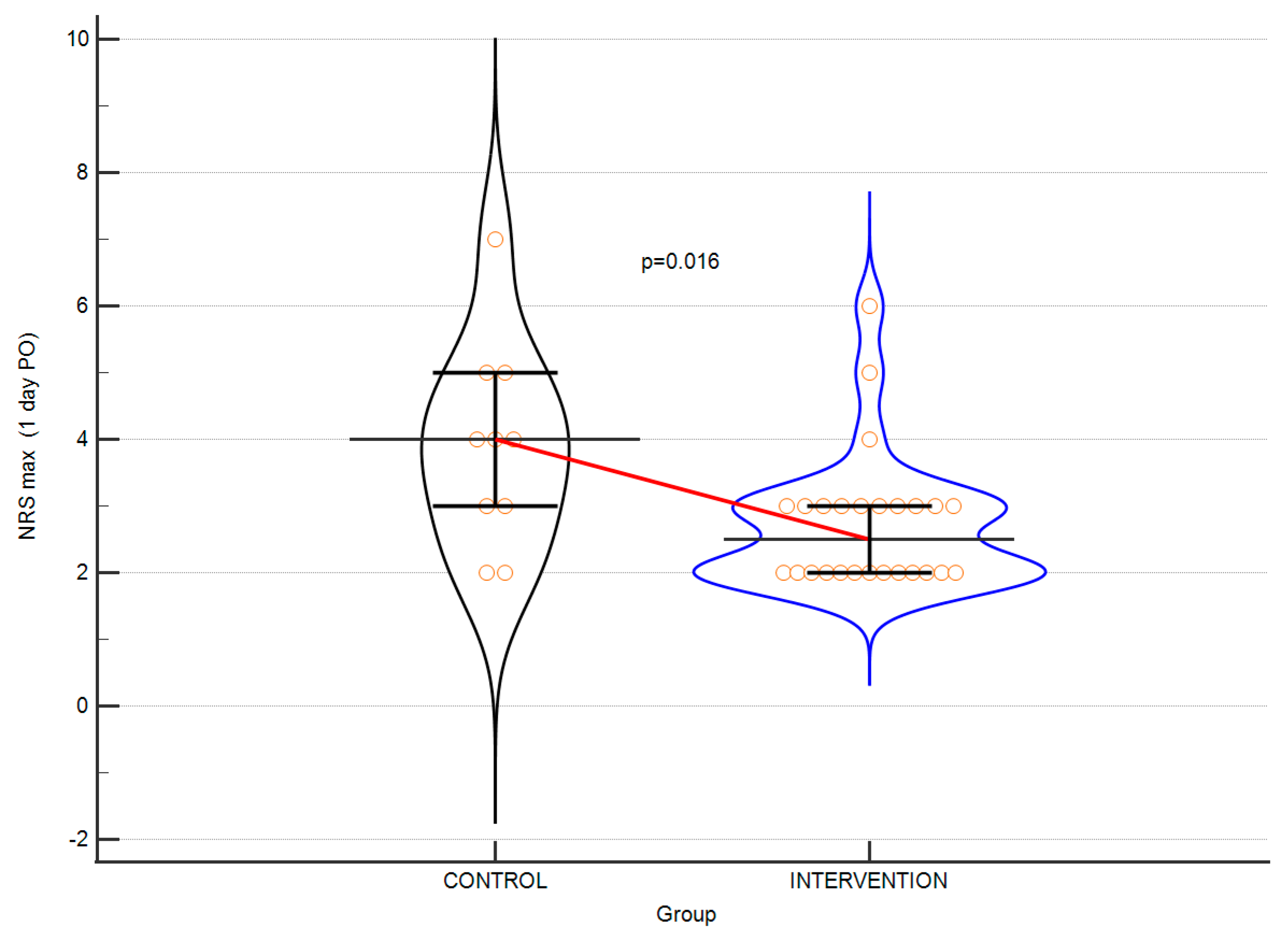
| MAX NRS 1, median (IQR) | 4. 0 (3.0–5.0) | 2.5 (2.0–3.0) | 0.015 |
| Length of hospitalisation (days) | 5 (50) | 4 (15) | 0.382 |
| Discontinuation of opioids 1 POD, n (%) | 3 (30) | 20 (76.9) | 0.009 |
| Discontinuation of opioids 2 POD, n (%) | 7 (70) | 6 (23.1) |
| Parameter | Control (n = 10) | Intervention (n = 26) | p |
|---|---|---|---|
| Adverse effects after pharmacotherapy n (%) | 2 (20) | 3 (11) | 0.516 |
| Complications after Nuss surgery n (%) | 5 (50) | 8 (31) | 0.283 |
| Complications after anaesthesia (early) | 0 | 0 | n.e. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacha, S.; Jarosz, K.; Kokot, K.; Biłas, J.; Skonieczna-Żydecka, K.; Gerus, S.; Kojder, K.; Biernawska, J. Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity. J. Pers. Med. 2023, 13, 1696. https://doi.org/10.3390/jpm13121696
Zacha S, Jarosz K, Kokot K, Biłas J, Skonieczna-Żydecka K, Gerus S, Kojder K, Biernawska J. Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity. Journal of Personalized Medicine. 2023; 13(12):1696. https://doi.org/10.3390/jpm13121696
Chicago/Turabian StyleZacha, Sławomir, Konrad Jarosz, Karolina Kokot, Jarosław Biłas, Karolina Skonieczna-Żydecka, Sylwester Gerus, Klaudyna Kojder, and Jowita Biernawska. 2023. "Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity" Journal of Personalized Medicine 13, no. 12: 1696. https://doi.org/10.3390/jpm13121696
APA StyleZacha, S., Jarosz, K., Kokot, K., Biłas, J., Skonieczna-Żydecka, K., Gerus, S., Kojder, K., & Biernawska, J. (2023). Benefits of the Erector Spinae Plane Block before Cryoanalgesia in Children Undergoing Surgery for Funnel Chest Deformity. Journal of Personalized Medicine, 13(12), 1696. https://doi.org/10.3390/jpm13121696







