Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up
Abstract
1. Introduction
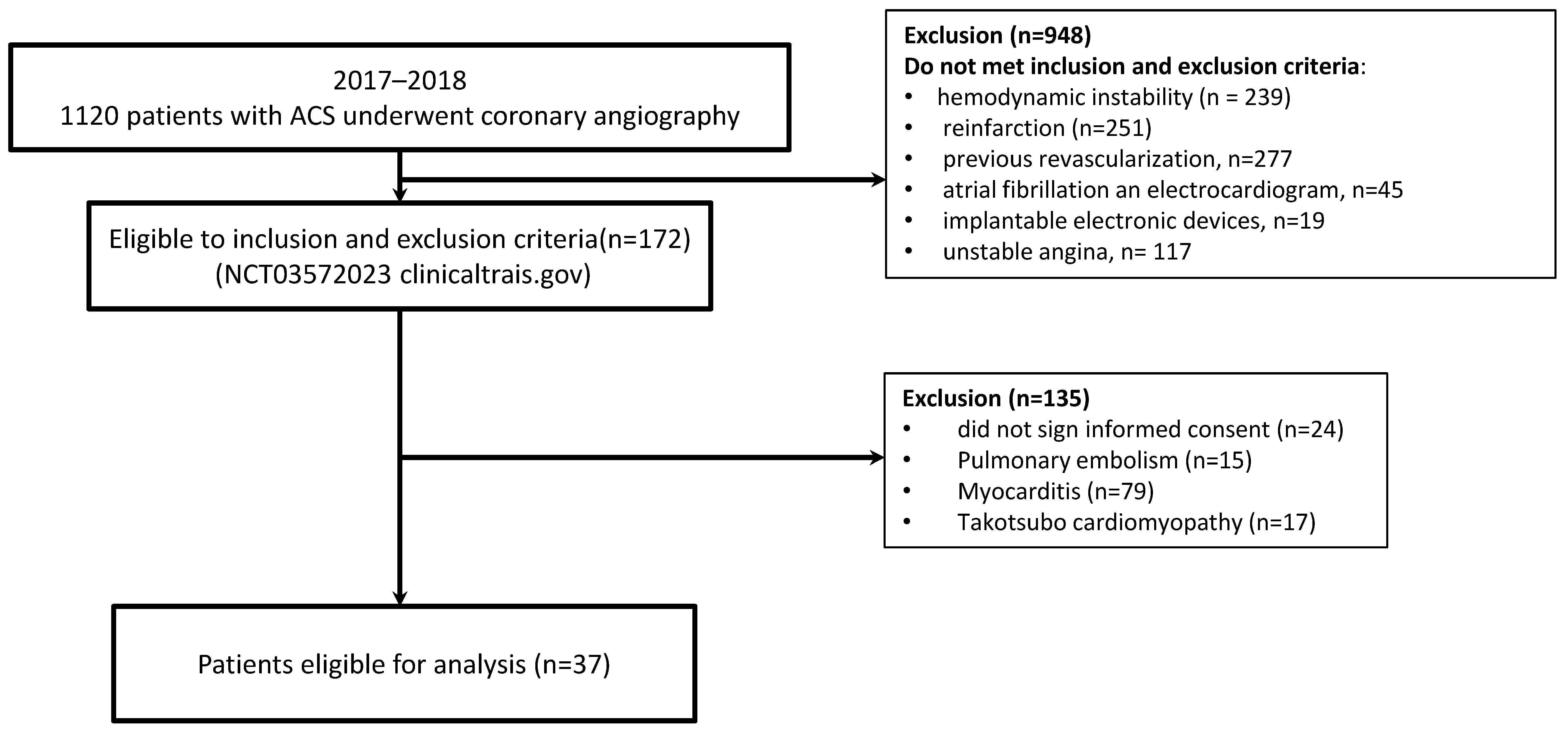
2. Materials and Methods
2.1. Invasive Coronary Angiography
2.2. Multidetector-Computed Tomography Coronary Angiography Protocol
2.3. Biochemical Analysis
2.4. Statistical Analysis
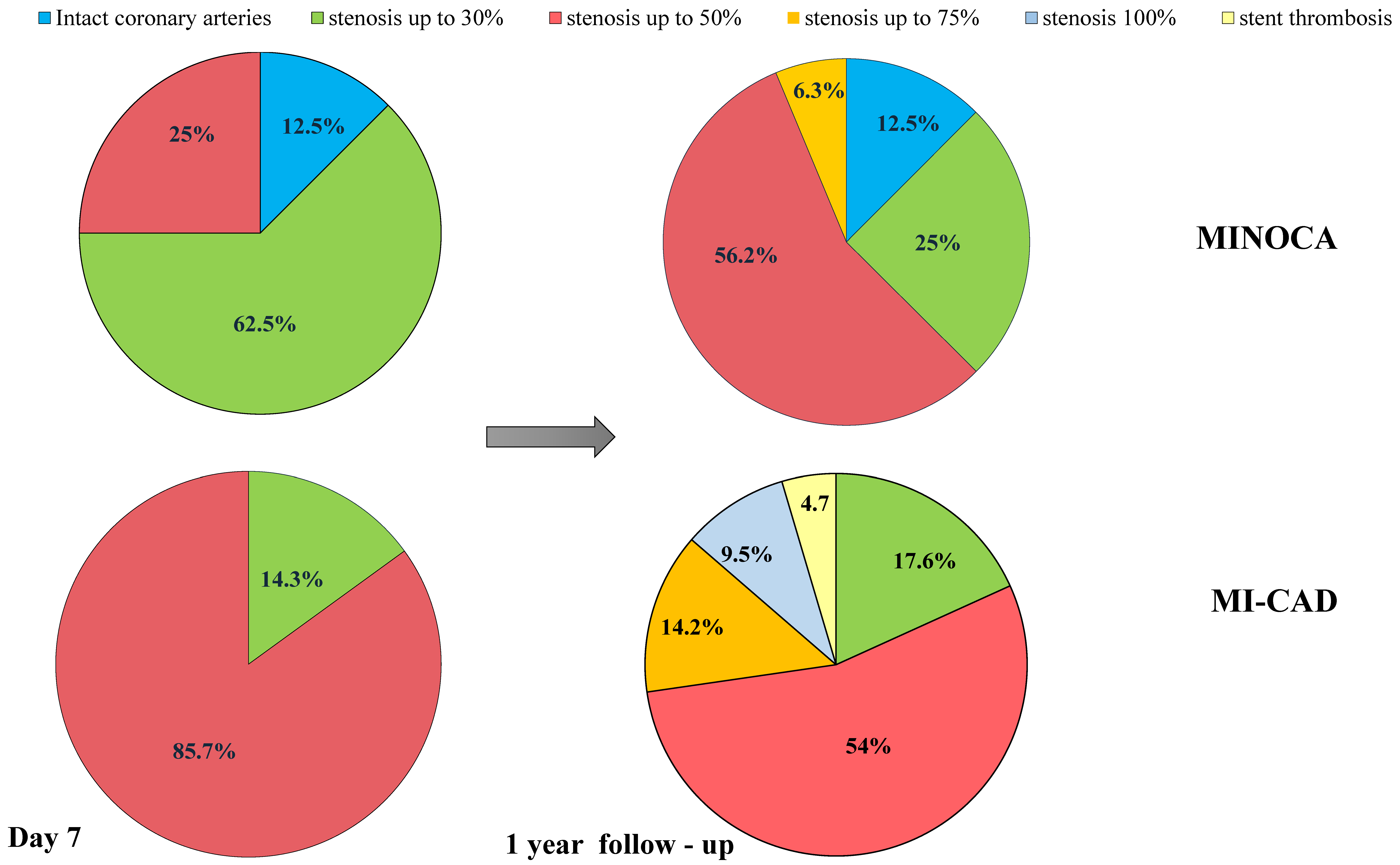
3. Results
Baseline Characteristics
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Journath, G.; Hambraeus, K.; Hagström, E.; Pettersson, B.; Löthgren, M. Predicted impact of lipid lowering therapy on cardiovascular and economic outcomes of Swedish atherosclerotic cardiovascular disease guideline. BMC Cardiovasc. Disord. 2017, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; Buyne, B.; Cristea, E.; Mintz, G.S.; Mhran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Guerra, F.; Pizzi, C.; Merlo, M.; Zilio, F.; Bianco, F.; Mancone, M.; Zaffalon, D.; Gioscia, R.; Bergamaschi, L.; et al. Characteristics of patients with recurrent acute myocardial infarction after MINOCA. Prog. Cardiovasc. Dis. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Kraaijenhof, J.M.; Mayr, M.; Nicholls, S.J.; Koenig, W.; Catapano, A.L.; Stroes, E.S.G. Proteomics and lipidomics in atherosclerotic cardiovascular disease risk prediction. Eur. Heart J. 2023, 44, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Lindhagen, L.; Baron, T.; Erlinge, D.; Hjort, M.; Jernberg, T.; Johnston, N.; Marko-Varga, G.; Rezeli, M.; Spaak, J.; et al. Sex-differences in circulating biomarkers during acute myocardial infarction: An analysis from the SWEDEHEART registry. PLoS ONE 2021, 16, e0249830. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Pepine, C.J.; Berry, C.; Camici, P.G. The role of a comprehensive two-step diagnostic evaluation to unravel the pathophysiology of MINOCA: A review. Int. J. Cardiol. 2021, 336, 1–7. [Google Scholar] [CrossRef]
- Lopez-Pais, J.; Coronel, B.I.; Gil, D.G.; Pascual, M.J.E.; Durán, B.A.; Peredo, C.G.M.; Vinués, C.M.; García, P.A.; Gonzalez-Juanatey, J.R.; García, J.M.; et al. Clinical characteristics and prognosis of myocardial infarction with non-obstructive coronary arteries (MINOCA): A prospective single-center study. Cardiol. J. 2022, 29, 798–806. [Google Scholar] [CrossRef]
- Hjort, M.; Eggers, K.M.; Lindhagen, L.; Lindhagen, L.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenbäck, C.; Frick, M.; et al. Increased inflammatory activity in patients 3 months after myocardial infarction with nonobstructive coronary arteries. Clin. Chem. 2019, 65, 1023–1030. [Google Scholar] [CrossRef]
- Chalikias, G.; Tziakas, D. Slow Coronary Flow: Pathophysiology, Clinical Implications, and Therapeutic Management. Angiology 2021, 72, 808–818. [Google Scholar] [CrossRef]
- Abbara, S.; Arbab-Zadeh, A.; Callister, T.Q.; Desai, M.Y.; Mamuya, W.; Thomson, L.; Weigold, W.G. SCCT guidelines for performance of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2009, 3, 190–204. [Google Scholar] [CrossRef]
- Zavadovsky, K.V.; Vorobyeva, D.A.; Mochula, O.V.; Mochula, A.V.; Maltseva, A.N.; Bayev, A.E.; Gulya, M.O.; Gimelli, A.; Ryabov, V.V. Myocardial Blood Flow and Flow Reserve in Patients With Acute Myocardial Infarction and Obstructive and Non-Obstructive Coronary Arteries: CZT SPECT Study. Front. Nucl. Med. 2022, 2, 935539. [Google Scholar] [CrossRef]
- Vorobeva, D.A.; Ryabov, V.V.; Lugacheva, J.G.; Zavadovsky, K.V.; Mochula, A.V. Relationships between indicators of prothrombotic activity and coronary microvascular dysfunction in patients with myocardial infarction with obstructive and non-obstructive coronary artery disease. BMC Cardiovasc. Disord. 2022, 22, 530. [Google Scholar] [CrossRef] [PubMed]
- Dieden, A.; Malan, L.; Mels, C.M.C.; Lammertyn, L.; Wentzel, A.; Nilsson, P.M.; Gudmundsson, P.; Jujic, A.; Magnusson, M. Exploring biomarkers associated with deteriorating vascular health using a targeted proteomics chip: The SABPA study. Medicine 2021, 100, e25936. [Google Scholar] [CrossRef] [PubMed]
- Sucato, V.; Testa, G.; Puglisi, S.; Evola, S.; Galassi, A.R.; Novo, G. Myocardial infarction with non-obstructive coronary arteries (MINOCA): Intracoronary imaging-based diagnosis and management. J. Cardiol. 2021, 77, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Vorobieva, D.A.; Lugacheva, Y.G.; Kapilevich, N.A.; Ryabov, V.V. Comparative analysis of prothrombotic activity in patients with myocardial infarction with and without obstructive coronary artery disease. Russ. J. Cardiol. 2021, 26, 3939. [Google Scholar] [CrossRef]
- Wassel, C.L.; Berardi, C.; Pankow, J.S.; Larson, N.B.; Decker, P.A.; Hanson, N.Q.; Tsai, M.Y.; Criqui, M.H.; Allison, M.A.; Bielinski, S.J. Soluble P-selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 405–411. [Google Scholar] [CrossRef]
- Kayikçioglu, M.; Can, L.; Mete-Erdem, N.; Kültürsay, H.; Payzin, S.; Kokuludag, A.; Türkoglu, C. Soluble P-selectin and the success of thrombolysis in acute myocardial infarction. Int. J. Cardiol. 2001, 79, 223–229. [Google Scholar] [CrossRef]
- Angeles-Martinez, J.; Posadas-Sanchez, R.; Bravo-Flores, E.; González-Salazar, M.D.C.; Vargas-Alarcón, G. Common Variants in IL-20 Gene are Associated with Subclinical Atherosclerosis, Cardiovascular Risk Factors and IL-20 Levels in the Cohort of the Genetics of Atherosclerotic Disease (GEA) Mexican Study. Biomolecules 2020, 10, 75. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hsieh, P.L.; Chou, W.C.; Hung, C.H.; Yang, H.L.; Chang, Y.C.; Chu, P.M.; Chang, M.S.; Chan, S.H. IL-20 promotes hypoxia/reoxygenation-induced mitochondrial dysfunction and apoptosis in cardio myocytes by upregulating oxidative stress by activating the PKC/NADPH oxidase pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165684. [Google Scholar] [CrossRef]
- Neri, M.; Fineschi, V.; Di Paolo, M. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef]
- Akhavanpoor, M.; Gleissner, C.A.; Gorbatsch, S.; Doesch, A.O.; Akhavanpoor, H.; Wangler, S.; Jahn, F.; Lasitschka, F.; Katus, H.A.; Erbel, C. CCL19 and CCL21 modulate the inflammatory milieu in atherosclerotic lesions. Drug Des. Dev. Ther. 2014, 8, 2359–2371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueland, T.; Aukrust, P.; Caidahl, K. CCL21 and prognosis in acute coronary syndrome. Aging 2019, 11, 9225–9226. [Google Scholar] [CrossRef] [PubMed]
- Damås, J.K.; Smith, C.; Øie, E.; Fevang, B.; Halvorsen, B.; Waehre, T.; Boullier, A.; Breland, U.; Yndestad, A.; Ovchinnikova, O.; et al. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: Possible pathogenic role in plaque destabilization. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.P.; Gullestad, L.; Fevang, B.; Holm, A.M.; Landrø, L.; Vinge, L.E.; Fiane, A.E.; Sandberg, W.J.; Otterdal, K.; Frøland, S.S. Increased expression of LIGHT/TNFSF14 and its receptors in experimental and clinical heart failure. Eur. J. Heart Fail. 2008, 10, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gu, Y.; Lai, X.; Gu, Q. LIGHT is increased in patients with coronary disease and regulates inflammatory response and lipid metabolism in oxLDL-induced THP-1 macrophages. Biochem. Biophys. Res. Commun. 2017, 490, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Rawat, U.; Kumar, P.; Mittal, P. Pleotropic effects of statins: The dilemma of wider utilization of statin. Egypt. Heart J. 2023, 75, 1. [Google Scholar] [CrossRef] [PubMed]
- Premnath, S.M.; Nanda, S.K.; Ray, L.; Arokiaraj, M.C. Effect of Statins on the Inflammatory Markers in Patients with Coronary Artery Disease. J. Lab. Physicians 2023, 15, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.R.; Fu, Q.; Sui, J.; Zhang, Q.; Wei, P.; Wu, Y.; Zhu, K.; Lu, Y.; Zong, B. Serum endothelial cell-specific molecule 1 (endocan) levels in patients with acute myocardial infarction and its clinical significance. Angiology 2017, 68, 354–359. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, C.; Wang, Y.; Mu, L.; Li, X.; Qi, Y.; Yang, J.; Ma, C. Soluble Vascular Cell Adhesion Molecule-1 as an Inflammation-Related Biomarker of Coronary Slow Flow. J. Clin. Med. 2023, 12, 543. [Google Scholar] [CrossRef]
- Chen, Y.; Nilsson, A.H.; Goncalves, I.; Edsfeldt, A.; Engström, G.; Melander, O.; Orho-Melander, M.; Rauch, U.; Tengryd, C.; Venuraju, S.M.; et al. Evidence for a protective role of placental growth factor in cardiovascular disease. Sci. Transl. Med. 2020, 12, eabc8587. [Google Scholar] [CrossRef]
- Stangret, A.; Dykacz, W.; Jabłoński, K.; Wesołowska, A.; Klimczak-Tomaniak, D.; Kochman, J.; Tomaniak, M. The cytokine trio—Visfatin, placental growth factor and fractalkine—And their role in myocardial infarction with non-obstructive coronary arteries (MINOCA). Cytokine Growth Factor Rev. 2023, S1359-6101(23)00052-7. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.D. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013, 512103. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Rai, V.; Agrawal, D.K. Role of oncostatin-M in ECM remodeling and plaque vulnerability. Mol. Cell Biochem. 2023, 478, 2451–2460. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Recio-Mayoral, A.; Rimoldi, O.E.; Camici, P.G.; Kaski, J.C. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc. Imaging 2013, 6, 660–667. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; Luca, G.D.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group. on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Armillotta, M.; Amicone, S.; Bergamaschi, L.; Angeli, F.; Rinaldi, A.; Paolisso, P.; Stefanizzi, A.; Sansonetti, A.; Impellizzeri, A.; Bodega, F.; et al. Predictive value of Killip classification in MINOCA patients. Eur. J. Intern. Med. 2023, 117, 57–65. [Google Scholar] [CrossRef]
- Xue, S.; Tang, H.; Zhao, G.; Fang, C.; Shen, Y.; Yan, D.; Yuan, Y.; Fu, W.; Shi, Z.; Tang, X.; et al. C-C motif ligand 8 promotes atherosclerosis via NADPH oxidase 2/reactive oxygen species-induced endothelial permeability increase. Free Radic. Biol. Med. 2021, 167, 181–192. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Ye, D.; Feng, Y.; Liu, M.; Xu, Y.; Wang, M.; Zhang, J.; Liu, J.; Zhao, M.; et al. The Role of CXC Chemokines in Cardiovascular Diseases. Front. Pharmacol. 2022, 12, 765768. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients, n% | MINOCA, n = 16 | MICAD, n = 21 | p-Value |
|---|---|---|---|
| Men, n (%) | 7 (43.7) | 17 (80.9) | 0.02 |
| Age, mean (Q25; Q75) | 66.0 (54.71) | 60 (56; 68) | 0.33 |
| Hypertension, n (%) | 13 (81.3) | 16 (76.1) | 0.71 |
| Dyslipidemia, n (%) | 14 (87.5) | 17 (80.9) | 0.89 |
| Overweight, n (%) | 4 (25) | 11 (52.3) | 0.15 |
| Family history of CAD, n (%) * | 7 (43.7) | 13 (61.9) | 0.27 |
| Smoking, n (%) | 5 (31.3) | 11 (52.3) | 0.26 |
| Diabetes mellitus, n (%) | 0 | 4 (19.0) | 0.02 |
| GFR, ml/min/1.73 m2, mean (Q25; Q75) | 71.5 (54.0; 80.0) | 79.0 (65.0; 89.0) | 0.20 |
| History of angina pectoris, n (%) | 10 (62.5) | 6 (28.5) | 0.04 |
| History of stroke, n (%) | 1 (5.2) | 2 (9.5) | 0.71 |
| Peripheral atherosclerosis, n (%) | 4 (25) | 7 (33.3) | 0.58 |
| Time of admission to the hospital, min, mean (Q25; Q75) | 390 (146.5; 870) | 180 (98; 240) | 0.02 |
| STEMI, n (%) | 10 (62.5) | 19 (90.4) | 0.01 |
| GRACE, risk, mean (Q25; Q75) | 2.0 (2.0; 3.5) | 2.3 (2.0; 5.0) | 0.26 |
| Thrombolytic therapy, n (%) | 3 (18.7) | 11 (52.3) | 0.007 |
| TIMI 2 flow, n (%) | 9 (56.3) | 1(4.7) | 0.01 |
| Wall motion score index, score | 1.0 (1.0; 1.2) | 1.2 (1.2; 1.5) | 0.04 |
| Left ventricular ejection fraction, % | 60.0 (45.0; 60.0) | 56.0 (50.0; 60.0) | 0.51 |
| Acute apical left ventricle aneurysm, n (%) | 3 (18.7) | 2 (9.5) | 0.62 |
| Indicator (Reference Range) | Day | MINOCA, n = 16 | MI-CAD, n = 21 | p-Value |
|---|---|---|---|---|
| Troponin I ng/mL (0.00–0.040) | 2 | 0.5 (0.11; 8.3) | 4.9 (1.0; 25.2) | 0.02 |
| 4 | 0.4 (0.07; 1.7) | 0.7 (0.5; 4.4) | 0.04 | |
| 7 | 0.08 (0.02; 0.2) | 0.4 (0.2; 0.9) | 0.0003 | |
| 1 year | 0.01 (0.01; 0.02) | 0.01 (0.01; 0.02) | 0.50 | |
| Cholesterol, mmol/L (<4.5) | 1 | 4.8 (4.2; 6.2) | 4.5 (3.9; 4.9) | 0.17 |
| 1 year | 4.4 (3.6; 5.7) | 3.6 (2.9; 4.3) | 0.01 | |
| Triglycerides, mmol/L (0.5–1.7) | 1 | 1.4 (0.9; 2.5) | 1.7(1.1; 2.0) | 0.88 |
| 1 year | 1.1 (0.7; 1.7) | 1.2 (0.7; 1.6) | 0.84 | |
| HDL-C, mmol/L (>1.0) | 1 | 1.2 (0.9; 1.5) | 1.1 (1.1; 1.3) | 0.58 |
| 1 year | 1.4 (1.2; 1.8) | 1.1 (0.9; 1.2) | 0.01 | |
| LDL-C, mmol/L (<2.5) | 1 | 2.7 (2.3; 4.0) | 2.6 (2.2; 2.8) | 0.23 |
| 1 year | 2.4 (1.5; 3.8) | 1.5 (1.3; 2.1) | 0.12 | |
| LDL/HDL (<2.5) | 1 | 2.8 (1.6; 3.0) | 2.1 (1.8; 2.3) | 0.39 |
| 1 year | 1.7 (0.78; 2.6) | 1.48 (0.9; 2.3) | 0.04 | |
| hsCRP, mg/L (<3.0) | 1 | 16.5 (3.8; 30.0) | 4.4 (3.8; 5.0) | 0.04 |
| 4 | 14.0 (4.8; 18.7) | 4.7 (3.9; 12.8) | 0.11 | |
| 7 | 5.3 (3.3; 10.0) | 4.0 (3.5; 13.1) | 0.84 | |
| 1 year | 3.7 (2.8; 10.1) | 3.1 (2.0; 4.0) | 0.23 |
| Indicator (Reference Range) | Day | MINOCA, n = 16 | MI-CAD, n = 21 | p-Value |
|---|---|---|---|---|
| CXCL6, pg/mL, Me (Q25; Q75) | 1 | 247.33 (213.34; 281.89) | 224.05 (167.99; 315.45) | 0.57 |
| 2 | 218.81 (201.17; 229.00) | 244.35 (201.31; 289.62) | 0.41 | |
| 4 | 236.81 (219.44; 252.11) | 218.88 (175.84; 246.74) | 0.26 | |
| 7 | 242.97 (226.40; 288.77) | 229.03 (214.07; 275.94) | 0.41 | |
| 1 year | 227.28 (208.75; 271.70) | 270.00 (147.70; 301.64) | 0.53 | |
| CCL-8, pg/mL, Me (Q25; Q75) | 1 | 45.9 (41.2; 56.9) | 40.3 (28.3; 47.1) | 0.49 |
| 2 | 42.7 (43.1; 53.8) | 39.5 (36.1; 45.2) | 0.06 | |
| 4 | 44.9 (40.8; 56.4) | 44.8 (39.6; 47.5) | 0.49 | |
| 7 | 39.8 (27.1; 63.3) | 38.9 (451; 54.4) | 0.44 | |
| 1 year | 47.1 (40.8; 64.4) | 49.5 (40.3; 52.3) | 0.54 | |
| CCL-15, pg/mL, Me (Q25; Q75) | 1 | 4931.0 (3317.5; 7496.5) | 3022.5 (2006.0; 4574.0) | 0.04 |
| 2 | 5683.5 (3284.0; 7168.0) | 3500.0 (2901.0; 4098.0) | 0.02 | |
| 4 | 5261.0 (3355.0; 6171.0) | 2606.0 (2389.0; 3851.0) | 0.04 | |
| 7 | 4906.5 (4199.5; 5733.5) | 3732.0 (2653.0; 3951.0) | 0.02 | |
| 1 year | 3694.0 (2623.5; 5428.0) | 2657.0 (2154.0; 3319.0) | 0.19 | |
| CCL-21, pg/mL, Me (Q25; Q75) | 1 | 96.9 (38.4; 192.9) | 193.8 (165.1; 200.9) | 0.08 |
| 2 | 171.0 (144.7; 221.3) | 154.9 (73.3; 174.9) | 0.18 | |
| 4 | 161.0 (84.0; 231.4) | 152.9 (143.3; 261.4) | 0.97 | |
| 7 | 110.7 (113.7; 275.6) | 110.2 (84.5; 196.2) | 0.26 | |
| 1 year | 184.7 (135.4; 267.0) | 90.5 (4.0; 148.9) | 0.02 | |
| IL-20, pg/mL, Me (Q25; Q75) | 1 | 59.2 (46.8; 84.1) | 48.9 (48.1; 60.8) | 0.25 |
| 2 | 63.5 (50.5; 90.2) | 40.0 (28.8; 48.9) | 0.005 | |
| 4 | 54.3 (45.0; 70.5) | 43.5 (41.7; 51.1) | 0.03 | |
| 7 | 57.9 (45.7; 73.3) | 55.3 (38.9; 58.8) | 0.34 | |
| 1 year | 58.2 (43.7; 76.6) | 56.9 (42.6; 69.6) | 0.92 | |
| Oncostatin M, pg/mL, Me (Q25; Q75) | 1 | 26.90 (7.44; 34.920) | 21.48 (14.83; 25.36) | 0.19 |
| 2 | 25.82 (7.44; 34.49) | 13.32 (9.12; 25.02) | 0.16 | |
| 4 | 29.63 (9.41; 38.56) | 16.56 (6.32; 23.83) | 0.04 | |
| 7 | 38.62 (17.45; 53.20) | 14.33 (6.91; 23.69) | 0.002 | |
| 1 year | 26.74 (17.71; 38.26) | 12.33 (7.24; 20.90) | 0.008 | |
| Placental GrowthFactor, pg/mL, Me (Q25; Q75) | 1 | 10.96 (5.41; 23.39) | 4.54 (0.34; 7.67) | 0.02 |
| 2 | 11.87 (5.34; 16.72) | 3.15 (0.32; 8.73) | 0.01 | |
| 4 | 8.07 (3.11; 16.86) | 2.79 (0.84; 11.36) | 0.12 | |
| 7 | 7.91 (5.20; 14.74) | 2.56 (0.27; 3.95) | 0.004 | |
| 1 year | 7.73 (4.08; 12.27) | 2.69 (1.35; 9.01) | 0.04 | |
| sP-Selectin, pg/mL, Me (Q25; Q75) | 1 | 69.45 (64.95; 79.81) | 78.04 (47.25; 117.54) | 0.04 |
| 2 | 67.15 (61.15; 85.24) | 86.79 (60.41; 120.79) | 0.03 | |
| 4 | 68.87 (44.31; 83.77) | 67.08 (44.81; 88.33) | 0.85 | |
| 7 | 73.48 (49.98; 79.81) | 66.74 (45.32; 87.43) | 0.87 | |
| 1 year | 82.63 (54.31; 92.93) | 67.75 (55.71; 94.66) | 0.77 | |
| LIGHT, pg/mL, Me (Q25; Q75) | 1 | 195.76 (114.82; 245.30) | 327.95 (194.89; 480.26) | 0.13 |
| 2 | 190.70 (167.49; 210.35) | 274.00 (197.02; 443.96) | 0.06 | |
| 4 | 199.45 (146.94; 305.82) | 212.29 (110.12; 311.65) | 0.82 | |
| 7 | 247.05 (182.33; 388.44) | 263.53 (177.80; 322.51) | 0.73 | |
| 1 year | 226.17 (114.03; 392.93) | 193.02 (83.02; 284.09) | 0.53 | |
| Endocan-1, pg/mL, Me (Q25; Q75) | 1 | 2467.50 (1542.0; 3489.0) | 1861.0 (1369.50; 2630.50) | 0.19 |
| 2 | 1650.50 (1551.0; 3744.0) | 1469.50 (1165.0; 3319.50) | 0.13 | |
| 4 | 1240.50 (858.11; 1895.0) | 1608.0 (1040.29; 2190.0) | 0.16 | |
| 7 | 1287.00 (856.64; 1935.0) | 1241.0 (1042.50; 1467.50) | 0.78 | |
| 1 year | 999.45 (786.86; 1171.0) | 1005.49 (861.91; 1410.0) | 0.26 | |
| sVCAM-1, pg/mL Me (Q25; Q75) | 1 | 734.6 (700.04; 876.4) | 612.09 (536.57; 739.7) | 0.02 |
| 2 | 696.6 (617.77; 744.1) | 606.67 (543.68; 701.8) | 0.08 | |
| 4 | 638.9 (569.93; 704.1) | 593.60 (503.21; 653.1) | 0.16 | |
| 7 | 674.6 (578.19; 714.5) | 623.20 (446.58; 686.9) | 0.16 | |
| 1 year | 763.4 (668.08; 904.9) | 600.47 (536.99; 643.0) | 0.03 |
| Indicator | Delta | MINOCA, n = 16 | MI-CAD, n = 21 | p-Value |
|---|---|---|---|---|
| CXCL6, pg/mL, Me (Q25; Q75) | 1 | −21.02 (−70.9; 15.74) | −10.50 (−56.1; 29.02) | 0.59 |
| 2 | 4.73 (−61.2; 45.25) | 50.15 (−58.6; 163.07) | 0.04 | |
| CCL-8, pg/mL, pg/mL Me (Q25; Q75) | 1 | 3.72 (0.61; 7.65) | 3.44 (−10.5; 13.6) | 0.97 |
| 2 | −0.92 (−16.4; 14.7) | 13.56 (2.28; 42.5) | 0.04 | |
| CCL-15, pg/mL, Me (Q25; Q75) | 1 | −133.17 (−1999.0; 380.0) | −118.50 (−1969.0; −8.50) | 0.39 |
| 2 | −154.0 (−598.0; 815.0) | −184.00 (−1177.0; 487.00) | 0.93 | |
| CCL-21, pg/mL, Me (Q25; Q75) | 1 | 76.6 (30.1; 126.3) | −91.75 (−145.4; −56.1) | 0.03 |
| 2 | 41.9 (−41.2; 124.6) | −6. 95 (−69.3; −45.2) | 0.12 | |
| IL-20, pg/mL, Me (Q25; Q75) | 1 | 0.45 (−5.5; 12.90) | 1.0 (−13.7; 10.21) | 0.38 |
| 2 | −1.75 (−7.0; 26.10) | 3.85 (−5.2; 54.35) | 0.42 | |
| Oncostatin M, pg/mL, Me (Q25; Q75) | 1 | −0.28 (−5.2; 8.23) | −4.26 (−15.1; 5.96) | 0.13 |
| 2 | −2.54 (−14.9; 3.23) | −4.61 (−5.9; 11.02) | 0.52 | |
| Placental Growth Factor, pg/mL, Me (Q25; Q75) | 1 | −1.19 (−8.1; 0.49) | 0.29 (−1.3; 4.51) | 0.17 |
| 2 | −1.84 (−2.5; 3.20) | 0.63 (−0.1; 8.38) | 0.11 | |
| sP-Selectin, pg/mL, Me (Q25; Q75) | 1 | 10.61 (−1.1; 12.04) | −7.87 (−52.1; 8.88) | 0.23 |
| 2 | 7.02 (−4.0; 26.97) | −7.31 (−8.5; 25.85) | 0.84 | |
| LIGHT, pg/mL, Me (Q25; Q75) | 1 | 42.49 (−90.9; 178.78) | −82.92 (−363.9; −14.69) | 0.03 |
| 2 | −73.68 (−101.4; 10.75) | 25.38 (−89.9; 105.33) | 0.72 | |
| Endocan-1, pg/mL, Me (Q25; Q75) | 1 | −1463.46 (−2423.0; −723.0) | −614.49 (−1526.5; −278.05) | 0.03 |
| 2 | −246.50 (−691.0; −23.18) | −2.50 (−210.2; 830.40) | 0.02 | |
| sVCAM-1, pg/mL Me (Q25; Q75) | 1 | 34.96 (−12.7; 95.74) | −15.96 (−65.1; 53.16) | 0.04 |
| 2 | 131.43 (−31.9; 213.46) | −16.55 (−44.5; 156.38) | 0.03 |
| Predictors | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| COR; 95% CI | p | AOR; 95% CI | p | |
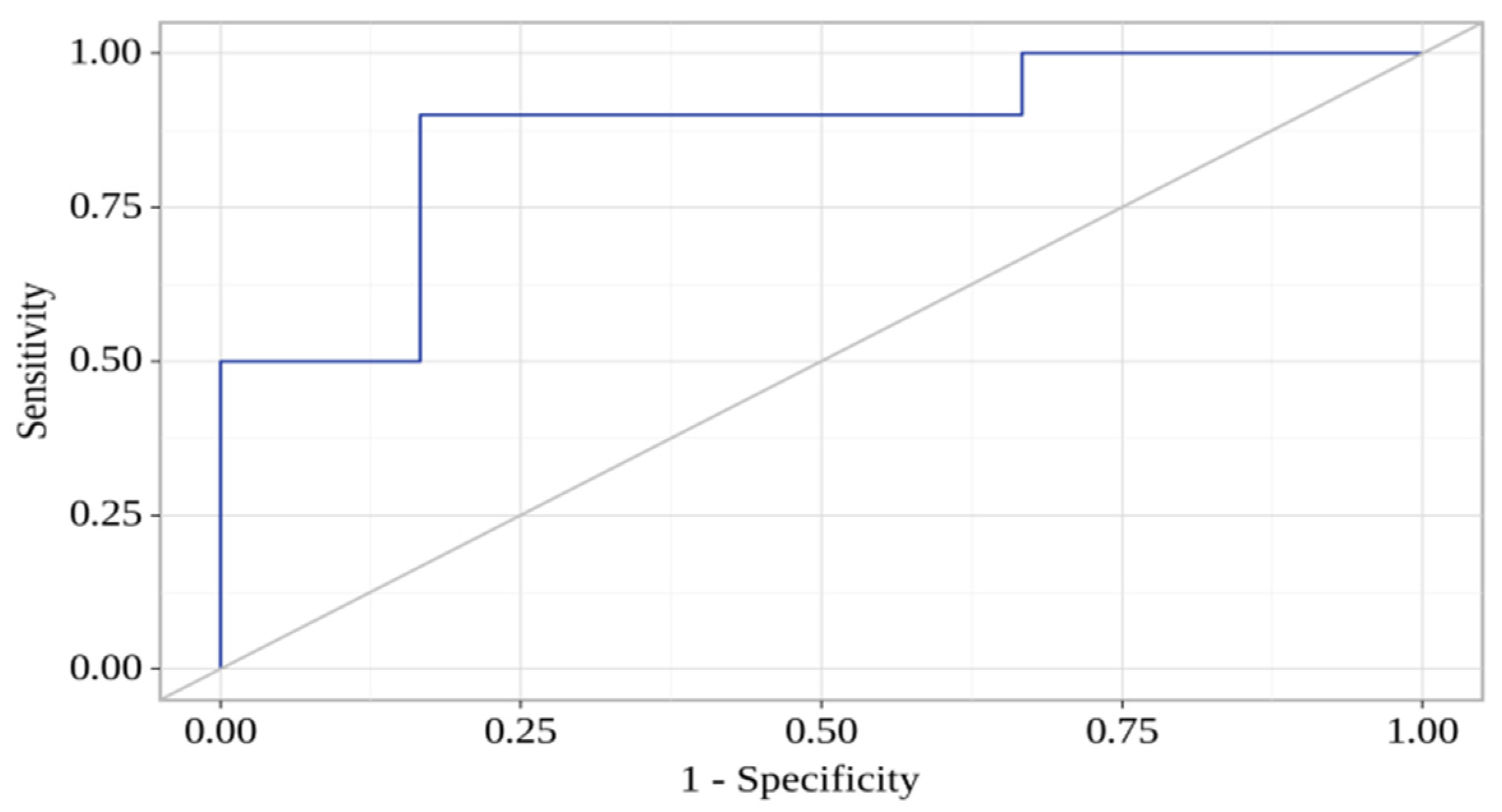
| sVCAM-1 (day 7) | 0.996; 0.991–1.001 | 0.04 * | 0.991; 0.984–0.998 | 0.02 * |
| CCL-21 (day 7) | 1.004; 0.997–1.011 | 0.02 * | 1.014; 1.002–1.026 | 0.02 * |
| LIGHT (day 1) | 1.005; 0.998–1.010 | 0.07 | 1.008; 1.001–1.015 | 0.06 |
| sP-Selectin (day 1) | 1.004; 0.997–1.011 | 0.29 | 1.014; 1.002–1.026 | 0.08 |
| Predictors | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| COR; 95% CI | p | AOR; 95% CI | p | |
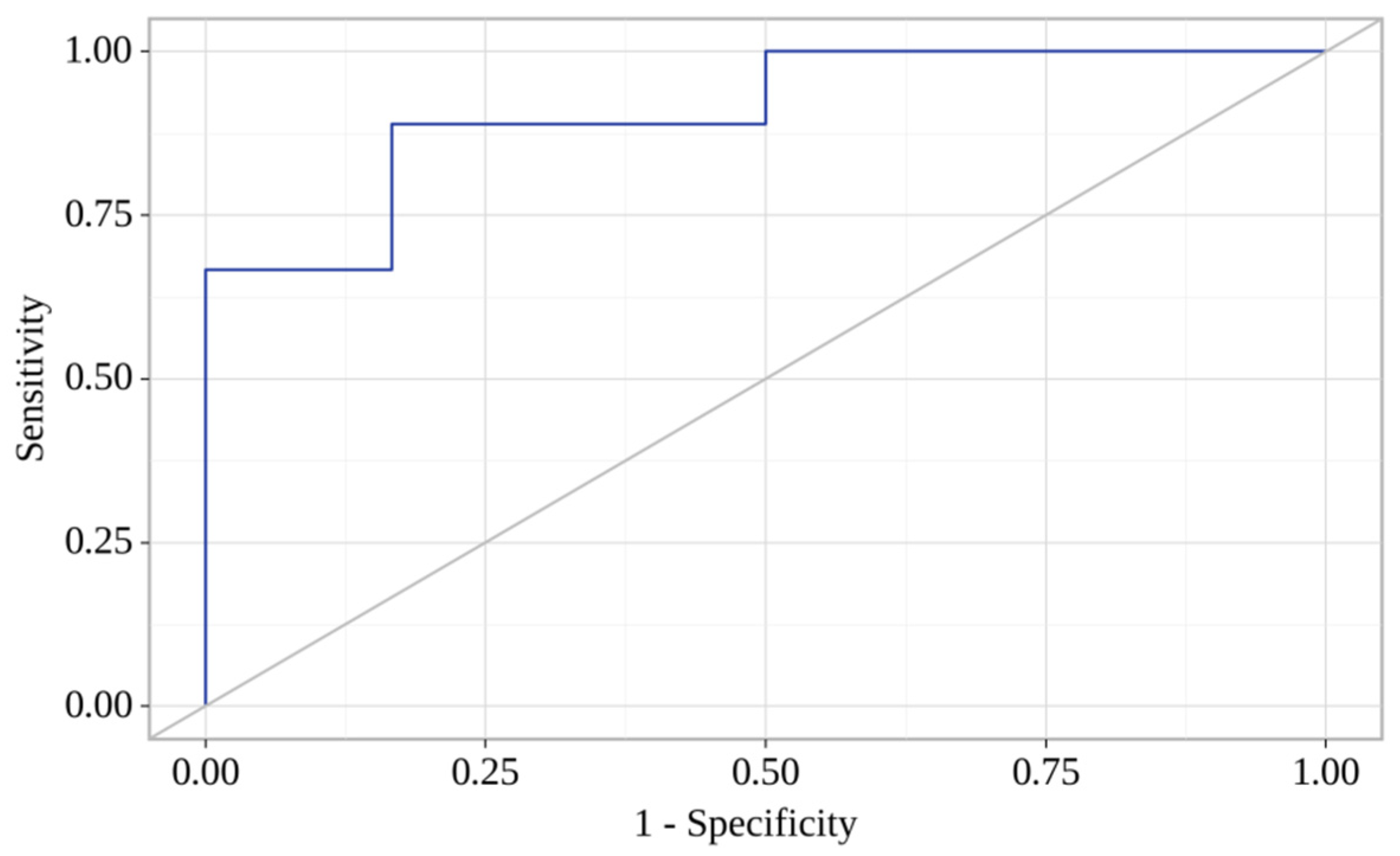
| CCL-8 (day 7) | 0.971; 0.944–0.999 | 0.04 * | 0.941; 0.852–1.039 | 0.02 * |
| CXCL-6 (day 7) | 1.016; 1.001–1.031 | 0.03 * | 1.035; 0.977–1.095 | 0.02 * |
| LIGHT (day 7) | 1.004; 0.997–1.011 | 0.19 | 1.018; 0.977–1.095 | 0.09 |
| IL-20 (day 7) | 1.000; 0.995–1.004 | 0.82 | 0.994; 0.987–1.001 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabov, V.V.; Vorobeva, D.A.; Kologrivova, I.V.; Suslova, T.E. Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up. J. Pers. Med. 2023, 13, 1669. https://doi.org/10.3390/jpm13121669
Ryabov VV, Vorobeva DA, Kologrivova IV, Suslova TE. Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up. Journal of Personalized Medicine. 2023; 13(12):1669. https://doi.org/10.3390/jpm13121669
Chicago/Turabian StyleRyabov, Vyacheslav V., Darya A. Vorobeva, Irina V. Kologrivova, and Tatiana E. Suslova. 2023. "Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up" Journal of Personalized Medicine 13, no. 12: 1669. https://doi.org/10.3390/jpm13121669
APA StyleRyabov, V. V., Vorobeva, D. A., Kologrivova, I. V., & Suslova, T. E. (2023). Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up. Journal of Personalized Medicine, 13(12), 1669. https://doi.org/10.3390/jpm13121669






