Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
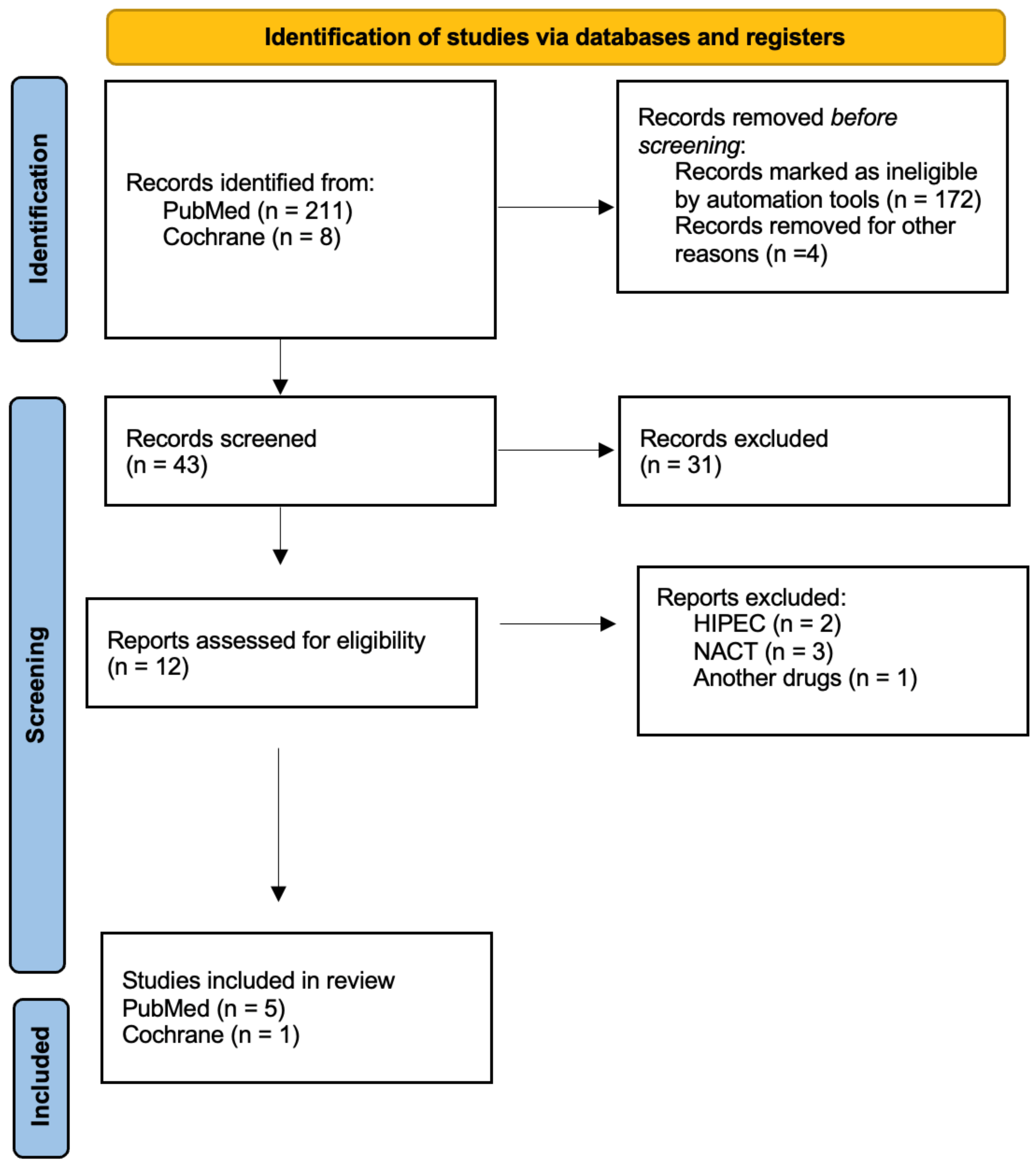
2.3. Selection Process
- Languages: English and Spanish.
- Date: published articles since 2013.
- Type of study: controlled trial, systematic review, and meta-analysis.
- Exclusion of the articles that use hyperthermic intraperitoneal chemotherapy, neoadjuvant chemotherapy, other drugs, patients in the initial stages of ovarian cancer, and patients with extra-abdominal disease.
2.4. Statistical Analysis
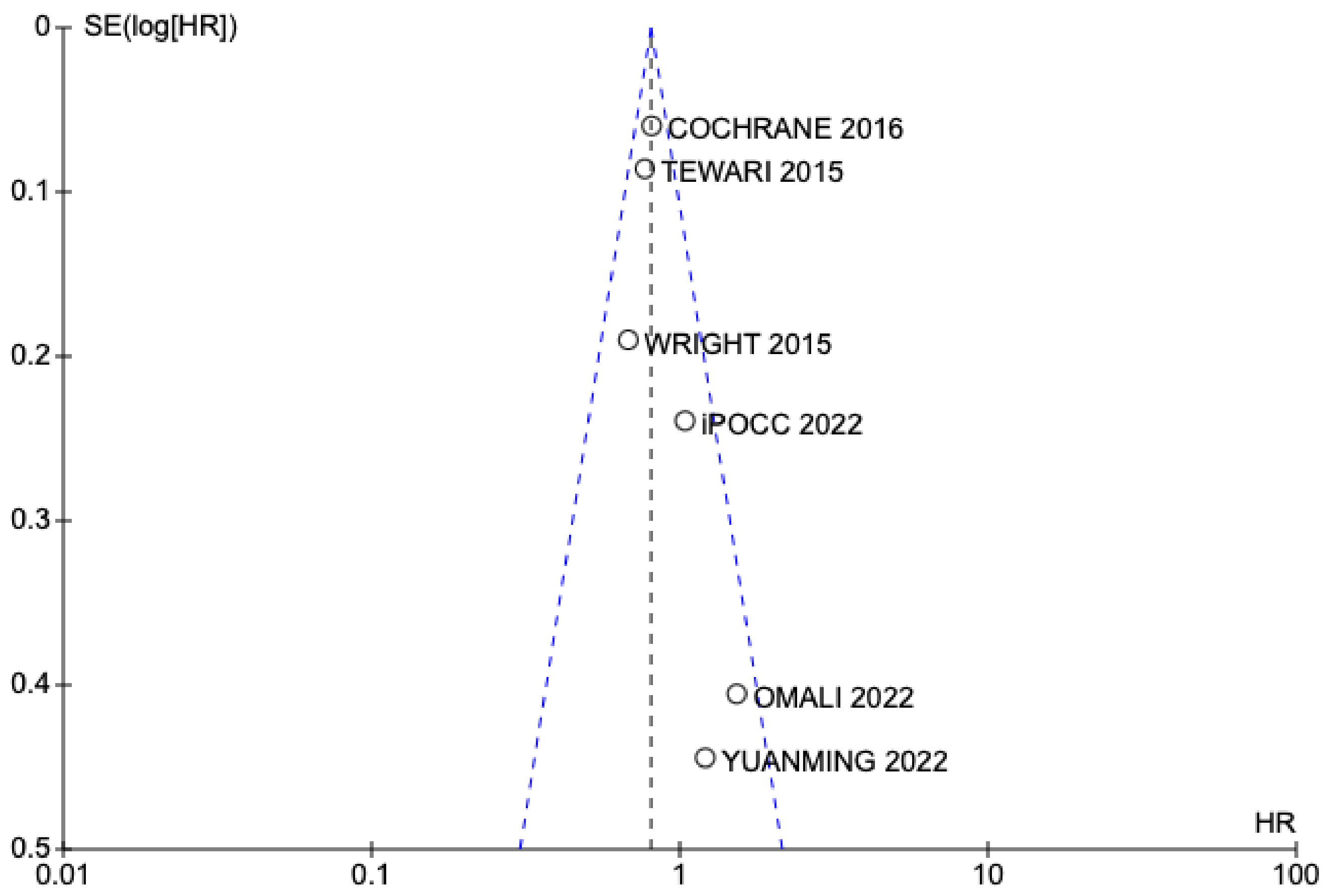
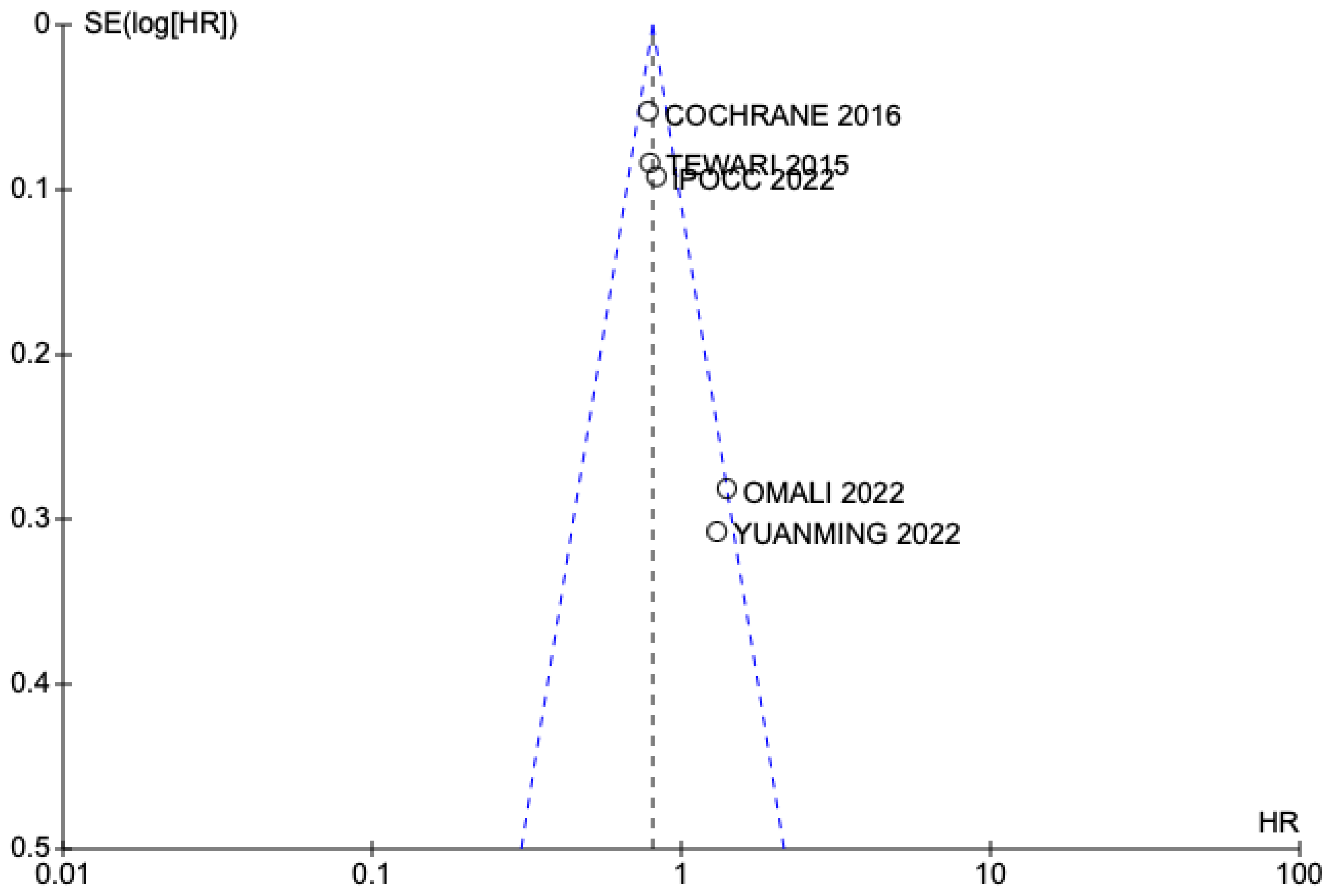
3. Results
3.1. Selected Studies
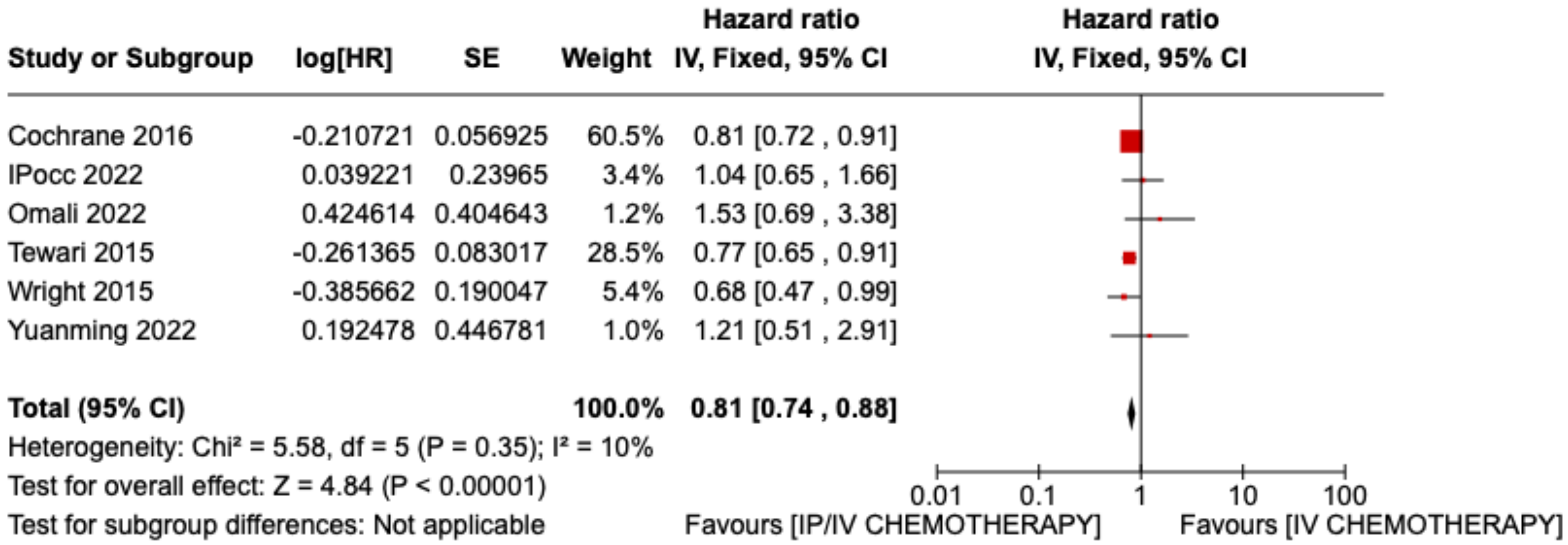
3.2. Results of Meta-Analysis on Overall Survival and Disease-Free Interval
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Monk, B.J.; Sood, A.K.; Herzog, T.J. Latest research and clinical treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Bristow, R.E. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: Redefining ‘optimal’ residual disease. Gynecol. Oncol. 2012, 125, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Brady, M.F.; McGuire, W.P.; Harper, P.G.; Alberts, D.S.; Friedlander, M.; Colombo, N.; Fowler, J.M.; Argenta, P.A.; Geest, K.D.; et al. Evaluation of New Platinum-Based Treatment Regimens in Advanced-Stage Ovarian Cancer: A Phase III Trial of the Gynecologic Cancer InterGroup. J. Clin. Oncol. 2009, 27, 1419. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Climent, M.T.; Segarra, B.; Maazouzi, Y.; Soriano, M.; Escrig, J.; on behalf MUAPOS Working Group. Outcome quality standards in advanced ovarian cancer surgery. World J. Surg. Oncol. 2020, 18, 309. [Google Scholar] [CrossRef]
- Llueca, A.; Climent, M.T.; Escrig, J.; Carrasco, P.; Serra, A.; MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery). Validation of three predictive models for suboptimal cytoreductive surgery in advanced ovarian cancer. Sci. Rep. 2021, 11, 8111. [Google Scholar] [CrossRef]
- Jones, N.L.; Chen, L.; Chatterjee, S.; Tergas, A.I.; Burke, W.M.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. National trends in extended procedures for ovarian cancer debulking surgery. Int. J. Gynecol. Cancer 2018, 28, 19–25. [Google Scholar] [CrossRef]
- Thigpen, T.; DuBois, A.; McAlpine, J.; DiSaia, P.; Fujiwara, K.; Hoskins, W.; Kristensen, G.; Mannel, R.; Markman, M.; Pfisterer, J.; et al. First-line therapy in ovarian cancer trials. Int. J. Gynecol. Cancer 2011, 21, 756–762. [Google Scholar] [CrossRef]
- Redondo, A.; Guerra, E.; Manso, L.; Martin-Lorente, C.; Martinez-Garcia, J.; Perez-Fidalgo, J.A.; Varela, M.Q.; Rubio, M.J.; Barretina-Ginesta, M.P.; Gonzalez-Martin, A. SEOM clinical guideline in ovarian cancer (2020). Clin. Transl. Oncol. 2021, 23, 961–968. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Java, J.J.; Salani, R.; Armstrong, D.K.; Markman, M.; Herzog, T.; Monk, B.J.; Chan, J.K. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2015, 33, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28, viii61–viii65. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.C.C. Update on the management and the role of intraperitoneal chemotherapy for ovarian cancer. Curr. Opin. Obs. Gynecol. 2014, 26, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; De Rosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar]
- Trimble, E.L.; Christian, M.C. Intraperitoneal chemotherapy for women with advanced epithelial ovarian carcinoma. Gynecol. Oncol. 2006, 100, 3–4. [Google Scholar] [CrossRef]
- Chambers, L.M.; Son, J.; Radeva, M.; Debernardo, R. Evaluation of non-completion of intraperitoneal chemotherapy in patients with advanced epithelial ovarian cancer. J. Gynecol. Oncol. 2019, 30, e93. [Google Scholar] [CrossRef]
- Markman, M. An update on the use of intraperitoneal chemotherapy in the management of ovarian cancer. Cancer J. 2009, 15, 105–109. [Google Scholar] [CrossRef]
- Petignat, P.; du Bois, A.; Bruchim, I.; Fink, D.; Provencher, D.M. Should intraperitoneal chemotherapy be considered as standard first-line treatment in advanced stage ovarian cancer? Crit. Rev. Oncol. Hematol. 2007, 62, 137–147. [Google Scholar] [CrossRef]
- Timmermans, M.; Sonke, G.S.; Van de Vijver, K.K.; van der Aa, M.A.; Kruitwagen, R.F.P.M. No improvement in long-term survival for epithelial ovarian cancer patients: A population-based study between 1989 and 2014 in the Netherlands. Eur. J. Cancer 2018, 88, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 17 September 2023).
- Fujiwara, K.; Aotani, E.; Hamano, T.; Nagao, S.; Yoshikawa, H.; Sugiyama, T.; Kigawa, J.; Aoki, D.; Katsumata, N.; Takeuchi, M.; et al. A randomized Phase II/III trial of 3 weekly intraperitoneal versus intravenous carboplatin in combination with intravenous weekly dose-dense paclitaxel for newly diagnosed ovarian, fallopian tube and primary peritoneal cancer. Jpn J. Clin. Oncol. 2011, 41, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2016, 2016, CD005340. [Google Scholar] [CrossRef] [PubMed]
- Pitiyarachchi, O.; Friedlander, M.; Java, J.J.; Chan, J.K.; Armstrong, D.K.; Markman, M.; Herzog, T.J.; Monk, B.J.; Backes, F.; Secord, A.A.; et al. What proportion of patients with stage 3 ovarian cancer are potentially cured following intraperitoneal chemotherapy? Analysis of the long term (≥10 years) survivors in NRG/GOG randomized clinical trials of intraperitoneal and intravenous chemotherapy in stage III ovarian cancer. Gynecol. Oncol. 2022, 166, 410–416. [Google Scholar] [PubMed]
- Wright, A.A.; Cronin, A.; Milne, D.E.; Bookman, M.A.; Burger, R.A.; Cohn, D.E.; Cristea, M.C.; Griggs, J.J.; Keating, N.L.; Levenback, C.F.; et al. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Oncol. 2015, 33, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, S.; Xu, J.; Xie, X.; Chen, Z. Modified Intraperitoneal Chemotherapy without Bevacizumab as a First-Line Therapy for Newly Diagnosed Advanced Epithelial Ovarian Cancer-Two Centers Experiences. Front. Med. 2022, 9, 846352. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- Markman, M.; Reichman, B.; Hakes, T.; Rubin, S.; Lewis, J.L.; Jones, W.; Barakat, R.; Curtin, J.; Almadrones, L.; Hoskins, W. Evidence Supporting the Superiority of Intraperitoneal Cisplatin Compared to Intraperitoneal Carboplatin for Salvage Therapy of Small-Volume Residual Ovarian Cancer. Gynecol. Oncol. 1993, 50, 100–104. [Google Scholar] [CrossRef]
- Esselen, K.M.; Rodriguez, N.; Growdon, W.; Krasner, C.; Horowitz, N.S.; Campos, S. Patterns of recurrence in advanced epithelial ovarian, fallopian tube and peritoneal cancers treated with intraperitoneal chemotherapy. Gynecol. Oncol. 2012, 127, 51–54. [Google Scholar] [CrossRef]
- Tanner, E.J.; Black, D.R.; Zivanovic, O.; Kehoe, S.M.; Dao, F.; Konner, J.A.; Barakat, R.R.; Lichtman, S.M.; Levine, D.A. Patterns of first recurrence following adjuvant intraperitoneal chemotherapy for stage IIIC ovarian cancer. Gynecol. Oncol. 2012, 124, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; Du Beshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Trimble, E.L.; Christian, M.C. National Cancer Institute-United States strategy regarding intraperitoneal chemotherapy for ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Chan, J.K. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for advanced ovarian cancer? Ann. Oncol. 2017, 28, viii40–viii45. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, L.B.; Huang, H.Q.; Armstrong, D.K.; Walker, J.L.; Cella, D.; Mackey, D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Skaznik-Wikiel, M.E.; Lesnock, J.L.; McBee, W.C.; Beriwal, S.; Zorn, K.K.; Richard, S.D.; Krivak, T.C.; Edwards, R.P. Intraperitoneal chemotherapy for recurrent epithelial ovarian cancer is feasible with high completion rates, low complications, and acceptable patient outcomes. Int. J. Gynecol. Cancer 2012, 22, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Goel, V.; Talwar, V.; Doval, D.C.; Raina, S.; Goyal, P.; Upadhyay, A.; Patnaik, N. Study of efficacy and safety of modified adjuvant intraperitoneal chemotherapy regimen in carcinoma ovary. Indian J. Cancer 2016, 53, 607–611. [Google Scholar]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Landrum, L.M.; Java, J.; Mathews, C.A.; Lanneau, G.S.; Copeland, L.J.; Armstrong, D.K.; Walker, J.L. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013, 130, 12–18. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Inoue, D.; Kurokawa, T.; Yoshida, Y. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J. Obs. Gynaecol. Res. 2020, 46, 1661–1671. [Google Scholar] [CrossRef]
- Tomao, F.; Panici, P.B.; Tomao, S. Intraperitoneal Chemotherapy in Advanced Ovarian Cancer: Old and Novel Questions. J. Clin. Oncol. 2019, 37, 3168–3169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Chen, P.; Liu, S.; Song, Z.; Ma, X. Effects of CytoReductive surgery plus hyperthermic IntraPEritoneal chemotherapy (HIPEC) versus CytoReductive surgery for ovarian cancer patients: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Llueca, M.; Ibañez, M.V.; Climent, M.T.; Serra, A.; Llueca, A. Effectiveness of Hyperthermic Intraperitoneal Chemotherapy Associated with Cytoreductive Surgery in the Treatment of Advanced Ovarian Cancer: Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Ibañez, M.V.; Cascales, P.; Gil-Moreno, A.; Bebia, V.; Ponce, J.; Fernandez, S.; Arjona-Sanchez, A.; Muruzabal, J.C.; Veiga, N.; et al. Neoadjuvant Chemotherapy plus Interval Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy (NIHIPEC) in the Treatment of Advanced Ovarian Cancer: A Multicentric Propensity Score Study. Cancers 2023, 15, 4271. [Google Scholar] [CrossRef] [PubMed]
- Koole, S.; Van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.E.; et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer 2020, 30, 888. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Rassy, E.; Moschetta, M.; Ghose, A.; Adeleke, S.; Sanchez, E.; Sheriff, M.; Chargari, C.; Pavlidis, N. BRCA Mutations in Ovarian and Prostate Cancer: Bench to Bedside. Cancers 2022, 14, 3888. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.L.; Churchman, M.; Gourley, C. Distinct implications of different BRCA mutations: Efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. Onco Targets Ther. 2017, 10, 2539–2551. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Martinelli, F.; Diana, A.; Franzese, E.; Centonze, S.; Pignata, S.; De Vita, F.; Orditura, M. PARP Inhibitors in First-Line Therapy of Ovarian Cancer: Are There Any Doubts? Front. Oncol. 2020, 1, 782. [Google Scholar]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Graybill, W.; Lorusso, D.; McCormick, C.C.; Freyer, G.; Backes, F.; Heitz, F.; Redondo, A.; et al. Progression-free survival and safety at 3.5 years of follow-up: Results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur. J. Cancer 2023, 189, 112908. [Google Scholar] [CrossRef]
- Swisher, E.M.; Aghajanian, C.; O’Malley, D.M.; Fleming, G.F.; Kaufmann, S.H.; Levine, D.A.; Birrer, M.J.; Moore, K.N.; Spirtos, N.M.; Shahin, M.S.; et al. Impact of homologous recombination status and responses with veliparib combined with first-line chemotherapy in ovarian cancer in the Phase 3 VELIA/GOG-3005 study. Gynecol. Oncol. 2022, 164, 245–253. [Google Scholar] [CrossRef]
- Ray-Coquard, I.L.; Leary, A.; Pignata, S.; Cropet, C.; Martin, A.J.G.; Bogner, G.; Yoshida, H.; Vergote, I.B.; Colombo, N.; Maenpaa, J.; et al. LBA29—Final overall survival (OS) results from the phase III PAOLA-1/ENGOT-ov25 trial evaluating maintenance olaparib (ola) plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced ovarian cancer (AOC). Ann. Oncol. 2022, 33, S1396–S1397. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Rohit, K.C.; Deo, S.V.S.; Ashwin, K.R. Pleura and Peritoneum. 2020. Available online: https://www.degruyter.com/journal/key/pp/html (accessed on 17 November 2023).

| Included Studies | Type of Trial | Participants | Interventions | Primary Outcome |
|---|---|---|---|---|
| Cochrane 2016 [25] | Systematic review | OS (2026) DFS (1311) | IP/IV chemotherapy vs. IV chemotherapy | OS DFS Toxicity |
| IPocc 2022 [24] | Randomised trial of superiority | 746 | Superiority of IP chemotherapy | DFS |
| Omali 2022 [26] | Three randomised trials: NRG/GOG 104 NRG/GOG 114 NRG/GOG 172 | 160 (long-term disease-free survivors (LTDFS) | IP/IV chemotherapy vs. IV chemotherapy | Determine independent prognostic factors of LTDFS. |
| Wright 2015 [27] | Prospective cohort study | 402 | IP/IV chemotherapy vs. IV chemotherapy | OS Toxicity |
| Yuanming 2022 [28] | Retrospective cohort study | 255 | IP/IV chemotherapy vs. IV chemotherapy | OS DFS Toxicity |
| Tewari 2015 [13] | Two randomised trials: NRG/GOG 114 NRG/GOG 172 | 876 | IP/IV chemotherapy vs. IV chemotherapy | Long-term survival and associated prognostic factors |
| Included Studies | Age (Years) | FIGO Stage (III and IV) | Serous Histology | Cytoreductive Surgery (None or <1 cm) | Chemotherapy Regimen | DFS (HR, CI 95%) | OS (HR, CI 95%) |
|---|---|---|---|---|---|---|---|
| IPocc 2022 [24] | - | 87% | 64.12% | 39.69% | IV: paclitaxel 80 mg/m2 + IP: carboplatin AUC 6 | 0.83 (0.69–0.99) | 0.81 (0.75–0.91) |
| Omali 2022 [26] | 57.2 | 100% | 68.4% | 33.6% | IV: cyclophosphamide or carboplatin + paclitaxel iv + IP: cisplatin | 1.40 (0.81–2.44) | 1.53 (0.69–3.38) |
| Wright 2015 [27] | 55–64 (37%) | 91% | 76% | 66% | IV: carboplatin + paclitaxel or docetaxel. IP: cisplatin | N/A | 0.68 (0.47–0.99) |
| Yuanming 2022 [28] | 53 | 100% | 90.5% | 100% | IV: carboplatin + IP: cisplatin 80 mg ip single dose or 75 mg/m2 every 3 weeks | 1.30 (0.71–2.37) | 1.21 (0.51–2.91) |
| Cochrane 2016 [25] | N/A | 0.78 (0.70–0.86) | 0.81 (0.72–0.91) | ||||
| Tewari 2015 [13] | >55 (485) | 100% | 72.5% | 63.9% | IV: cisplatin + paclitaxel or carboplatin intensive + IP: cisplatin + paclitaxel or paclitaxel | 0.79 (0.67–0.93) | 0.77 (0.65–0.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent, M.T.; Serra, A.; Balaguer, C.; Llueca, A. Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis. J. Pers. Med. 2023, 13, 1636. https://doi.org/10.3390/jpm13121636
Climent MT, Serra A, Balaguer C, Llueca A. Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis. Journal of Personalized Medicine. 2023; 13(12):1636. https://doi.org/10.3390/jpm13121636
Chicago/Turabian StyleCliment, Maria Teresa, Anna Serra, Carolina Balaguer, and Antoni Llueca. 2023. "Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis" Journal of Personalized Medicine 13, no. 12: 1636. https://doi.org/10.3390/jpm13121636
APA StyleCliment, M. T., Serra, A., Balaguer, C., & Llueca, A. (2023). Should We Abandon Intraperitoneal Chemotherapy in the Treatment of Advanced Ovarian Cancer? A Meta-Analysis. Journal of Personalized Medicine, 13(12), 1636. https://doi.org/10.3390/jpm13121636