Abstract
(1) Background: Approximately 30% of schizophrenia patients are known to be treatment-resistant. For these cases, more personalized approaches must be developed. Virtual reality therapeutic approaches such as avatar therapy (AT) are currently undergoing investigations to address these patients’ needs. To further tailor the therapeutic trajectory of patients presenting with this complex presentation of schizophrenia, quantitative insight about the therapeutic process is warranted. The aim of the study is to combine a classification model with a regression model with the aim of predicting the therapeutic outcomes of patients based on the interactions taking place during their first immersive session of virtual reality therapy. (2) Methods: A combination of a Linear Support Vector Classifier and logistic regression was conducted over a dataset comprising 162 verbatims of the immersive sessions of 18 patients who previously underwent AT. As a testing dataset, 17 participants, unknown to the dataset, had their first immersive session presented to the combinatory model to predict their clinical outcome. (3) Results: The model accurately predicted the clinical outcome for 15 out of the 17 participants. Classification of the therapeutic interactions achieved an accuracy of 63%. (4) Conclusion: To our knowledge, this is the first attempt to predict the outcome of psychotherapy patients based on the content of their interactions with their therapist. These results are important as they open the door to personalization of psychotherapy based on quantitative information about the interactions taking place during AT.
1. Introduction
1.1. Schizophrenia and Treatment Resistance
A recent study estimated that, in 2019, about 418 million disability-adjusted life years are caused by mental disorders, with a worldwide economic burden evaluated at USD 5 trillion [1]. Amongst mental health disorders, schizophrenia has a relatively small prevalence of less than 1% but significantly contributes to this global burden of mental disorders [2,3]. This psychotic disorder was originally defined by Eugen Bleuler in 1908 [4,5]. Schizophrenia is a functional psychotic condition marked by the presence of delusional beliefs, hallucinations, and disruptions in cognition, perception, and behavior [6,7]. Hallucinations are more often auditory in schizophrenia and are also part of an ensemble of symptoms known as positive symptoms [8,9]. This constellation of symptoms includes hallucinations, delusions, and cognitive impairments. This medical condition is not benign considering that people with schizophrenia often have a life expectancy reduction of 10 to 25 years because they have a greater suicide risk and more physical health issues than the general population [10]. Moreover, there is a higher risk of violence when the symptoms are not addressed, including hetero-aggression, victimization, and self-harm [11,12]. Indeed, violent offenses, including homicides, are also more likely in schizophrenia and other psychotic disorders [13]. However, most of the extra risk appears to be mitigated by concomitant drug usage [14,15,16]. Patients suffering from schizophrenia and benefiting from treatment usually reduce their risk of self-harm and violence [17,18]. They also experience improvements in their quality of life and life expectancy [19].
In schizophrenia, the treatment course usually includes psychopharmacological and psychotherapeutic approaches [20,21]. As dopamine is a relevant neurotransmitter involved in the positive symptoms observed in schizophrenia, the psychopharmacological component most often includes dopaminergic receptor antagonists, also known as antipsychotics [22,23]. However, about 30% of people with schizophrenia will not respond adequately to these medications and will be referred to as treatment-resistant schizophrenia (TRS) patients [24]. For those patients, most clinical guidelines support that a failure to respond to two antipsychotics warrants a trial of clozapine: a second-generation antipsychotic [25]. This approach is the current standard treatment for patients suffering from TRS. However, it is estimated that 40–70% of patients with TRS have persistent symptoms despite clozapine use [26,27]. Such symptoms include persistent auditory hallucinations, which represent the most prevalent and disabling symptoms in schizophrenia. Trials of cognitive behavioral therapy (CBT) have been recommended as an adjunctive approach in the treatment of positive and persistent positive symptoms in TRS [28]. While this form of therapy is an interesting avenue to reduce positive symptoms in people suffering from TRS, results are mitigated, hence why new therapeutic strategies are currently emerging [29].
1.2. Current Innovation: Virtual Reality Therapy
Virtual reality therapy (VRT) for patients suffering from TRS is also known as avatar therapy (AT). This psychotherapeutic approach, developed by Julian Leff and his team in 2008, involves the use of an immersive virtual reality system in which TRS patients interact with an avatar, which virtually represents their main persistent auditory verbal hallucination while being controlled and animated by the therapist [30].
Numerous studies have shown that AT is useful in reducing auditory and verbal hallucinations [31,32]. AT is also a psychotherapeutic treatment developed at the Centre de Recherche de Institut universitaire en santé mentale de Montréal (CR-IUSMM). Studies are undergoing to evaluate its efficacy compared to that of CBT [32]. Nine weekly therapy sessions are planned as part of the therapeutic process, most of which are derived from other relational therapeutic approaches and CBT [32]. To accurately depict the patient’s own representation of their most upsetting speech hallucination (persistent auditory hallucinations; “voice”), the therapist and the patient work together to create an avatar during the first session using Unity software. The design of the avatar allows for the consideration of a wide range of traits, including gender, facial features, breadth, voice, and height. Patients then engage with the avatar during a portion of the following eight sessions while wearing a virtual reality headset. The therapist controls the animation of the avatar and operates an external speech modulator system to control their voice modulation.
Pilot studies examining the effects of AT showed that patients with TRS improved by significantly lowering the frequency of persistent auditory hallucinations as well as the distress associated with these symptoms, with large effect sizes [31,32]. Moreover, this therapy also significantly improved their quality of life [33]. To better document and analyze the content of the immersive portions (dialogues between the avatar and the patient), two main qualitative studies were conducted. Five key themes emerged from an initial qualitative investigation of the discourse elements of AT among the patients: emotional responses to voices, beliefs about voices and schizophrenia, self-perceptions, coping mechanisms, and aspirations [34]. Then, a second in-depth investigation by Beaudoin and her team provided further details. The verbatims (immersive session transcripts) of 18 patients who received AT were analyzed for content. Positive techniques (comprising six sub-themes) and confrontational techniques (comprising eight sub-themes) were the two main core interactions identified for the avatar [35]. The patients’ self-perceptions, emotional reactions, goals, coping strategies, and beliefs were all highlighted as five major themes comprising 14 sub-themes. Qualitative data are vast and insightful, and they can lead to the generation of novel hypotheses [35]. However, they lacks the quantitative equivalent required to identify the precise components of therapy that may help patients achieve favorable outcomes.
Considering the above limitations and potential human biases involved in the classification of verbal interactions, automated classification approaches have been attempted to analyze new verbatims of patients who underwent AT by classifying every interaction into one of the sub-themes identified by Beaudoin and her team. Several machine learning classifiers were compared [36]. In the end, the linear support vector machine classifier (LSVC) performed best for classification of therapeutic interactions taking place in AT [37].
AT being a novel approach for which access is currently limited to research participants, a better understanding of the therapeutic outcomes and predictors of such outcomes could be helpful when assessing which patients will benefit from the therapy. Considering that therapeutic components (i.e., verbal interactions between the patient and their avatar) can be qualitatively and quantitatively assessed, it could be beneficial to assess their predictive power of the reduction of persistent auditory hallucinations and consequently better personalize the treatment of TRS patients.
1.3. Precision Medicine Using Predictive Approaches
Modern medicine encompasses several precision medicine avenues, including the use of artificial intelligence to conduct an array of tasks: helping clinicians establish diagnosis, choosing treatment plans, outcome prediction, etc. [38,39,40]. Although precision medicine lags in psychiatry compared to other areas of medicine, it has been highlighted as an avenue to help patients and clinicians in achieving personalization of treatment plans [41]. A recent example is the implementation of a crisis predictor from the exploration of 581 656 medical records for patients suffering from various psychiatric disorders [42]. The model, developed by Garriga and his team, predicted crises with a sensitivity of 58% and a specificity of 85%, achieving an area under the receiver operating characteristic curve of 0.797 and an area under the precision-recall curve of 0.159 [42]. As for treatment responses, a relevant review identified eight studies about patients suffering from depression in which implementations of machine learning models have shown good treatment response prediction (up to 80% accuracy), frequently surpassing usual regression techniques [43]. Although this literature is scarce, several reviews on the application of machine learning to psychiatry and psychology support the idea that this avenue could provide more personalized care for patients [44]. Such use of machine learning in VRT could provide an insight as to which patients are more likely to benefit from this approach earlier or later in their recovery process. Moreover, such tools could also help therapists in planning their immersive sessions more effectively and ultimately conduct better tailored therapeutic sessions to help each patient in achieving favorable outcomes.
1.4. Objective and Hypothesis
By combining a classification model with a regression model, the aim of the study is to predict patients’ therapeutic outcome based on the interactions taking place in their first AT therapeutic session. The classification model is used to classify a verbatim into the right interaction themes, and the regression model is used to determine the predictive value of the newly annotated verbatim. Given the prior use of automated classification algorithms in virtual reality therapy, along with the binary nature of the therapeutic outcome, it is hypothesized that this process can be employed to predict a patient’s therapeutic outcome. To the best of our knowledge, this is the first attempt to predict the outcome of a patient in psychotherapy based on the content of their interactions with their therapist.
2. Materials and Methods
2.1. Participants and Recruitment
Data from participants involved in previous studies were used for the purpose of this investigation. These individuals were signed up for the clinical trial with the NCT03585127 identifier that was listed on ClinicalTrials.gov (accessed on 12 September 2023) [32]. All of them underwent six to ten one-hour psychotherapy sessions, eight of which involved interaction with an avatar that represented their auditory verbal hallucinations, while the creation of the avatar took place during the first session. The immersive portion of the therapeutic sessions between the patient and the avatar lasted between 15 and 50 min. Recruitment occurred at the CR-IUSMM between 2017 and 2022; participants were either referred by their treating team or self-referred. Inclusion criteria included an age of 18 or older and a diagnosis of TRS, characterized by a persistent auditory hallucination despite two or more trials of dopaminergic antagonists. The dataset consisted of treatment interventions for 18 patients, and the prediction power was tested using data from 17 other participants not included in the initial dataset. Therefore, for these 17 patients, the therapeutic outcome was known to the therapists but was unknown to the predictive algorithm.
2.2. Dataset: Corpus of Avatar Therapy and Features
A dataset comprising a total of 162 handwritten treatment transcripts of 18 patients who received VRT between 2017 and 2020 at our institution, corresponding to up to 10 therapy sessions per patient, was developed. The transcripts were written in Canadian French. The 27 themes listed in Beaudoin et al. 2021 were used to hand annotate transcripts [35]. This qualitative analysis of AT was carried out in previous research. Every one of the distinct interactions was individually coded by two study assistants. The same two research assistants cross-validated the robustness of the coding grid. Annotations were performed using the qualitative data analysis program QDA Miner version 5 (Provalis Research) [45]. Then, these were retrieved as text files from QDA Miner and categorized under two conceptual databases, Avatar and Patient, in order to optimize the automatic categorization. These text files contained between one and forty interactions of the same topic. The conceptual datasets were created in accordance with Figure 1’s representation.
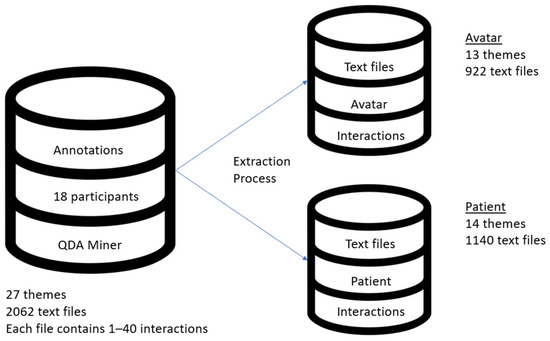
Figure 1.
AT dataset and visual representation of Avatar and Patient conceptual databases.
In this study, the individual interactions, presented in Table 1 for the avatar and the patient, were used as features for the classification algorithm and the predictive algorithm. Both implementations are presented below.

Table 1.
Summary of interactions for the avatar and the patients in VRT as defined by Beaudoin et al.
2.3. Overview of the Predictive Approach
This study combines an automated classification algorithm with a logistic regression. The classification model was trained based on the AT dataset for each conceptual database. Then, an unannotated verbatim for the first immersive session of a participant who previously underwent AT (but unknown to the AT dataset) was presented to the classification model. Once automated classification of each interaction between the avatar and the patient was achieved, the frequency for each interaction was compiled and passed through the logistic regression model. A prediction of the outcome could then be achieved. The overall flow of this predictive approach is presented in Figure 2.
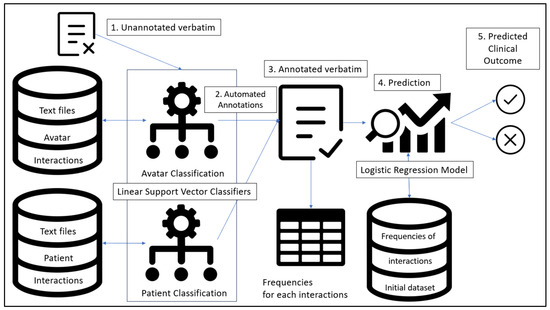
Figure 2.
General flow and elements of the combination of LSVC for each conceptual database combined with a regression algorithm.
2.4. Automated Classification of Verbatims
2.4.1. Previous Work
A support vector machine (SVM) was implemented as per previous work on avatar therapy [37]. This machine learning method is used for both regression and classification problems. It operates by identifying the ideal hyperplane in a high-dimensional feature space that best divides several classes [46]. This hyperplane is set up to maximize the distance (margin) between the classes, which enhances the model’s ability to generalize to new data. SVMs are a well-liked option for text classification jobs because of their capacity to handle high-dimensional, sparse, and non-linear data [46].
In this study, a linear form of SVM was combined to a term frequency-inverse document frequency statistic (TF-IDF). Compared to various SVMs with a tokenizer combination, a TF-IDF performs best with text categorization [47]. The TfidfVectorizer class, available in the Scitkit-Learn open library, was chosen for the TF-IDF tokenization as it allows for the conversion of raw text (extracted interactions from interview transcripts) into numerical vectors [48]. Stop-words can be accounted for by customizing vectorizers. The features were expected to be linearly separable since the classification categories were created so that text entities would be divided based on their inherent qualities, which are fundamentally distinct and specified in Beaudoin et al. (2021). A GridSearchCV (GSCV) was employed to guarantee the LSVC algorithm’s optimal performance and to improve search tactics [48]. The benefit of a GSCV is that it allows the user to test for various hyper-parameters and cross-validate the LSVC’s classification to find the optimal set of LSVC parameters and TfidVectorizer parameter variables. The full implementation details, including implementation parameters and hypertuning, can be found in Hudon et al. [37].
2.4.2. Linear Support Vector Classifier
The LSVC employs a linear kernel as opposed to regular SVM [49]. A kernel is a mathematical function that transforms data into a higher-dimensional feature space and is used in several machine learning techniques [50]. Kernels are crucial to algorithms’ capacity to solve complex problems that might be difficult or even impossible to handle in the original input space [50]. When the data can be separated linearly, a linear kernel is thus applied. Scikit-Learn’s SVC class of the SVM library, which has the specification to use a linear kernel, is the implementation of the SVC used in this work [48].
2.5. Prediction of Patient’s Outcome
In this study, the outcome was measured based on the change in auditory hallucinations, measured using the Psychotic Symptom Rating Scales (PSYRATS) auditory hallucinations subscale [51]. The PSYRATS is a clinical evaluation instrument used to gauge how severe and specific psychotic symptoms are in psychotic individuals. The auditory hallucinations subscale is comprised of 11 items: frequency, duration, controllability, loudness, location, amount and degree of negative content, severity and intensity of distress, beliefs about the origin of voices, and disruption [51]. A participant who experienced a decrease of 20% in the PSYRATS auditory hallucination subscale was defined as being a good responder, whereas other participants were referred to as non-responders.
2.6. Data Analysis and Validation
2.6.1. Training and Cross-Validation
For each conceptual database, a partitioning method was implemented, with 70% of the annotated documents being used to train the LSVC and the remaining 30% being used for testing. The goal was to determine a statistical likelihood for the LSVC, represented by a classification predictive score, which would indicate how well an interaction might be classified. To follow suggested design practices, the training and testing sets were purposefully kept apart [52]. The predictive score reflects the average accuracy as determined by the F1-Score. The K-Fold model from the Scikit-Learn module was used to build a tenfold cross-validation strategy for both the logistic regression algorithm and the linear support vector algorithm.
2.6.2. Classification Analysis
Information on the classification performance of each topic, including accuracy, recall, and F1-Score for each method, was gathered using the Classification Report tool from the Scikit-Learn metrics module [48]. The F1-Score depicts the accuracy of theme categorization, recall of the sensitivity of the prediction, and precision of the positive predictive value. To provide a comprehensive evaluation of classification accuracy, the F1-Score, a widely used metric in text classification, finds a compromise between precision and recall [53]. Therefore, the harmonic mean of recall and accuracy is the F1-Score [53].
2.7. Prediction Analysis
Considering the binary outcome of virtual reality therapy (good responder versus non-responders), a logistic regression was implemented. The interactions between avatar and the patient as defined above were used as features to determine the outcome of the regression. The LogisticRegressionCV class, which is a logistic regression with build-in cross-validation from the Scikit-Learn library, was used [48]. The adjusted R2 score was representative of our predictive score. A score of 1 would indicate that the model explains all the variation of the dependent variable around its mean compared to a score of 0, which means that the model does not explain at all the observed variations. Collinearity between the different variables was accounted for in the logistic regression algorithm by providing the variance inflation factor (VIF) for each feature.
To build the logistic regression model, the average frequency for each type of interaction for the first set of participants was used to construct a second dataset used for predictive purposes. This dataset contained all the frequencies of the interaction themes of the 18 participants who previously completed AT. Considering that the interaction themes are the features of the predictive model, this approach is needed to predict the potential therapeutic outcome of a newly annotated verbatim. The data used for this are available in Supplementary Material Table S1.
Finally, to predict patients’ outcomes, automatically annotated verbatims of the first immersive session of 17 participants (second set of participants) were used. The frequency of each type of interaction was calculated and used by the model to conclude if the participant was forecasted to be a good responder or a non-responder. Statistical significance is defined by a likelihood ratio p-value smaller than 0.05 [54].
3. Results
3.1. Sample Characteristics
Interactions taking place in the verbatims of 18 patients were used to construct the initial interaction dataset, from which interaction frequencies were used to make the prediction of the outcome. The characteristics of the sampled patients can be found in Table 2.

Table 2.
Characteristics of sampled patients for the first set of participants included in previously published studies. N = 18.
3.2. Performance of the Classification Algorithm
The average performance of the LSVC for the automatic annotation of the verbatims of the second set of participants can be found in Table 3. The precision score ranges from 0.62 to 0.67, the recall ranges from 0.58 to 0.65, and the F1-Score ranges from 0.60 to 0.65. Classification scores for participants 007 and 016 are the lowest, whereas the average accuracy score for annotation is 63% (as per the F1-Score). The classification of avatar themes performed better than patient interaction themes (70% versus 62%). Sample performances for each theme and class balances for each feature are found in Supplementary Material Table S2.

Table 3.
Average performances of each participant on the Avatar conceptual database for the metrics. N = 17.
3.3. Performance of the Predictive Algorithm
The logistic regression model achieved an adjusted R2 performance of 0.736 with a likelihood p-value of 0.04. From the first immersive session verbatim of the 17 participants, a total of 15 had a predicted outcome corresponding to their true outcome (88.2% accurate predictions). Errors occurred for participants 003 and 016. Amongst the previously identified 27 themes, 16 were selected for the model when accounting for collinearity and relevancy. Coefficients of the features as well as model performances are found in Appendix A. Logistic regression score and outcomes are presented for each participants in Table 4.

Table 4.
Comparisons of the true outcome to the predicted outcome for the verbatim of the first immersive session of the 17 participants.
4. Discussion
This study aimed to combine a classification model with a regression model to predict patients’ treatment outcome based on interactions in their first AT immersive session. The verbatims (first immersive session) of 17 participants who previously underwent AT were automatically annotated based on a corpus from previous participants of AT, and a prediction was effectuated based on the frequency of each type of interaction that took place during the first VR session. As a result, the combination of the models predicted accurately the outcome of 15 out of 17 participants with an average accuracy score for the automated annotations of 63%.
Prediction of psychotherapeutic outcome is a potentially interesting avenue to personalize treatments for patients suffering from severe mental illnesses. However, prior works on the topic of predicting patient outcomes using patient risk factors and demographics were mostly inconclusive [55,56]. Patient factors alone cannot be the sole elements to predict patients’ outcomes as therapeutic processes are highly dependent on the therapeutic alliances and patient–therapist interactions. A five-year longitudinal study protocol highlighted the importance of considering the therapist’s interpersonal skills in relation with the outcome of the psychotherapy [57]. Other elements of the psychotherapeutic process are also in the process of being modeled. For example, a recent study trained several machine learning algorithms on patients’ self-reported side effects of psychotherapy to predict performances of the psychotherapeutic outcomes. They achieved an accuracy of 79.7% using Random Forest-based machine learning classifiers [58]. However, such implementation of prediction classifiers is to be used with caution as numerous biases might be implied such as class imbalances and the definition of the therapeutic outcome in their context of relevance. When compared to other fields of medicine, where terms (e.g., signs and symptoms) may be used to help categorization, psychotherapy such as AT often utilizes a larger range of words and contextual sentences. This might explain why the automated classification does not reach perfect accuracy.
As for the predictive performance, few studies address predictive indicators of therapeutic outcomes based on therapeutic interactions. However, a recent literature review on the potential uses of machine learning to predict responses to CBT for different mental health disorders identified algorithm performances ranging from 67.3% up to 87.0% [59]. The performance of our model (88.2%) may differ from this range of performances because AT differs from CBT and uses a protocolized approach for patients suffering from TRS. However, the complexity of TRS and the variety of interactions between the therapists and the patients might account for the lower classification scores which is often observed when the elements of the corpus are overlapping or imbalanced. Another explanation could be that AT is based on an important role-playing relationship between the therapist and the patient, necessarily less linear in its approach, which could explain the low classification accuracy.
Limitations
The performance trend for the LSVC is to be re-evaluated when additional patients are added to the dataset. Of note, transcripts used for this study’s analysis were written in Canadian French, and consequently, finding vectorizers that included stop-words particularly for the Canadian French language was challenging. Stop-words are terms that are often not included in the tokenization process because their meanings are either vague or unimportant. The accuracy of the analysis could have been impacted by the lack of suitable stop-words for Canadian French, which could lead to irrelevant terms being included in the word vectors and distorting the final findings. Finally, it is of importance to note that the performance of the regression algorithm was based on a limited dataset which might affect its performance and is dependent of the classification algorithm.
5. Conclusions
To conclude, this study demonstrated that classification algorithms such as LSVC can be combined with a predictive algorithm to predict patients’ outcomes for AT. Out of 17 participants unknown to the original dataset, the outcomes of 15 were accurately predicted based on the frequency of their interactions during their first immersive session. Automated classifications of the interactions taking place during the immersive session achieved performances comparable to previous studies on the subject. These results present an interesting avenue for the personalization of patients’ experiences with AT as the therapist might use this insight between the immersive sessions to help prepare their next sessions to enhance patients’ likelihood of achieving a favorable outcome. This could be carried out by identifying interactions linked to a positive outcome and encouraging such interactions. To the best of our knowledge, this is the first implementation of a predictor based on content elements of the therapeutic process. This opens the door to future studies to explore the possibility of using such classifiers in different psychotherapeutic contexts or by mixing potential predictive elements such as emotional content, therapeutic alliance, and patients’ characteristics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13121660/s1, Table S1: Frequencies of all avatar and patient themes across avatar therapy; Table S2: Sample performances for each theme and class balances for each feature.
Author Contributions
Conceptualization, A.H., M.B., K.P., S.P. and A.D.; methodology, A.H. and A.D.; validation, A.H. and A.D.; formal analysis, A.H.; investigation, A.H.; data curation, A.H.; writing—original draft preparation, A.H. and M.B.; writing—review and editing, A.H., M.B., K.P., S.P. and A.D.; supervision, K.P., S.P. and A.D.; project administration, K.P.; funding acquisition, K.P., S.P. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was indirectly supported indirectly by Le Fonds de recherche du Québec—Santé (FRQS); Otsuka Canada Pharmaceutical Inc.; Chaire Eli Lilly Canada de recherche en schizophrénie; MEI (Ministère de l’Économie et de l’Innovation); Services et recherches psychiatriques AD; Fonds d’excellence en recherche Apogée Canada. These fundings bodies had no part in the data collection, analysis, interpretation of data, and in writing the manuscript.
Institutional Review Board Statement
This study was approved by the institutional ethical committee, and written informed consent was obtained from all patients. Patients that are part of this study were selected based on the proof-of-concept trial from Percy du Sert’s 2018 study and Dellazizzo’s 2021 study. The trial was conducted in accordance with the Declaration of Helsinki and was approved by the institutional ethical committee (CER IPPM 16-17-06). We obtained written informed consent from all patients.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study containing patients’ verbatims are not publicly available due to patients’ privacy but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Logistic regression model’s performances.
Table A1.
Logistic regression model’s performances.
| Model | Logistic Regression |
|---|---|
| Pseudo R-Squared | 0.736 |
| Likelihood ratio p-value | 0.04 |
| intercept | −4.651781763166642 |
| coefficients: | |
| Self-appraisal (patient) | 0.04883651593641261 |
| Self-affirmation (patient) | 0.02567784838758098 |
| Beliefs (avatar) | −0.09561273366103458 |
| Negative (patient) | −0.11364269902464413 |
| Psychotherapeutic interventions (neutral) | 0.010654286427180373 |
| Other beliefs (patient) | 0.029167922584813755 |
| Provocation (avatar) | 0.1866938254993158 |
| Negation (patient) | 0.1231509841333093 |
| Prevention (patient) | −0.31748282158545243 |
| Accusations (avatar) | −0.12466383099943076 |
| Positive (patient) | 0.4499983951894208 |
| Questions about coping mechanisms (avatar) | −0.35225305832560383 |
| Counterattack (patient) | 0.10198904940934386 |
| Questions about self-perceptions (avatar) | 0.4672076538702732 |
| Reinforcement (avatar) | −0.005131226285317009 |
| Maliciousness of the voice (patient) | −0.154875181322441 |
References
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the global burden of mental disorders and their economic value. EClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef]
- Janoutová, J.; Janácková, P.; Serý, O.; Zeman, T.; Ambroz, P.; Kovalová, M.; Varechová, K.; Hosák, L.; Jirík, V.; Janout, V. Epidemiology and risk factors of schizophrenia. Neuroendocrinol. Lett. 2016, 37, 1–8. [Google Scholar]
- Mueser, K.T.; McGurk, S.R. Schizophrenia. Lancet 2004, 363, 2063–2072. [Google Scholar] [CrossRef]
- Ashok, A.H.; Baugh, J.; Yeragani, V.K. Paul Eugen Bleuler and the origin of the term schizophrenia (SCHIZOPRENIEGRUPPE). Indian J. Psychiatry 2012, 54, 95–96. [Google Scholar] [CrossRef]
- Orsolini, L.; Pompili, S.; Volpe, U. Schizophrenia: A Narrative Review of Etiopathogenetic, Diagnostic and Treatment Aspects. J. Clin. Med. 2022, 11, 5040. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Humpston, C.S.; Broome, M.R. Thinking, believing, and hallucinating self in schizophrenia. Lancet Psychiatry 2020, 7, 638–646. [Google Scholar] [CrossRef]
- Lim, A.; Hoek, H.W.; Deen, M.L.; Blom, J.D.; GROUP Investigators. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr. Res. 2016, 176, 493–499. [Google Scholar] [CrossRef]
- Montagnese, M.; Leptourgos, P.; Fernyhough, C.; Waters, F.; Larøi, F.; Jardri, R.; McCarthy-Jones, S.; Thomas, N.; Dudley, R.; Taylor, J.-P.; et al. A Review of Multimodal Hallucinations: Categorization, Assessment, Theoretical Perspectives, and Clinical Recommendations. Schizophr. Bull. 2021, 47, 237–248. [Google Scholar] [CrossRef]
- Laursen, T.M.; Munk-Olsen, T.; Vestergaard, M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry 2012, 25, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Girasek, H.; Nagy, V.A.; Fekete, S.; Ungvari, G.S.; Gazdag, G. Prevalence and correlates of aggressive behavior in psychiatric inpatient populations. World J. Psychiatry 2022, 12, 1–23. [Google Scholar] [CrossRef]
- Cho, W.; Shin, W.S.; An, I.; Bang, M.; Cho, D.Y.; Lee, S.H. Biological Aspects of Aggression and Violence in Schizophrenia. Clin. Psychopharmacol. Neurosci. 2019, 17, 475–486. [Google Scholar] [CrossRef]
- Tiihonen, J.; Isohanni, M.; Räsänen, P.; Koiranen, M.; Moring, J. Specific major mental disorders and criminality: A 26-year prospective study of the 1966 northern Finland birth cohort. Am. J. Psychiatry 1997, 154, 840–845. [Google Scholar] [CrossRef]
- Manseau, M.; Bogenschutz, M. Substance Use Disorders and Schizophrenia. Focus (Am. Psychiatr. Publ.) 2016, 14, 333–342. [Google Scholar] [CrossRef]
- Hudon, A.; Dellazizzo, L.; Phraxayavong, K.; Potvin, S.; Dumais, A. Association Between Cannabis and Violence in Community-Dwelling Patients with Severe Mental Disorders: A Cross-sectional Study Using Machine Learning. J. Nerv. Ment. Dis. 2023, 211, 88–94. [Google Scholar] [CrossRef]
- Fazel, S.; Långström, N.; Hjern, A.; Grann, M.; Lichtenstein, P. Schizophrenia, substance abuse, and violent crime. JAMA 2009, 301, 2016–2023. [Google Scholar] [CrossRef]
- Wimberley, T.; MacCabe, J.H.; Laursen, T.M.; Sørensen, H.J.; Astrup, A.; Horsdal, H.T.; Gasse, C.; Støvring, H. Mortality and Self-Harm in Association with Clozapine in Treatment-Resistant Schizophrenia. Am. J. Psychiatry 2017, 174, 990–998. [Google Scholar] [CrossRef]
- Kasckow, J.; Felmet, K.; Zisook, S. Managing suicide risk in patients with schizophrenia. CNS Drugs 2011, 25, 129–143. [Google Scholar] [CrossRef][Green Version]
- Guo, X.; Zhang, Z.; Zhai, J.; Fang, M.; Hu, M.; Wu, R.; Liu, Z.; Zhao, J. Effects of antipsychotic medications on quality of life and psychosocial functioning in patients with early-stage schizophrenia: 1-year follow-up naturalistic study. Compr. Psychiatry 2012, 53, 1006–1012. [Google Scholar] [CrossRef]
- Kokurcan, A.; Güriz, S.O.; Karadağ, H.; Erdi, F.; Örsel, S. Treatment strategies in management of schizophrenia patients with persistent symptoms in daily practice: A retrospective study. Int. J. Psychiatry Clin. Pract. 2021, 25, 238–244. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Mental Health (UK). Psychosis and Schizophrenia in Adults: Treatment and Management; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Scott, J.G. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef]
- Novak, G.; Seeman, M.V. Dopamine, Psychosis, and Symptom Fluctuation: A Narrative Review. Healthcare 2022, 10, 1713. [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Bittner, R.A.; Reif, A.; Qubad, M. The ever-growing case for clozapine in the treatment of schizophrenia: An obligation for psychiatrists and psychiatry. Curr. Opin. Psychiatry 2023, 36, 327–336. [Google Scholar] [CrossRef]
- Chakrabarti, S. Clozapine resistant schizophrenia: Newer avenues of management. World J. Psychiatry 2021, 11, 429–448. [Google Scholar] [CrossRef]
- Shah, P.; Iwata, Y.; Brown, E.E.; Kim, J.; Sanches, M.; Takeuchi, H.; Nakajima, S.; Hahn, M.; Remington, G.; Gerretsen, P.; et al. Clozapine response trajectories and predictors of non-response in treatment-resistant schizophrenia: A chart review study. Eur. Arch Psychiatry Clin. Neurosci. 2020, 270, 11–22. [Google Scholar] [CrossRef]
- Ryan, M.; Sattenspiel, D.; Chianese, A.; Rice, H. CE: Original Research: Cognitive Behavioral Therapy for Symptom Management in Treatment-Resistant Schizophrenia. Am. J. Nurs. 2022, 122, 24–33. [Google Scholar] [CrossRef]
- Morrison, A.P.; Pyle, M.; Gumley, A.; Schwannauer, M.; Turkington, D.; MacLennan, G.; Norrie, J.; Hudson, J.; Bowe, S.E.; French, P.; et al. Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): An assessor-blinded, randomised controlled trial. Lancet Psychiatry 2018, 5, 633–643. [Google Scholar] [CrossRef]
- Leff, J.; Williams, G.; Huckvale, M.; Arbuthnot, M.; Leff, A.P. Avatar therapy for persecutory auditory hallucinations: What is it and how does it work? Psychosis 2014, 6, 166–176. [Google Scholar] [CrossRef]
- Craig, T.K.; Rus-Calafell, M.; Ward, T.; Leff, J.P.; Huckvale, M.; Howarth, E.; Emsley, R.; Garety, P.A. AVATAR therapy for auditory verbal hallucinations in people with psychosis: A single-blind, randomised controlled trial. Lancet Psychiatry 2018, 5, 31–40. [Google Scholar] [CrossRef]
- Dellazizzo, L.; Potvin, S.; Phraxayavong, K.; Dumais, A. One-year randomized trial comparing virtual reality-assisted therapy to cognitive-behavioral therapy for patients with treatment-resistant schizophrenia. NPJ Schizophr. 2021, 7, 9. [Google Scholar] [CrossRef]
- Beaudoin, M.; Potvin, S.; Phraxayavong, K.; Dumais, A. Changes in Quality of Life in Treatment-Resistant Schizophrenia Patients Undergoing Avatar Therapy: A Content Analysis. J. Pers. Med. 2023, 13, 522. [Google Scholar] [CrossRef]
- Dellazizzo, L.; du Sert, O.P.; Phraxayavong, K.; Potvin, S.; O’Connor, K.; Dumais, A. Exploration of the dialogue components in Avatar Therapy for schizophrenia patients with refractory auditory hallucinations: A content analysis. Clin. Psychol. Psychother. 2018, 25, 878–885. [Google Scholar] [CrossRef]
- Beaudoin, M.; Potvin, S.; Machalani, A.; Dellazizzo, L.; Bourguignon, L.; Phraxayavong, K.; Dumais, A. The therapeutic processes of avatar therapy: A content analysis of the dialogue between treatment-resistant patients with schizophrenia and their avatar. Clin. Psychol. Psychother. 2021, 28, 500–518. [Google Scholar] [CrossRef]
- Hudon, A.; Phraxayavong, K.; Potvin, S.; Dumais, A. Comparing the Performance of Machine Learning Algorithms in the Automatic Classification of Psychotherapeutic Interactions in Avatar Therapy. Mach. Learn. Knowl. Extr. 2023, 5, 1119–1131. [Google Scholar] [CrossRef]
- Hudon, A.; Beaudoin, M.; Phraxayavong, K.; Dellazizzo, L.; Potvin, S.; Dumais, A. Implementation of a machine learning algorithm for automated thematic annotations in avatar: A linear support vector classifier approach. Health Inform. J. 2022, 28, 14604582221142442. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Kitsios, F.; Kamariotou, M.; Syngelakis, A.I.; Talias, M.A. Recent Advances of Artificial Intelligence in Healthcare: A Systematic Literature Review. Appl. Sci. 2023, 13, 7479. [Google Scholar] [CrossRef]
- Fakhoury, M. Artificial Intelligence in Psychiatry. Adv. Exp. Med. Biol. 2019, 1192, 119–125. [Google Scholar] [CrossRef]
- Garriga, R.; Mas, J.; Abraha, S.; Nolan, J.; Harrison, O.; Tadros, G.; Matic, A. Machine learning model to predict mental health crises from electronic health records. Nat. Med. 2022, 28, 1240–1248. [Google Scholar] [CrossRef]
- Sajjadian, M.; Lam, R.W.; Milev, R.; Rotzinger, S.; Frey, B.N.; Soares, C.N.; Parikh, S.V.; Foster, J.A.; Turecki, G.; Müller, D.J.; et al. Machine learning in the prediction of depression treatment outcomes: A systematic review and meta-analysis. Psychol Med. 2021, 51, 2742–2751. [Google Scholar] [CrossRef]
- Chen, Z.S.; Kulkarni, P.P.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern views of machine learning for precision psychiatry. Patterns 2022, 3, 100602. [Google Scholar] [CrossRef]
- QDA Miner, Version 5; Provalis Research: Montreal, QC, Canada, 2016.
- Ben-Hur, A.; Weston, J. A user’s guide to support vector machines. Methods Mol. Biol. 2010, 609, 223–239. [Google Scholar] [CrossRef]
- Busagala, L.S.P.; Ohyama, W.; Wakabayashi, T.; Kimura, F. Multiple feature-classifier combination in automated text classification. In Proceedings of the 2012 10th IAPR International Workshop on Document Analysis Systems (DAS), Gold Cost, QLD, Australia, 27–29 March 2012; pp. 43–47. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, É. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Longato, E.; Acciaroli, G.; Facchinetti, A.; Maran, A.; Sparacino, G. Simple Linear Support Vector Machine Classifier Can Distinguish Impaired Glucose Tolerance Versus Type 2 Diabetes Using a Reduced Set of CGM-Based Glycemic Variability Indices. J. Diabetes Sci. Technol. 2020, 14, 297–302. [Google Scholar] [CrossRef]
- Müller, K.R.; Mika, S.; Rätsch, G.; Tsuda, K.; Schölkopf, B. An introduction to kernel-based learning algorithms. IEEE Trans. Neural Netw. 2001, 12, 181–201. [Google Scholar] [CrossRef]
- Woodward, T.S.; Jung, K.; Hwang, H.; Yin, J.; Taylor, L.; Menon, M.; Peters, E.; Kuipers, E.; Waters, F.; Lecomte, T.; et al. Symptom dimensions of the psychotic symptom rating scales in psychosis: A multisite study. Schizophr. Bull. 2014, 40 (Suppl. S4), S265–S274. [Google Scholar] [CrossRef]
- Wei, Q.; Dunbrack, R.L., Jr. The role of balanced training and testing data sets for binary classifiers in bioinformatics. PLoS ONE 2013, 8, e67863. [Google Scholar] [CrossRef]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On evaluation metrics for medical applications of artificial intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef] [PubMed]
- Riedle, B.; Neath, A.A.; Cavanaugh, J.E. Reconceptualizing the p-value from a likelihood ratio test: A probabilistic pairwise comparison of models based on Kullback-Leibler discrepancy measures. J. Appl. Stat. 2020, 47, 2582–2609. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Shamout, F.; Zhu, T.; Clifton, D.A. Machine Learning for Clinical Outcome Prediction. IEEE Rev. Biomed. Eng. 2021, 14, 116–126. [Google Scholar] [CrossRef]
- Schöttke, H.; Flückiger, C.; Goldberg, S.B.; Eversmann, J.; Lange, J. Predicting psychotherapy outcome based on therapist interpersonal skills: A five-year longitudinal study of a therapist assessment protocol. Psychother. Res. 2017, 27, 642–652. [Google Scholar] [CrossRef]
- Yao, L.; Xu, Z.; Zhao, X.; Chen, Y.; Liu, L.; Fu, X.; Chen, F. Therapists and psychotherapy side effects in China: A machine learning-based study. Heliyon 2022, 8, e11821. [Google Scholar] [CrossRef]
- Vieira, S.; Liang, X.; Guiomar, R.; Mechelli, A. Can we predict who will benefit from cognitive-behavioural therapy? A systematic review and meta-analysis of machine learning studies. Clin. Psychol. Rev. 2022, 97, 102193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).