Abstract
Collagen VI-related disorders constitute a spectrum of severities from the milder Bethlem myopathy (BM) to the Ullrich congenital muscular dystrophy (UCMD), which is more severe, and an intermediate form characterized by muscle weakness that begins in infancy. Affected children are able to walk, although walking becomes increasingly difficult starting in early adulthood. They develop contractures in the ankles, elbows, knees, and spine in childhood. In some affected cases, the respiratory muscles are weakened, requiring mechanical ventilation, particularly during sleep. Individuals with collagen VI-related myopathy are at risk of restrictive lung disease and sleep-disordered breathing due to the development of scoliosis associated with neuromuscular weakness. Typical signs of respiratory failure are not always present, and some patients are unaware that their respiratory muscles have become weaker. Here, we report a case of an intermediate form of collagen VI-related myopathy confirmed by next-generation sequencing. The girl presented morning headache, irritability, and aggressiveness, and because of these main symptoms, she was referred by the neurologist for respiratory evaluation. The result of spirometry was associated with hypoventilation shown during sleep studies, indicating the necessity to initiate home non-invasive ventilation (NIV) with immediate improvement in the symptoms. Neuromuscular disorders (NMDs) have a great impact on sleep, but only very few studies evaluating sleep quality in young patients with collagen VI-related myopathy have been described. Daytime symptoms of sleep-disordered breathing may include irritability, emotional lability, and poor attentiveness, but these can be overseen by the severity of other complex medical problems in patients with collagen VI-related myopathy. We underline the importance of the close monitoring of respiratory function, sleep evaluation, and decision making to support the NIV treatment of other collagen VI-related myopathy variant-specific patients. Early recognition of sleep disturbances and initiation of respiratory support can preserve or enhance the quality of life for patients and their caregivers. Routine screening for identification of emotional distress should be instituted in the clinical practice using validated psychological measures in a multidisciplinary approach with different intervention strategies for both patient and parent when necessary.
1. Introduction
Collagen VI-related myopathy is a disease entity with a broad clinical spectrum that affects the skeletal muscles and connective tissue, caused by mutations in the α-chains of collagen-VI-related genes (COL6A1, COL6A2, and COL6A3) [1]. Collagen VI is a crucial part of the extracellular matrix, which, together with the surrounding basement membrane and cells, creates a microfibrillar structure. In many tissues, the extracellular matrix is essential for cell attachment, stability, and repair. Collagen VI-related disorders constitute a spectrum of severities from the milder Bethlem Myopathy (BM) to the Ullrich congenital muscular dystrophy (UCMD), which is more severe. Both BM and UCMD are now recognized as collagen VI-related myopathies, which have a wide range of clinical manifestations and were formerly thought to be distinct disease entities [1,2]. Given that the same gene mutation can cause a variety of clinical symptom severities, determining the genotype–phenotype association in this myopathy is particularly challenging. The diagnosis is made by using a combination of clinical presentation, muscle magnetic resonance imaging, muscle biopsy, and genetic analysis, which can be challenging to evaluate [3]. BM has a late onset and is often characterized by contractures of the ankle and long finger flexors, with modest phenotypic and a slow or stagnant progression. Hypotonia and postponed motor breakthroughs appear in early childhood [4]. More than two-thirds of those affected by BM remain freely mobile indoors while they are reliant on supportive means of transportation outdoors due to the sluggish onset of weakening. The intermediate form of collagen VI-related dystrophy is characterized by muscle weakness that begins in infancy (Table 1). They develop contractures in their fingers, elbows, shoulders, and ankles in childhood.

Table 1.
The spectrum of clinical collagen VI-related conditions [5].
Table 1.
The spectrum of clinical collagen VI-related conditions [5].
| CONDITION | Bethlem Muscular Dystrophy (BMD) | Intermediate Form (Severe BMD/Mild UCMD) | Ullrich Congenital Muscular Dystrophy (UCMD) |
|---|---|---|---|
| Onset | Late onset | Late–early | Early onset, congenital |
| The main phenotype | Proximal muscle weakness and joint contractures | Not clearly defined | Congenital weakness, hypotonia, proximal joint contractures, and striking hyperlaxity of distal joints |
| Clinical picture | Mild hypotonia and weakness may be present congenitally. Hypotonia and delayed motor milestones occur in early childhood By adulthood, there is evidence of proximal weakness and contractures of the elbows, Achilles tendons, and long finger flexors | Individuals do not achieve the ability to run, jump, or climb stairs without the use of a railing The contractures are variable | Prenatal phenotype with decreased fetal movements was frequently reported |
| Prognosis | The progression of weakness is slow or static. More than 2/3 of affected individuals older than age 50 years remain independently ambulatory indoors while relying on supportive means for mobility outdoors | Independent ambulation past age 11 years | Progressive |
| Respiratory assessment | Respiratory involvement is not a consistent feature. | Respiratory insufficiency that is later in onset than in UCMD and results in the need for non-invasive ventilation in the form of bilevel positive airway pressure by the late teens to early 20s | Early and severe respiratory insufficiency occurs in all individuals, resulting in the need for non-invasive ventilation in the form of bilevel-positive airway pressure by age 11 years |
The assessments listed in Table 2 are recommended to assess the severity of the disease and the needs of a person with collagen VI-related myopathy.
Management of Clinical Manifestations in Collagen VI-Related Myopathies
Scoliosis management and the treatment of Achilles tendon contractures are needed in individuals with collagen VI-related myopathy under the direction of an orthopedic specialist. Sometimes surgical treatment for scoliosis is required, and coordination between orthopedic, intensive care, and pulmonary specialists is necessary. Achilles tendon surgical procedures can increase the range of movements [3,4,6]. Physical therapy is recommended for joint stretches to improve the quality of life [5,7,8]. Neuromuscular disorders have a great impact on sleep. Sleep-disordered breathing is accurately detected by polysomnography (PSG), which also defines the severity of the condition. Muscle weakness with respiratory muscle dysfunction leads to low chest wall compliance with a decrease in lung volume, which causes hypoventilation [9,10,11]. Respiratory surveillance for nighttime hypoventilation is an important aspect of clinical therapy. Nocturnal hypercapnia can be predicted by forced vital capacity lower than 60% on spirometry [12]. During polysomnography (PSG), non-invasive ventilation (NIV) should be initiated using bilevel positive airway pressure (BiPAP), with pressures adjusted to provide adequate breathing, and then used constantly throughout the night. During recurrent PSG monitoring, it is important to carefully monitor and modify the nocturnal BiPAP pressures.

Table 2.
Based on the severity of collagen VI-related myopathy and the system involved, the evaluation recommendation was summarized for the better management of patients [4,6,9,11,12].
Table 2.
Based on the severity of collagen VI-related myopathy and the system involved, the evaluation recommendation was summarized for the better management of patients [4,6,9,11,12].
| System Involved | Evaluation | Recommendation |
|---|---|---|
| Muscle | Muscle weakness and how it affects movement and function, delayed motor milestones | Neuromuscular examination |
| Joints | Joint contractures are examined, especially to check for asymmetry in Achilles tendon contractures | Physiotherapy assessment |
| Skeletal | Rib cage deformities due to stiffness, scoliosis, respiratory muscle weakness | Orthopedic surgeon and pulmonary specialists’ evaluation |
| Pulmonary | Forced vital capacity must be constantly monitored, and sitting and supine positions will be used for pulmonary function tests PSG, CO2 monitoring | Pulmonary specialist and respiratory physiotherapy evaluation |
| Cardiovascular | Cardiologic abnormalities such as cardiomyopathy or symptomatic arrhythmia | Cardiac examination, echocardiogram, and ECG |
| Laboratory determinations | Genetic analysis | Consultation with clinical geneticist and genetic counselor |
| Skin | Cutaneous abnormalities such as keloids, keratosis pilaris, and soft/velvety skin | Specialist consultation |
2. Methods
We made a retrospective review of the medical record of a patient suffering from collagen VI-related myopathy. We measured body weight, height, arm span, body mass index (BMI), spirometry parameters (FVC-forced vital capacity and FEV1-forced expiratory volume in the first second as a percent predicted), and Cobb angle as indicators of growth status and respiratory function. Arm span was used as a stand-in for height for determining BMI and spirometry standards [9,13]. Spirometry data were collected using tools and procedures that adhered to the American Thoracic Society/European Respiratory Society’s norms Vitalograph 6800 Pneumotrac Spirometer with Spirotrac Software [13].
The nighttime video PSG was performed using System Alice 6 LDx, Phillips Respironics, with the software Sleepware G3 version 3.9.6.0. Transcutaneous CO2 (TcCO2) values were obtained over the entire PSG recording period of 485 min using the Radiometer Monitor system.
Respiratory parameters, including apnea–hypopnea index (AHI) and sleep stages, were scored according to the American Academy of Sleep Medicine (AASM) criteria.
We collected the following respiratory parameters from the sleep study: total recording time (TRT), total sleep time (TST), apnea–hypopnea index (AHI), oxygen desaturation index (ODI), oxygen saturation nadir, and transcutaneous CO2 (TcCO2). This study was approved by the local ethical committee of the Regina Maria Healthcare Network Romania, No.372/31.05.2023, in accordance with the Declaration of Helsinki, and parents gave their written informed consent.
3. Case Presentation
We present a 12-year-old girl who was first referred to the sleep unit of the Pediatric Department in Ponderas Academic Hospital Bucharest for respiratory evaluation and sleep study using PSG, mainly for symptoms consisting of morning headache, irritability, and aggressive behavior.
From her history, we retained that she was born at term, from a normal pregnancy of a non-consanguineous healthy family. She presented with motor developmental delay, acquiring independent walking at the age of one year and a half with normal cognition. Starting with the second year of life, she showed chronic, progressive motor deficit involving all four limbs, clinically expressed as difficulties with both running and walking.
Her current clinical exam showed weight = 43.5 kg (percentile 50), height = 159 cm (percentile 90) (arm span was used as a surrogate measurement for height), BMI = 17.2 kg/m2, thoracic dextroscoliosis with an asymmetric posture, with elevated right hip and bilateral high foot arches with hammertoes. The mental status was normal, although she appeared to be hesitating and somehow anxious; no psychiatric diagnosis was made. The clinical cardiovascular assessment was normal. The neurological phenotype consisted of muscle atrophy involving the upper limbs and the lower third of her thigh, with chronic, progressive muscle weakness, lower limbs being more affected (grade 3 on the Medical Research Council Scale for Muscle Strength scale) than upper limbs (grade 4, Medical Research Council Scale for Muscle Strength scale), with positive Gowers maneuver and joint retraction on both ankles (right side greater than left side) and shoulders. Cervical muscle weakness was observed, especially involving the neck flexors. Also, the patient presented with a particular gait with a tendency of asymmetric digitigrade walking, especially on the right limb, poor exercise tolerance, and could walk for distances no longer than 200 m, raising complaints of muscle fatigue and diffuse pain.
Molecular genetic testing was performed via sequence analysis and deletion/duplication testing of 71 gene panels associated with myopathies. A heterozygous variant in the COL6A2 gene c.784 G > T, p.(Gly262Cys) was detected, confirming the genetic diagnosis. It is classified as a likely pathogenic variant (class 2) according to the recommendation of the American College of Medical Genetics and Genomics guideline. This gene is related to an autosomal dominant and recessive Bethlem myopathy 1 (BM; MIM #120250) and Ullrich congenital muscular dystrophy 1 (UCMD; MIM #158810). Although Sanger sequencing was not performed, her parents are clinically unaffected; thus, it is very probable to be the novo mutation.
A comprehensive assessment of the thorax and lungs was performed. The plain X-rays of the spine showed dextroscoliosis of the thoracic spine with 65 degrees Cobb angle and levoscoliosis of the lower thoracic and lumbar spine with 42 degrees Cobb angle along with axial dorsolumbar vertebral rotation (Figure 1). The sagittal alignment of the cervical spine was preserved. Also, a spirometry was performed 3 months before her previous admission, which showed altered respiratory parameters: FVC = 52.9% and FEV1 = 56.06% (in July 2019). At that moment, she was also evaluated via venous blood gas analysis: pH = 7.30, pCO2 = 56 mmHg.
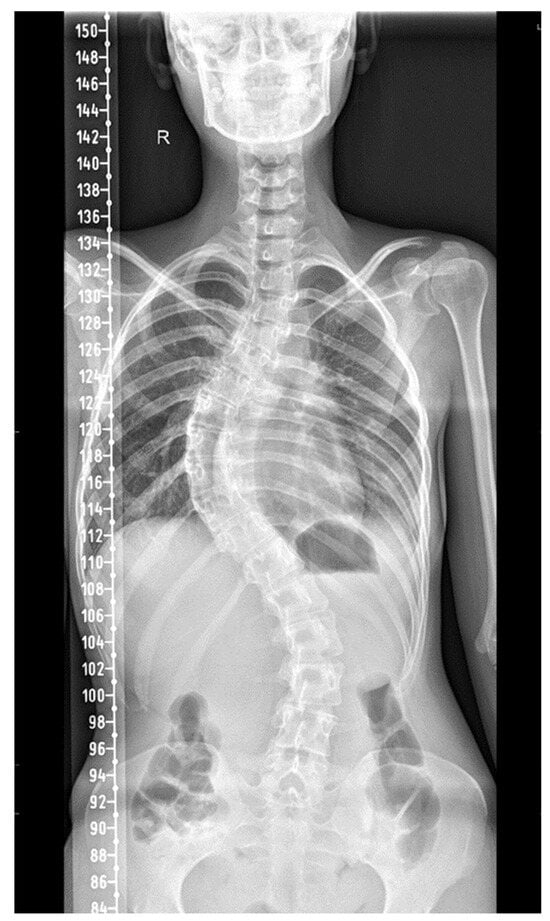
Figure 1.
Thoracic dextroscoliosis and levoscoliosis of the lower thoracic and lumbar spine. (R = right side).
The results of the first PSG performed in September 2019 showed an apnea–hypopnea index AHI = 0.5/h, REMindex = 3.2/h, and maximum transcutaneous carbon dioxide pressure (PtcCO2) of 50.8 mmHg during sleep. Transcutaneous CO2 (TcCO2) values were obtained over the entire PSG recording period of 485 minutes using the Radiometer Monitor system, version 1.3.0. Details of the PSG parameters are given in Table 3.

Table 3.
Polysomnography findings.
Low FVC was associated with increased respiratory effort with paradoxical breathing, low amplitude thoracic movements during sleep, and nocturnal hypercapnia, followed by daytime symptoms such as morning headaches, restless sleep, tiredness, and difficulty concentrating at school. All data were relevant for the diagnosis of sleep-related hypoventilation and justified the recommendation of NIV during sleep.
After 2 months, during her second visit to our department, a new PSG study was performed with BiPAP ST titration based on an acclimatization period with nasal mask Wisp X1 S/M. Using the manual titration protocol [14] and the patient’s compliance, expiratory positive airway pressure (EPAP)/inspiratory positive airway pressure (IPAP) was increased gradually to EPAP = 5 cmH20 and IPAP = 14 cmH20, back-up rate = 18 breathings/min, and Tinspiratory = 1 s (Table 3).
A markedly favorable evolution was observed by the patient’s mother after the initiation of BPAP ST therapy during sleep, with the patient sleeping much better, with no aggressive behavior during daytime, with better school results, with no headaches, and improved tolerance to effort (which was visible during physical therapy).
The compliance card and therapy report showed that the patient wore the device 79.7% of the time in the first 5 months (138 days) after BiPAP therapy initiation, with an average of 6 h 36 min used each day and a maximum of 11 h 20 min (Table 4).

Table 4.
The compliance card and therapy report in the first 5 months after BiPAP therapy was initiated.
The next two PSG assessments demonstrated a satisfactory improvement both from a clinical perspective and by the resulting measurements (Table 3). The patient continues to be monitored multidisciplinary, including the evolution and the treatment of her scoliosis.
4. Discussion
Based on the clinical presentation and her evolution in time, our patient showed characteristics consistent with an intermediate form of collagen VI-related myopathy.
In a recent study, Marinella et al. [15] proposed a diagnostic flowchart to assess the real pathogenicity of variants in collagen VI genes. The authors conclude that a multistep integration of clinical and molecular data allowed the identification of about 3% of those patients harboring pathogenetic collagen VI variants [15].
Nalini et al. prospectively evaluated nine cases of classical UCMD, primarily describing the motor milestones and other clinical phenotypic characteristics as histopathological and immunohistochemical findings [16]. They drew attention to the importance of prenatal diagnosis.
Highly developed research centers have contributed to the discovery of the connection between mitochondrial dysfunction [17,18] and defective autophagy [17,19] in the pathogenesis of collagen VI-related myopathies.
Merlini et al. conducted a cohort study in patients affected by collagen VI-related myopathies, providing clinical and genetic information and presenting quantitative data on muscle strength and contractures that can be valuable in clinical trials [17]. Their results show that respiratory failure can be a part of the clinical spectrum even in ambulatory patients, confirming the relationship between FVC and the three common collagen VI-related myopathies phenotypes acknowledged in previous studies [20,21]. They concluded that the age at which ventilation was initiated significantly differed among the three phenotypes.
Another study showed that respiratory surveillance is necessary for possible nocturnal hypoventilation, prophylaxis, and treatment of pulmonary infections [22].
The authors point out that assisted cough, non-invasive ventilation, and tracheostomy ventilation are treatments successfully applied in UCMD.
In a medical system that is ill-prepared for managing this kind of case and does not have a well-developed pediatric pulmonology network, the physician’s methodology is oriented toward early diagnosis. Therefore, in this case, by monitoring and improving the patient’s quality of life, an attempt was made to compensate for the major deficiencies of the medical system. Close contact with the patient, regular check-ups, and cultivating a trust-based relationship with the patient’s family were the priorities in this case, showing visible results upon its development.
NMDs have a great impact on sleep [23,24], but we found very few studies evaluating sleep quality in patients with collagen VI-related myopathy. They are especially related to the initiation of NIV in a patient who presented morning headache, irritability, and aggressiveness as main symptoms.
Sleep-disordered breathing has been documented in children with neuromuscular weakness disorders that cause restrictive lung illness [9]. The severity of sleep-disordered breathing is dependent, in part, on lung volume, with a greater tendency for desaturation as lung volume is decreased [9].
Wang et al. mentioned in the Consensus Statement on Standard of Care for Congenital Muscular Dystrophies that the regular monitoring of pulmonary function can predict potential changes in a patient’s health [25]. The authors describe the indications and the utility of nocturnal oximetry, nocturnal CO2 monitoring, blood gas, and polysomnography including CO2 monitoring in the evaluation of respiratory function.
Measurements of absolute lung volumes in older children with NMD consistently show significant decreases in vital capacity and functional residual capacity. Scoliosis is one of the most common and severe complications of virtually all conditions associated with muscle weakness, impacting respiratory mechanics in several ways [11,23] and additionally contributing to restrictive lung expansion, favoring the progression toward hypoventilation. Patients with scoliosis had lower minimal SpO2 values than the general population, and they experienced more apnea and hypopnea episodes during sleep than the control group [26].
The symptoms of nocturnal hypoventilation are divided into two categories [23,24,27]:
- Daytime symptoms: fatigue, excessive daytime sleepiness, morning headaches, fatigue upon awakening, reliance on alarms, frequent sleeping during weekends;
- Nighttime symptoms: snoring, apneas, restlessness, frequent awakenings, nocturia.
Our patient experienced agitated sleep, fatigue, difficult awakenings, dyspnea, morning headaches, concentration difficulties, decreased school performance, hypersomnolence, and altered mental status with normal SatO2 during wakefulness.
Sleep-disordered breathing symptoms due to muscle weakness have a slow progression and therefore present a high risk of being missed. In other words, it is possible that some patients do not realize they have lower respiratory muscle strength and that they may initially have subtle hypoventilation symptoms. Therefore, physical examination is not sufficient for the diagnosis of sleep-disordered breathing, and careful periodic monitoring is required, adapted to the progression of each case with collagen VI-related myopathy.
Polysomnography plays an important role in the diagnosis of sleep-disordered breathing in neuromuscular diseases and in optimizing non-invasive ventilation settings [23].
In our patient, we performed an initial PSG with TcCO2 and a systematic follow-up with PSGs after NIV initiation to evaluate the benefits of respiratory support for the progression of the underlying disease.
For monitoring gas exchanges during wakefulness and sleep, the non-invasive method of blood gas measurement is recommended. In more advanced stages, hypoxemia and CO2 retention may occur during the daytime. The earliest signs of hypoventilation occur during sleep, first in REM and then in NREM, with a subsequently progressive evolution, causing a decrease in Sat O2 and an increase in CO2 blood saturation [27,28,29].
Maximal PtcCO2 > 50 mmHg, with 3% oxygen desaturation index > 1.4 events/h and/or lung function data, has been used as a criterion to initiate NIV [28]. Besides these criteria for CPAP/NIV, others include the patient’s respiratory status and disease, abnormal daytime and nocturnal gas exchange, sleep and/or lung function data, or other parameters [29].
In our case with collagen VI-related myopathy, initiation criteria for BPAP therapy consisted of sleep hypoventilation symptoms, nocturnal hypercapnia, lung function with low FVC, and increased respiratory effort. We performed the titration PSG in order to determine the level of IPAP, EPAP, and backup rate. Therapeutic age-appropriate education played an important role and included both the patient and the caregiver.
NIV was associated with an improvement in sleep-related breathing disorder symptoms, nocturnal gas exchange, and improvement in the child’s and family’s quality of life. The patient’s compliance with therapy was good, especially after observing the positive effects of BPAP.
Darke et al. raised the issue that behavior, social, and communicational problems are common in children with neuromuscular diseases [30]. They draw attention to the fact that there is a higher likelihood that parents will seek advice about their children’s behavioral difficulties from the educational sector or at the muscle clinic than from mental health professionals.
Therefore, screening should be considered in order to identify children who are at high risk of emotional disorders and mental health problems.
Limitations of this study are due to one case report information. In our study, pediatric pulmonologists played the role of facilitators of emotional health via the relationship based on trust between the patient and the family. Another limitation of our study is that we could not evaluate this patient using validated psychological measures. However, the patient is still being monitored and evaluated by a multidisciplinary team, so further data on her condition will be available in the future.
5. Conclusions
In patients with collagen VI-related myopathy, the caregivers and the medical team need to be aware of the possibility of unrecognized progressive respiratory problems.
Daytime symptoms with morning headaches, tiredness, irritability, and difficulty concentrating at school can be relevant to identifying sleep-related hypoventilation and recommendations for NIV during sleep.
According to the literature, the data integration of PSG results with clinical evaluation is important for the diagnosis and treatment of sleep-disordered breathing, with the final goal of preserving or improving the quality of life of patients and their caregivers.
In our patient’s case, it has been demonstrated how the patient–medical doctor relationship can be beneficial for this patient’s future. This opens up the possibility for educational programs geared toward medical doctors, which emphasize early diagnosis and a proactive approach to the evolution of cases and promote emotional health care among patients with collagen VI-related myopathy and their families.
Further studies will need to address mental health research. Routine screening for the identification of emotional distress should be instituted in the clinical practice in a multidisciplinary approach with different intervention strategies for both patient and parent when necessary.
Author Contributions
Conceptualization, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; methodology, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; Software, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; Validation, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; formal analysis, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; investigation, M.O., A.G., L.B., G.J., C.P., M.A.-D., F.M. and D.C.G.; resources, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; data curation, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; writing—original draft preparation, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; writing—review and editing, M.O., A.G., V.P., G.J., C.P., F.M. and D.C.G.; visualization, M.O., A.G., L.B., G.J., C.P., M.A.-D., F.M. and D.C.G.; supervision, M.O., A.G., V.P., G.J., C.P., F.M. and D.C.G.; project administration, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G.; funding acquisition, M.O., A.G., L.B., G.J., C.P., F.M. and D.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Regina Maria Healthcare Network Romania, No.372/31.05.2023.
Informed Consent Statement
Written informed consent was obtained from the parents of the patient for publication of this article and any accompanying images.
Data Availability Statement
All data supporting this article are publicly available and can be retrieved from repositories and platforms such as PubMed.
Acknowledgments
The authors have stated that they have no interests that might be perceived as posing a conflict or bias.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Di Martino, A.; Cescon, M.; D’Agostino, C.; Schilardi, F.; Sabatelli, P.; Merlini, L.; Faldini, C. Collagen VI in the Musculoskeletal System. Int. J. Mol. Sci. 2023, 24, 5095. [Google Scholar] [CrossRef]
- Bushby, K.M.; Collins, J.; Hicks, D. Collagen type VI myopathies. Adv. Exp. Med. Biol. 2014, 802, 185–199. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, H.Y.; Park, H.J.; Kim, S.H.; Kim, S.M.; Choi, Y.C. Clinical, Pathologic, and Genetic Features of Collagen VI-Related Myopathy in Korea. J. Clin. Neurol. 2017, 13, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Villar-Quiles, R.N.; Donkervoort, S.; de Becdelièvre, A.; Gartioux, C.; Jobic, V.; Foley, A.R.; McCarty, R.M.; Hu, Y.; Menassa, R.; Michel, L.; et al. Clinical and Molecular Spectrum Associated with COL6A3 c.7447A>G p.(Lys2483Glu) Variant: Elucidating its Role in Collagen VI-related Myopathies. J. Neuromuscul. Dis. 2021, 8, 633–645. [Google Scholar] [CrossRef]
- Foley, A.R.; Mohassel, P.; Donkervoort, S. Collagen VI-Related Dystrophies. 2004 Jun 25 [Updated 2021 Mar 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1503/ (accessed on 12 July 2023).
- Le Goff, L.; Meilleur, K.G.; Norato, G.; Rippert, P.; Jain, M.; Fink, M.; Foley, A.R.; Waite, M.; Donkervoort, S.; Bönnemann, C.G.; et al. Responsiveness and Minimal Clinically Important Difference of the Motor Function Measure in Collagen VI-Related Dystrophies and Laminin Alpha2-Related Muscular Dystrophy. Arch. Phys. Med. Rehabil. 2021, 102, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, D.S. An evaluation of a continuing education program for family caregivers of ventilator-dependent children with spinal muscular atrophy (SMA). Children 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, T.; Komaki, H.; Okada, M.; Hayashi, Y.K.; Nonaka, I.; Sugai, K.; Sasaki, M.; Nishino, I. Rapidly progressive scoliosis and respiratory deterioration in Ullrich congenital muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 2013, 84, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Viprey, M.; Trang, H.; Pomedio, M.; Bessaci-Kabouya, K.; Sabouraud, P.; Cheliout-Heraut, F.; Mauran, P. Early Onset of Sleep-Disordered Breathing in Two Children With SEPN1-Related Myopathies. J. Clin. Sleep Med. 2017, 13, 1105–1108. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Verhulst, S. Obstructive sleep isordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef]
- Striegl, A.; Chen, M.L.; Kifle, Y.; Song, K.; Redding, G. Sleep-disordered breathing in children with thoracic insufficiency syndrome. Pediatr. Pulmonol. 2010, 45, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Lutter, R.; Kabitz, H.J.; Henke, C.; Herkenrath, S.; Randerath, W.; Young, P.; Dreher, M.; Görlich, D.; Boentert, M. Respiratory Muscle Function Tests and Diaphragm Ultrasound Predict Nocturnal Hypoventilation in Slowly Progressive Myopathies. Front Neurol. 2021, 12, 731865. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Swenson, E.R. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Kushida, C.A.; Chediak, A.; Berry, R.B.; Brown, L.K.; Gozal, D.; Iber, C.; Parthasarathy, S.; Quan, S.F.; Rowley, J.A. Positive Airway Pressure Titration Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2008, 4, 157–171. [Google Scholar] [PubMed]
- Marinella, G.; Astrea, G.; Buchignani, B.; Cassandrini, D.; Doccini, S.; Filosto, M.; Galatolo, D.; Gallone, S.; Giannini, F.; Lopergolo, D.; et al. A Schematic Approach to Defining the Prevalence of COL VI Variants in Five Years of Next-Generation Sequencing. Int. J. Mol. Sci. 2022, 23, 14567. [Google Scholar] [CrossRef] [PubMed]
- Nalini, A.; Gayathri, N.; Santosh, V. Ullrich congenital muscular dystrophy: Report of nine cases from India. Neurol. India. 2009, 57, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Merlini, L.; Sabatelli, P.; Gualandi, F.; Redivo, E.; Di Martino, A.; Faldini, C. New Clinical and Immunofluorescence Data of Collagen VI-Related Myopathy: A Single Center Cohort of 69 Patients. Int. J. Mol. Sci. 2023, 24, 12474. [Google Scholar] [CrossRef] [PubMed]
- Irwin, W.A.; Bergamin, N.; Sabatelli, P.; Reggiani, C.; Megighian, A.; Merlini, L.; Braghetta, P.; Columbaro, M.; Volpin, D.; Bressan, G.M.; et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet. 2003, 35, 367–371. [Google Scholar]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L.; et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar]
- Foley, A.R.; Quijano-Roy, S.; Collins, J.; Straub, V.; McCallum, M.; Deconinck, N.; Mercuri, E.; Pane, M.; D’Amico, A.; Bertini, E.; et al. Natural history of pulmonary function in collagen VI-related myopathies. Brain J. Neurol. 2013, 136, 3625–3633. [Google Scholar]
- van der Kooi, A.J.; de Voogt, W.G.; Bertini, E.; Merlini, L.; Talim, F.B.; Ben Yaou, R.; Urtziberea, A.; de Visser, M. Cardiac and pulmonary investigations in Bethlem myopathy. Arch. Neurol. 2006, 63, 1617–1621. [Google Scholar]
- Merlini, L.; Bernardi, P. Therapy of collagen VI-related myopathies (Bethlem and Ullrich). Neurotherapeutics 2008, 5, 613–618. [Google Scholar] [CrossRef]
- Aboussouan, L.S. Sleep-disordered Breathing in Neuromuscular Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 979–989. [Google Scholar] [CrossRef]
- Oros, M.; Baranga, L.; Plaiasu, V.; Cozma, S.R.; Neagos, A.; Paduraru, L.; Necula, V.; Martu, C.; Dima-Cozma, L.C.; Gheorghe, D.C. Obstructing Sleep Apnea in Childrenwith Genetic Disorders—A Special Need for Early Multidisciplinary Diagnosis and Treatment. J. Clin. Med. 2021, 10, 2156. [Google Scholar] [CrossRef]
- Wang, C.H.; Bonnemann, C.G.; Rutkowski, A.; Sejersen, T.; Bellini, J.; Battista, V.; Florence, .J.M.; Schara, U.; Schuler, P.M.; Wahbi, K.; et al. International Standard of Care Committee for Congenital Muscular Dystrophy. Consensus statement on standard of care for congenital muscular dystrophies. J. Child. Neurol. 2010, 25, 1559–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, H.; Chen, C.; Tan, H.; Lin, Y.; Li, Z.; Shen, J. Does Scoliosis Affect Sleep Breathing? World Neurosurg. 2018, 118, e946–e950. [Google Scholar] [CrossRef]
- Amaddeo, A.; Frapin, A.; Fauroux, B. Long-term non-invasive ventilation in children. Lancet Respir. Med. 2016, 4, 999–1008. [Google Scholar] [PubMed]
- Voulgaris, A.; Antoniadou, M.; Agrafiotis, M.; Steiropoulos, P. Respiratory Involvement in Patients with Neuromuscular Diseases: A Narrative Review. Pulm. Med. 2019, 2019, 2734054. [Google Scholar] [CrossRef] [PubMed]
- Fauroux, B.; Abel, F.; Amaddeo, A. ERS statement on paediatric long-term noninvasive respiratory support. Eur. Respir. J. 2022, 59, 2101404. [Google Scholar] [CrossRef]
- Darke, J.; Bushby, K.; Le Couteur, A.; McConachie, H. Survey of behaviour problems in children with neuromuscular diseases. Eur. J. Paediatr. Neurol. 2006, 10, 129–134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).