Factors Associated with Spontaneous Preterm Birth after Ultrasound-Indicated Cerclage
Abstract
:1. Introduction
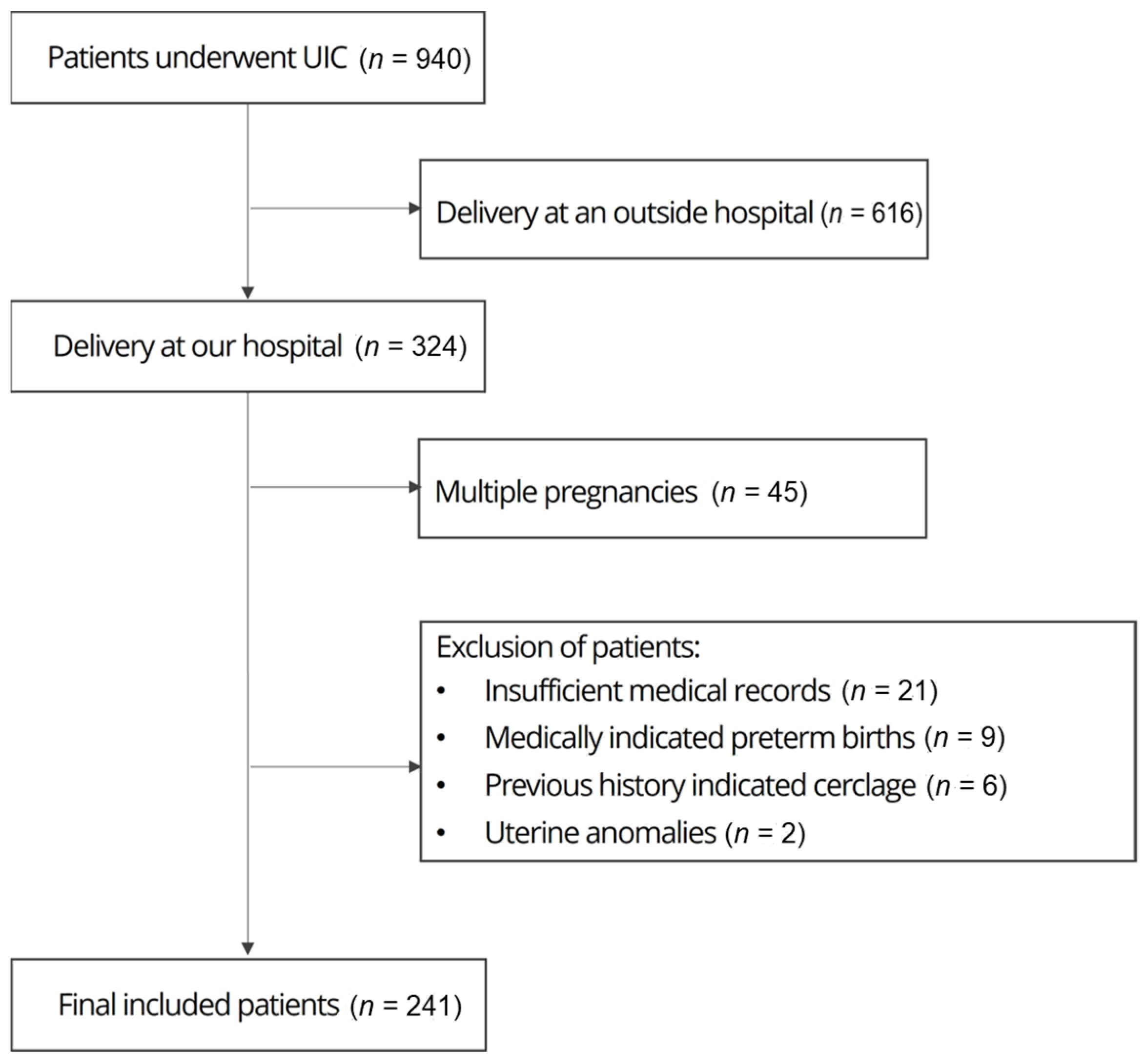
2. Materials and Methods
3. Results
Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, K.; Horiuchi, T.; Kusuda, S.; Kabe, K.; Itani, Y.; Nakamura, T.; Fujimura, M.; Matsuo, M. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics 2009, 123, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Shim, J.W.; Jin, H.S.; Bae, C.W. Changes in Survival Rate for Very-Low-Birth-Weight Infants in Korea: Comparison with Other Countries. J. Korean Med. Sci. 2015, 30, S25–S34. [Google Scholar] [CrossRef]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N. Engl. J. Med. 1996, 334, 567–573. [Google Scholar] [CrossRef]
- Hibbard, J.U.; Tart, M.; Moawad, A.H. Cervical length at 16-22 weeks’ gestation and risk for preterm delivery. Obstet. Gynecol. 2000, 96, 972–978. [Google Scholar]
- Owen, J.; Hankins, G.; Iams, J.D.; Berghella, V.; Sheffield, J.S.; Perez-Delboy, A.; Egerman, R.S.; Wing, D.A.; Tomlinson, M.; Silver, R.; et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am. J. Obstet. Gynecol. 2009, 201, 375.e1–375.e8. [Google Scholar] [CrossRef]
- Berghella, V.; Ciardulli, A.; Rust, O.A.; To, M.; Otsuki, K.; Althuisius, S.; Nicolaides, K.H.; Roman, A.; Saccone, G. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: Systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound. Obstet. Gynecol. 2017, 50, 569–577. [Google Scholar] [CrossRef]
- Gulersen, M.; Bornstein, E.; Domney, A.; Blitz, M.J.; Rafael, T.J.; Li, X.; Krantz, D.; Rochelson, B. Cerclage in singleton gestations with an extremely short cervix (≤10 mm) and no history of spontaneous preterm birth. Am. J. Obstet. Gynecol. MFM 2021, 3, 100430. [Google Scholar] [CrossRef] [PubMed]
- Althuisius, S.M.; Dekker, G.A.; Hummel, P.; Bekedam, D.J.; van Geijn, H.P. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): Therapeutic cerclage with bed rest versus bed rest alone. Am. J. Obstet. Gynecol. 2001, 185, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Rafael, T.J.; Szychowski, J.M.; Rust, O.A.; Owen, J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: A meta-analysis. Obstet. Gynecol. 2011, 117, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Souka, A.P.; Papamihail, M.; Pilalis, A. Very short cervix in low-risk asymptomatic singleton pregnancies: Outcome according to treatment and cervical length at diagnosis. Acta Obstet. Gynecol. Scand. 2020, 99, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Boelig, R.C.; Dugoff, L.; Roman, A.; Berghella, V.; Ludmir, J. Predicting asymptomatic cervical dilation in pregnant patients with short mid-trimester cervical length: A secondary analysis of a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2019, 98, 761–768. [Google Scholar] [CrossRef]
- Pan, M.; Fang, J.N.; Wang, X.X.; Zhang, J.; Lin, Z. Predictors of cerclage failure in singleton pregnancies with a history of preterm birth and a sonographic short cervix. Int. J. Gynaecol. Obstet. 2022, 156, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Türkyılmaz, G.; Karaaslan, O. The Effectiveness of Rescue Cervical Cerclage: A Retrospective Observational Study. Eastern J. Med. 2020, 25, 439–443. [Google Scholar] [CrossRef]
- Son, G.H.; Chang, K.H.; Song, J.E.; Lee, K.Y. Use of a uniconcave balloon in emergency cerclage. Am. J. Obstet. Gynecol. 2015, 212, 114.e1–114.e4. [Google Scholar] [CrossRef]
- Terkildsen, M.F.; Parilla, B.V.; Kumar, P.; Grobman, W.A. Factors associated with success of emergent second-trimester cerclage. Obstet. Gynecol. 2003, 101, 565–569. [Google Scholar]
- Fuchs, F.; Senat, M.V.; Fernandez, H.; Gervaise, A.; Frydman, R.; Bouyer, J. Predictive score for early preterm birth in decisions about emergency cervical cerclage in singleton pregnancies. Acta Obstet. Gynecol. Scand. 2012, 91, 744–749. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet. Gynecol. 2014, 123, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Gagnon, R.; Delisle, M.F. No. 373-cervical insufficiency and cervical cerclage. J. Obstet. Gynaecol. Can. 2019, 41, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Shennan, A.H.; Story, L. Cervical cerclage: Green-top Guideline No. 75. BJOG 2022, 129, 1178–1210. [Google Scholar] [CrossRef] [PubMed]
- Poggi, S.H.; Vyas, N.; Pezzullo, J.C.; Landy, H.J.; Ghidini, A. Therapeutic cerclage may be more efficacious in women who develop cervical insufficiency after a term delivery. Am. J. Obstet. Gynecol. 2009, 200, 68.e1–68.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jun, H.A.; Kim, H.B.; Kang, S.W. Interleukin-6, but not relaxin, predicts outcome of rescue cerclage in women with cervical incompetence. Am. J. Obstet. Gynecol. 2004, 191, 784–789. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, D.J.; Kwak-Kim, J. Upregulated amniotic fluid cytokines and chemokines in emergency cerclage with protruding membranes. Am. J. Reprod. Immunol. 2011, 66, 310–319. [Google Scholar] [CrossRef]
- Aguin, E.; Aguin, T.; Cordoba, M.; Aguin, V.; Roberts, R.; Albayrak, S.; Kruger, M.; Bahado-Singh, R. Amniotic fluid inflammation with negative culture and outcome after cervical cerclage. J. Matern. Fetal. Neonatal. Med. 2012, 25, 1990–1994. [Google Scholar] [CrossRef]
- Lee, K.N.; Park, K.H.; Kim, Y.M.; Cho, I.; Kim, T.E. Prediction of emergency cerclage outcomes in women with cervical insufficiency: The role of inflammatory, angiogenic, and extracellular matrix-related proteins in amniotic fluid. PLoS ONE 2022, 17, e0268291. [Google Scholar] [CrossRef]
- Son, G.H.; You, Y.A.; Kwon, E.J.; Lee, K.Y.; Kim, Y.J. Comparative analysis of midtrimester amniotic fluid cytokine levels to predict spontaneous very pre-term birth in patients with cervical insufficiency. Am. J. Reprod. Immunol. 2016, 75, 155–161. [Google Scholar] [CrossRef]
- Chalupska, M.; Kacerovsky, M.; Stranik, J.; Grergor, M.; Maly, J.; Jacobsson, B.; Musilova, I. Intra-amniotic infection and sterile intra-amniotic inflammation in cervical insufficiency with prolapsed fetal membranes: Clinical implications. Fetal. Diagn. Ther. 2021, 48, 58–69. [Google Scholar] [CrossRef]
- Monsanto, S.P.; Daher, S.; Ono, E.; Pendeloski, K.P.; Traina, E.; Mattar, R.; Tayade, C. Cervical cerclage placement decreases local levels of proinflammatory cytokines in patients with cervical insufficiency. Am. J. Obstet. Gynecol. 2017, 217, 455.e1–455.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, X.; Yan, J.; Zhang, J.; Yang, D.; Pan, M. Predictive value of serum inflammatory markers for histological chorioamnionitis among women with preterm premature rupture of membranes after undergoing cervical cerclage. Clinics 2023, 78, 100292. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Park, K.H.; Lee, S.Y.; Ryu, A.; Joo, J.K.; Park, J.W. Predicting out-comes of emergency cerclage in women with cervical insufficiency using inflammatory markers in maternal blood and amniotic fluid. Int. J. Gynaecol. Obstet. 2016, 132, 165–169. [Google Scholar] [CrossRef]
- Weiner, C.P.; Lee, K.Y.; Buhimschi, C.S.; Christner, R.; Buhimschi, I.A. Proteomic biomarkers that predict the clinical success of rescue cerclage. Am. J. Obstet. Gynecol. 2005, 192, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, Y.; Miura, H.; Sato, A.; Onodera, Y.; Sato, N.; Shimizu, D.; Kumazawa, Y.; Sanada, H.; Hirano, H.; Terada, Y. Neutrophil elastase in amniotic fluid as a predictor of preterm birth after emergent cervical cerclage. Acta Obstet. Gynecol. Scand. 2016, 95, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J.; Shah, P.; Ohlsson, A.; Shah, V.; Murphy, K.E.; McDonald, S.D.; Hutton, E.; et al. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 2010, 341, c3428. [Google Scholar] [CrossRef] [PubMed]
- Torloni, M.R.; Betrán, A.P.; Daher, S.; Widmer, M.; Dolan, S.M.; Menon, R.; Bergel, E.; Allen, T.; Merialdi, M. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. J. Matern. Fetal. Neonatal. Med. 2009, 22, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Durmuş, B.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Jaddoe, V.W. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013, 21, 1046–1055. [Google Scholar] [CrossRef]
- Hall, L.F.; Neubert, A.G. Obesity and pregnancy. Obstet. Gynecol. Surv. 2005, 60, 253–260. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Lu, X.; Xi, W.; Li, Z. Maternal early pregnancy body mass index and risk of preterm birth. Arch. Gynecol. Obstet. 2011, 284, 813–819. [Google Scholar] [CrossRef]
- Räisänen, S.; Gissler, M.; Saari, J.; Kramer, M.; Heinonen, S. Contribution of risk factors to extremely, very and moderately preterm births register-based analysis of 1,390,742 singleton births. PLoS ONE 2013, 8, e60660. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Wise, P.H.; Mayo, J.; Carmichael, S.L.; Ley, C.; Lyell, D.J.; Shachar, B.Z.; Melsop, K.; Phibbs, C.S.; Stevenson, D.K.; et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr. Perinat. Epidemiol. 2014, 28, 302–311. [Google Scholar] [CrossRef]
- Shachar, B.Z.; Mayo, J.A.; Lee, H.C.; Carmichael, S.L.; Stevenson, D.K.; Shaw, G.M.; Gould, J.B. Effects of race/ethnicity and BMI on the association between height and risk for spontaneous preterm birth. Am. J. Obstet. Gynecol. 2015, 213, 700.e1–700.e9. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Saijo, Y.; Yoshioka, E.; Sato, Y.; Kato, Y.; Nagaya, K.; Takahashi, S.; Ito, Y.; Kobayashi, S.; Miyashita, C.; et al. Association between maternal multimorbidity and preterm birth, low birth weight and small for gestational age: A prospective birth cohort study from the Japan Environment and Children’s Study. BMJ Open. 2023, 13, e069281. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Hart, J.E.; Agwu, O.C.; Fisher, B.M.; West, N.A.; Gibbs, R.S. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 428.e1–428.e9. [Google Scholar] [CrossRef]
- Yalvac, S.; Esin, S.; Kocak, O.; Yirci, B.; Kandemir, O. Effect of body mass index on latency periods after history-indicated cervical cerclage. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 121–125. [Google Scholar] [CrossRef]
- Poggi, S.H.; Vyas, N.A.; Pezzullo, J.C.; Landy, H.J.; Ghidini, A. Does increasing body mass index affect cerclage efficacy. J. Perinatol. 2012, 32, 777–779. [Google Scholar] [CrossRef]
- Farinelli, C.K.; Wing, D.A.; Szychowski, J.M.; Owen, J.; Hankins, G.; Iams, J.D.; Sheffield, J.S.; Perez-Delboy, A.; Berghella, V.; Guzman, E.R.; et al. Association between body mass index and pregnancy outcome in a randomized trial of cerclage for short cervix. Ultrasound. Obstet. Gynecol. 2012, 40, 669–673. [Google Scholar] [CrossRef]
| Characteristic | Total Patients (n = 241) |
|---|---|
| Age (years) | 34.1 ± 4.2 |
| Primipara | 128 (53.1) |
| Preterm birth history | 38 (15.8) |
| Preterm birth < 32 weeks | 27 (11.2) |
| Full-term birth history | 82 (34.0) |
| Height (cm) | 161.3 ± 5.3 |
| Prepregnancy body weight (kg) | 59.6 ± 13.0 |
| Prepregnancy BMI (kg/m2) | 22.8 ± 4.5 |
| Hypertensive disease | 7 (2.9) |
| Gestational or overt diabetes | 32 (13.3) |
| Gestational weeks at operation (weeks) | 22.1 ± 3.1 |
| Cervical length at operation (mm) | 14.6 ± 5.8 |
| Cervical length < 10 mm | 65 (27.0) |
| Presence of cervical funnel | 169 (70.1) |
| Preoperative laboratory results | |
| ESR (mm/h) | 36.4 ± 17.4 |
| CRP (mg/L) | 7.7 ± 13.2 |
| WBC count (/μL) | 9706 ± 2345 |
| Repeat cerclage | 15 (6.2) |
| Gestational weeks at delivery (weeks) | 33.2 ± 6.3 |
| Birthweight (kg) | 2.3 ± 1.1 |
| Characteristic | Preterm Group (n = 138) | Full-Term Group (n = 103) | p Value |
|---|---|---|---|
| Age (years) | 33.9 ± 4.2 | 34.3 ± 4.1 | 0.508 |
| Primipara | 80 (58.0) | 48 (46.6) | 0.118 |
| Preterm birth history | 21 (15.2) | 17 (16.5) | 0.859 |
| Full-term birth history | 39 (28.3) | 43 (41.7) | 0.039 |
| Height (cm) | 161.5 ± 5.4 | 161.1 ± 5.3 | 0.602 |
| Prepregnancy body weight (kg) | 61.7 ± 14.7 | 56.7 ± 9.6 | 0.003 |
| Prepregnancy BMI (kg/m2) | 23.5 ± 5.0 | 21.8 ± 3.6 | 0.003 |
| Gestational weeks at operation (weeks) | 21.7 ± 3.2 | 22.7 ± 3.0 | 0.020 |
| Cervical length at operation (mm) | 13.3 ± 5.9 | 16.1 ± 5.6 | <0.001 |
| Cervical length < 10 mm | 50 (36.2) | 15 (14.6) | <0.001 |
| Presence of cervical funnel | 104 (75.4) | 65 (63.1) | 0.047 |
| Preoperative laboratory results | |||
| ESR (mm/h) | 39.6 ± 18.7 | 31.3 ± 14.0 | <0.001 |
| CRP (mg/L) | 9.6 ± 12.9 | 5.2 ± 13.4 | 0.011 |
| WBC count (/μL) | 10,004.6 ± 2550.8 | 9216.4 ± 1949.1 | 0.009 |
| Repeat cerclage | 12 (8.7) | 3 (2.9) | 0.103 |
| Gestational weeks at delivery (weeks) | 29.4 ± 5.8 | 38.5 ± 1.0 | <0.001 |
| Birthweight (kg) | 1.6 ± 0.9 | 3.2 ± 0.4 | <0.001 |
| Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Gestational weeks at operation (weeks) | 0.897 | 0.817–0.985 | 0.023 |
| Cervical length at operation (mm) | 0.915 | 0.870–0.963 | 0.001 |
| Obesity | 4.067 | 1.081–15.305 | 0.038 |
| Preoperative ESR (mm/h) | 1.030 | 1.011–1.049 | 0.002 |
| Full-term birth history | 1.256 | 0.689–2.288 | 0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-N.; Yun, S.; Park, S.-Y.; Kim, K.; Lee, K.-Y.; Lee, J.J.; Son, G.-H. Factors Associated with Spontaneous Preterm Birth after Ultrasound-Indicated Cerclage. J. Pers. Med. 2023, 13, 1678. https://doi.org/10.3390/jpm13121678
Lee K-N, Yun S, Park S-Y, Kim K, Lee K-Y, Lee JJ, Son G-H. Factors Associated with Spontaneous Preterm Birth after Ultrasound-Indicated Cerclage. Journal of Personalized Medicine. 2023; 13(12):1678. https://doi.org/10.3390/jpm13121678
Chicago/Turabian StyleLee, Kyong-No, Sangho Yun, So-Yoon Park, Kyoungseon Kim, Keun-Young Lee, Jae Jun Lee, and Ga-Hyun Son. 2023. "Factors Associated with Spontaneous Preterm Birth after Ultrasound-Indicated Cerclage" Journal of Personalized Medicine 13, no. 12: 1678. https://doi.org/10.3390/jpm13121678
APA StyleLee, K.-N., Yun, S., Park, S.-Y., Kim, K., Lee, K.-Y., Lee, J. J., & Son, G.-H. (2023). Factors Associated with Spontaneous Preterm Birth after Ultrasound-Indicated Cerclage. Journal of Personalized Medicine, 13(12), 1678. https://doi.org/10.3390/jpm13121678





