Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Approval and Patient Enrollment
2.2. CABG Surgical Procedure and Post-Operative Care
2.3. Blood Sampling, Biochemistry, and Hemorheology
3. Statistical Analysis
4. Results
4.1. Grafting Details and Post-Operative Care
4.2. Hemorheological Characteristics
4.3. Data of Blood Biochemistry
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Diodato, M.; Chedrawy, E.G. Coronary artery bypass graft surgery: The past, present, and future of myocardial revascularisation. Surg. Res. Pract. 2014, 2014, 726158. [Google Scholar] [CrossRef]
- Perrotti, A.; Ecarnot, F.; Monaco, F.; Dorigo, E.; Monteleone, P.; Besch, G.; Chocron, S. Quality of life 10 years after cardiac surgery in adults: A long-term follow-up study. Health Qual. Life Outcomes 2019, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Montrief, T.; Koyfman, A.; Long, B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am. J. Emerg. Med. 2018, 36, 2289–2297. [Google Scholar] [CrossRef]
- Biancari, F.; Mikkola, R.; Heikkinen, J.; Lahtinen, J.; Airaksinen, K.J.; Juvonen, T. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2012, 41, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.A.; Harky, A. Complications of coronary artery bypass grafting. Int. J. Med. Rev. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Sarkar, M.; Prabhu, V. Basics of cardiopulmonary bypass. Indian J. Anaesth. 2017, 61, 760. [Google Scholar] [CrossRef]
- Tu, L.N.; Hsieh, L.; Kajimoto, M.; Charette, K.; Kibiryeva, N.; Forero, A.; Hampson, S.; Marshall, J.A.; O’Brien, J.; Scatena, M. Shear stress associated with cardiopulmonary bypass induces expression of inflammatory cytokines and necroptosis in monocytes. JCI Insight 2021, 6, e141341. [Google Scholar] [CrossRef]
- Lowe, G.; Welsh, P.; Rumley, A.; Sattar, N.; Preedy, V.; Hunter, J. Inflammatory cytokines and cardiovascular risk. In Cytokines; St. Helier Science Publishers: Jersey, UK, 2011; pp. 235–250. [Google Scholar]
- Alexy, T.; Pais, E.; Wenby, R.B.; Mack, W.J.; Hodis, H.N.; Kono, N.; Wang, J.; Baskurt, O.K.; Fisher, T.C.; Meiselman, H.J. Abnormal blood rheology and chronic low grade inflammation: Possible risk factors for accelerated atherosclerosis and coronary artery disease in Lewis negative subjects. Atherosclerosis 2015, 239, 248–251. [Google Scholar] [CrossRef]
- Wiewiora, M.; Piecuch, J.; Glűck, M.; Slowinska-Lozynska, L.; Sosada, K. The effects of weight loss surgery on blood rheology in severely obese patients. Surg. Obes. Relat. Dis. 2015, 11, 1307–1314. [Google Scholar] [CrossRef]
- Sloop, G.D.; De Mast, Q.; Pop, G.; Weidman, J.J.; Cyr, J.A.S. The role of blood viscosity in infectious diseases. Cureus 2020, 12, e7090. [Google Scholar] [CrossRef]
- Schmidt, A.; da Silva Junior, T.; Pazin-Filho, A.; Otávio Murta Júnior, L.; César Almeida-Filho, O.; Gallo-Júnior, L.; Antonio Marin-Neto, J.; Carlos Maciel, B. Effects of changing blood viscosity and heart rate on vena contracta width as evaluated by color Doppler flow mapping. An in vitro study with a pulsatile flow model. Echocardiography 2008, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Jung, J.M. New method of hematocrit correction of whole blood viscosity. Int. Commun. Heat Mass Transf. 2014, 57, 221–227. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.H.; Lee, J.H.; Yun, Y.-M.; Choi, D.-H.; Kim, H.Y. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 20. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D.; Harris, K.; Koenig, W.; Ben-Shlomo, Y.; Thorand, B.; Peters, A.; Meisinger, C.; Imhof, A.; Tunstall-Pedoe, H.; Peters, S.A. Plasma viscosity, immunoglobulins and risk of cardiovascular disease and mortality: New data and meta-analyses. J. Clin. Pathol. 2023, 208223. [Google Scholar] [CrossRef]
- Duyuler, P.T.; Duyuler, S.; İleri, M.; Demir, M.; Dolu, A.K.; Başyiğit, F. Evaluation of whole blood viscosity in patients with aortic sclerosis. J. Tehran Univ. Heart Cent. 2017, 12, 6. [Google Scholar]
- DeFoe, G.R.; Ross, C.S.; Olmstead, E.M.; Surgenor, S.D.; Fillinger, M.P.; Groom, R.C.; Forest, R.J.; Pieroni, J.W.; Warren, C.S.; Bogosian, M.E. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Ann. Thorac. Surg. 2001, 71, 769–776. [Google Scholar] [CrossRef]
- Groom, R.C. High or low hematocrits during cardiopulmonary bypass for patients undergoing coronary artery bypass graft surgery? An evidence-based approach to the question. Perfusion 2002, 17, 99–102. [Google Scholar] [CrossRef]
- Nollert, G.; Sperling, J.; Sakamoto, T.; Jaeger, B.; Jonas, R. Higher hematocrit improves liver blood flow and metabolism during cardiopulmonary bypass in piglets. Thorac. Cardiovasc. Surg. 2001, 49, 226–230. [Google Scholar] [CrossRef]
- Kensey, K.R. The mechanistic relationships between hemorheological characteristics and cardiovascular disease. Curr. Med. Res. Opin. 2003, 19, 587–596. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Richter, W.O.; Schwandt, P. Contribution of fibrinogen and lipoproteins to plasma viscosity in hypercholesterolemia and hypertriglyceridemia: Evaluation by selective depletion of low-density lipoproteins or fibrinogen. Metabolism 2000, 49, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Ramunni, A.; Petrarulo, F.; Grasso, C.; Papagni, S.; Brescia, P. Acute and chronic effects of therapeutic apheresis. Atheroscler. Suppl. 2013, 14, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Seo, S.W.; Jeong, Y.; Park, J.E.; Kim, G.H.; Noh, Y.; Cho, H.; Kim, H.J.; Yoon, C.W.; Byong, S.Y. Blood viscosity in subcortical vascular mild cognitive impairment with versus without cerebral amyloid burden. J. Stroke Cerebrovasc. Dis. 2014, 23, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, Y.I.; Lee, D.-H.; Park, C.-M.; Moon, H.-W.; Hur, M.; Kim, J.Q.; Yun, Y.-M. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin. Biochem. 2013, 46, 139–142. [Google Scholar] [CrossRef]
- Cho, Y.I.; Cho, D.J.; Rosenson, R.S. Endothelial shear stress and blood viscosity in peripheral arterial disease. Curr. Atheroscler. Rep. 2014, 16, 404. [Google Scholar] [CrossRef]
- Li, R.-Y.; Cao, Z.-G.; Li, Y.; Wang, R.-T. Increased whole blood viscosity is associated with silent cerebral infarction. Clin. Hemorheol. Microcirc. 2015, 59, 301–307. [Google Scholar] [CrossRef]
- Başkurt, O.K.; Mat, F. Importance of measurement temperature in detecting the alterations of red blood cell aggregation and deformability studied by ektacytometry: A study on experimental sepsis in rats. Clin. Hemorheol. Microcirc. 2000, 23, 43–49. [Google Scholar]
- Ali-Hassan-Sayegh, S.; Mirhosseini, S.J.; Haddad, F.; Karimi-Bondarabadi, A.A.; Shahidzadeh, A.; Weymann, A.; Popov, A.-F.; Sabashnikov, A. Protective effects of corticosteroids in coronary artery bypass graft surgery alone or combined with valvular surgery: An updated and comprehensive meta-analysis and systematic review. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 825–836. [Google Scholar] [CrossRef]
- Baker, W.L.; White, C.M.; Kluger, J.; Denowitz, A.; Konecny, C.P.; Coleman, C.I. Effect of perioperative corticosteroid use on the incidence of postcardiothoracic surgery atrial fibrillation and length of stay. Heart Rhythm 2007, 4, 461–468. [Google Scholar] [CrossRef]
- de Souza Brito, F.; Mehta, R.H.; Lopes, R.D.; Harskamp, R.E.; Lucas, B.D., Jr.; Schulte, P.J.; Tardif, J.-C.; Alexander, J.H. Nonsteroidal anti-inflammatory drugs and clinical outcomes in patients undergoing coronary artery bypass surgery. Am. J. Med. 2017, 130, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.; Bykov, K.; Choudhry, N.K.; Bateman, B.T. Non-steroidal anti-inflammatory drug administration after coronary artery bypass surgery: Utilization persists despite the boxed warning. Pharmacoepidemiol. Drug Saf. 2015, 24, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, W.; Guo, X.; Wu, Y.; Li, C.; Lu, Z.; Su, G.; Li, X.; Liu, Z.; Guo, R. Ginkgo biloba extract for patients with early diabetic nephropathy: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013, 689142. [Google Scholar]
- Saydam, O.; Atay, M.; Serefli, D.; Doganci, S.; Kumabasar, U.; Yılmaz, M.; Dogan, R.; Demircin, M. Preoperative low-molecular-weight heparin prophylaxis associated with increased heparin resistance frequency in on-pump coronary artery bypass graft surgery. Cardiol. Res. Pract. 2019, 2019, 4310407. [Google Scholar] [CrossRef]
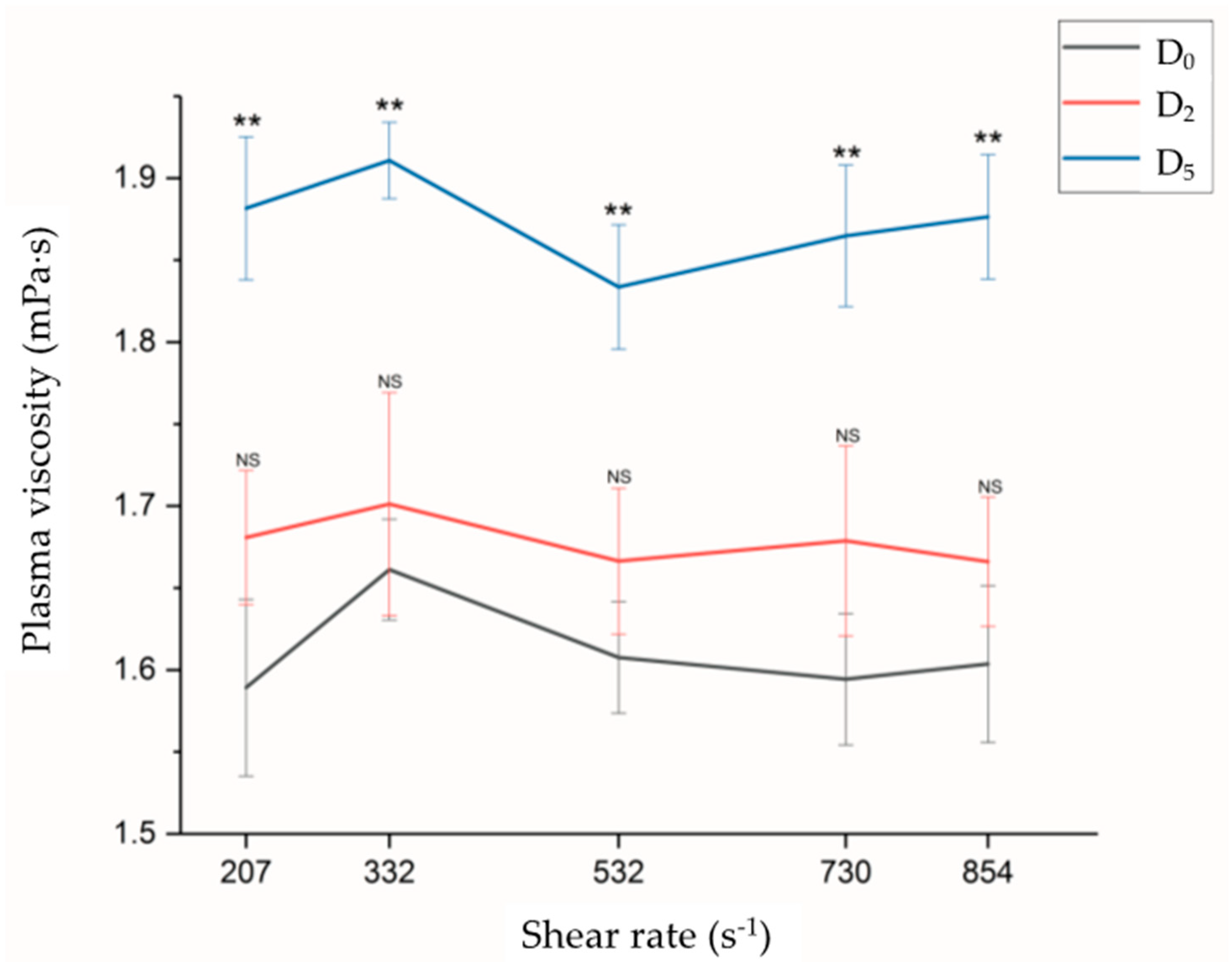
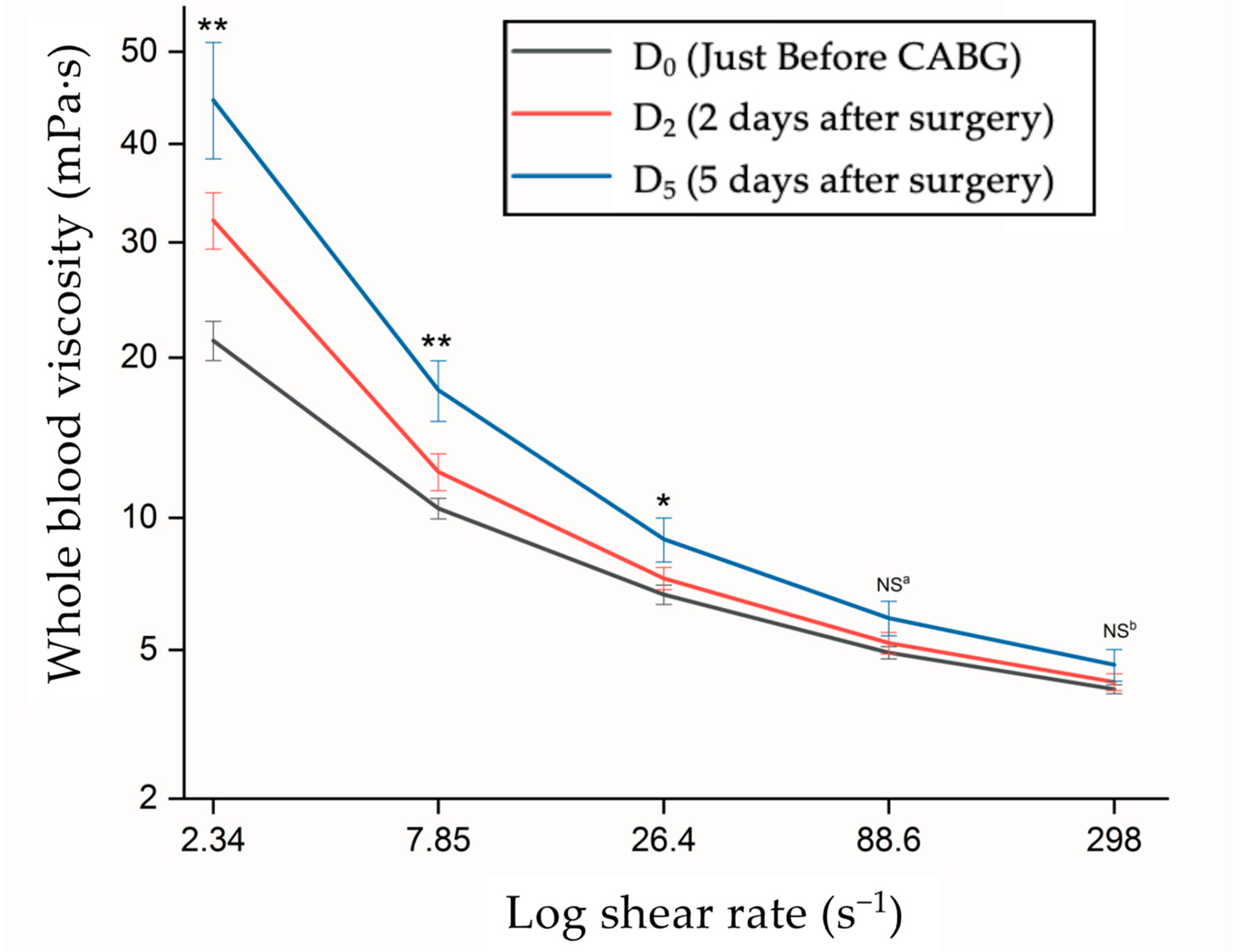
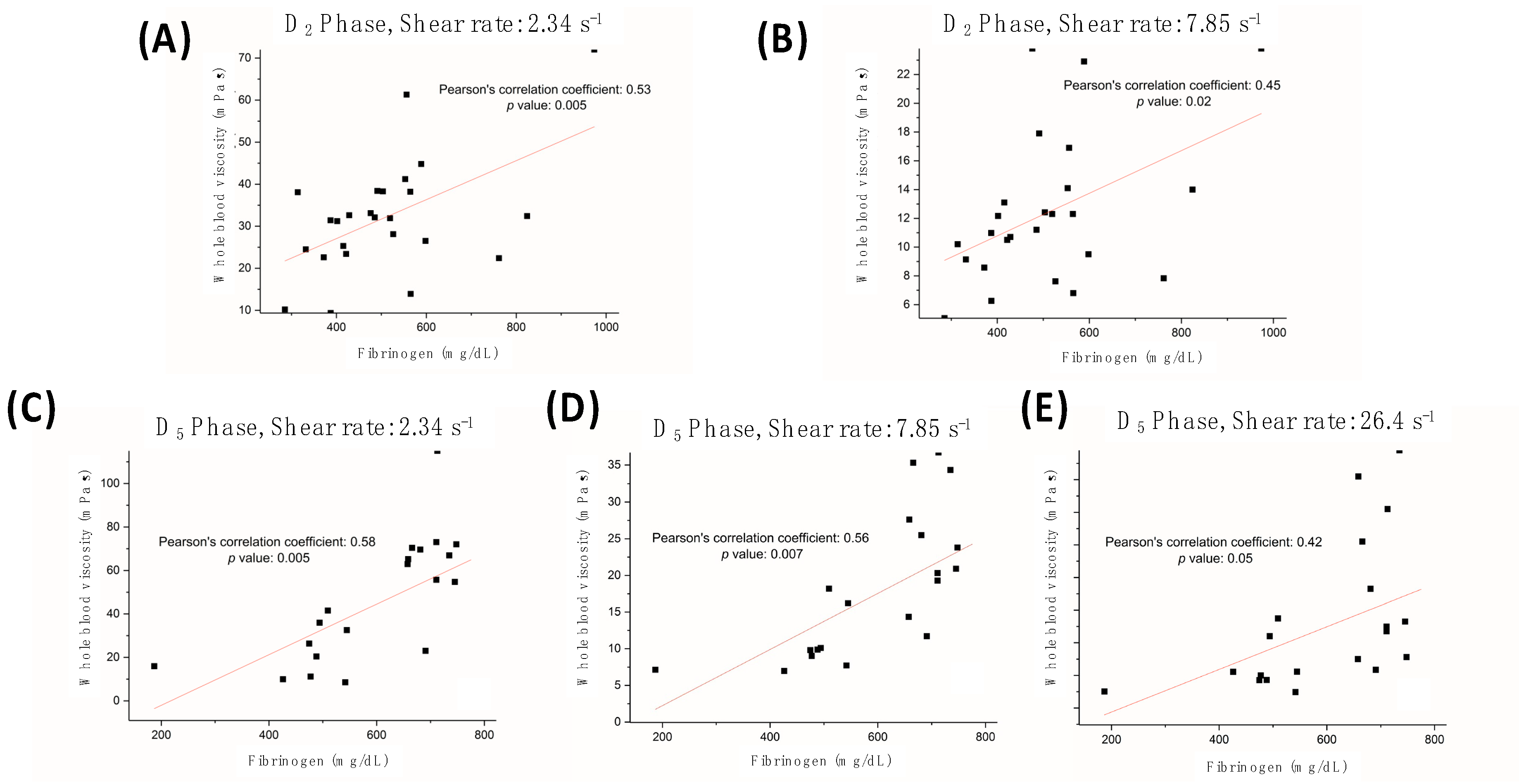
| Characteristics | All Patients (N = 25) Case (%) |
|---|---|
| Male/Female | 17/8 |
| Age (years) | 63.1 ± 3.1 |
| BMI (kg/m2) | 24.16 ± 0.81 |
| Smoking | 16 (64%) |
| Alcohol consumption | 3 (12%) |
| LVEF (%) | 57.6 ± 2.96 |
| Hypertension | 16 (64%) |
| Hyperlipidemia | 15 (60%) |
| Diabetes | 9 (36%) |
| Stroke | 4 (16%) |
| COPD | 1 (4%) |
| Aspirin administration | 20 (80%) |
| Clopidogrel administration | 4 (16%) |
| Parameters | All Patients (N = 25) Number (%) |
|---|---|
| Peri-operative variables | |
| Graft number | 3.81 ± 0.23 |
| LIMA harvesting | 23 (92%) |
| Cross-clamping time (min) | 55.4 ± 13.1 |
| Pump time (min) | 124.0 ± 8.3 |
| Procedure time (min) | 344.8 ± 17.2 |
| Packed red blood cells (PRBC, units) | 3.76 ± 0.60 |
| Fresh frozen plasma (FFP, units) | 4.44 ± 1.00 |
| Platelet concentrate (units) | 1.44 ± 0.58 |
| Post-operative outcomes | |
| 24 h drainage amount (mL) | 236.7 ± 48.9 |
| Packed red blood cells (PRBC, units) | 0.84 ± 0.37 |
| Fresh frozen plasma (FFP, units) | 1.12 ± 0.44 |
| Platelet concentrate (units) | 0.08 ± 0.28 |
| Ventilation period (days) | 1.40 ± 0.32 |
| ICU stay (days) | 2.75 ± 0.98 |
| Hospital stay (days) | 10.90 ± 4.18 |
| Lung atelectasis | 1 (4%) |
| Acute kidney injury | 2 (8%) |
| Parameters | D0 | D2 | D5 | p Value | Post Hoc Tukey-HSD Test with a Significant Difference |
|---|---|---|---|---|---|
| White blood cell (103/uL) | 6.63 ± 2.23 | 9.61 ± 3.17 | 8.48 ± 2.45 | <0.001 | D5 versus D0, D2 versus D0 |
| Platelet (103/uL) | 189.4 ± 44.7 | 129.8 ± 25.6 | 267.2 ± 94.0 | <0.001 | D5 versus D0, |
| Hemoglobin (g/dL) | 12.71 ± 1.90 | 10.48 ± 1.36 | 9.89 ± 1.08 | <0.001 | D5 versus D0, |
| Hematocrit (%) | 37.76 ± 5.13 | 30.63 ± 4.52 | 29.98 ± 3.62 | <0.001 | -- |
| Prothrombin time (s) | 10.63 ± 1.40 | 10.94 ± 0.73 | 11.34 ± 2.16 | 0.26 | -- |
| Partial thromboplastin time (PTT) (s) | 29.60 ± 2.87 | 28.89 ± 3.02 | 31.37 ± 3.84 | 0.02 | -- |
| International normalized ratio | 0.97 ± 0.24 | 1.05 ± 0.09 | 1.07 ± 0.24 | 0.20 | -- |
| Creatine kinase (U/L) | 86.5 ± 44.5 | 756.8 ± 383.9 | NA | <0.001 | -- |
| Troponin-I (ng/mL) | 0.77 ± 1.11 | 6.23 ± 4.84 | NA | <0.001 | -- |
| Aspartate aminotransferase (U/L) | 26.7 ± 14.2 | 55.8 ± 20.9 | NA | <0.001 | -- |
| Alanine transaminase (U/L) | 22.8 ± 11.4 | 28.0 ± 16.2 | NA | 0.20 | -- |
| Total bilirubin (mg/dL) | 0.94 ± 0.50 | 1.08 ± 0.39 | NA | 0.25 | -- |
| Blood urea nitrogen (mg/dL) | 20.8 ± 7.5 | 21.0 ± 9.0 | 20.8 ± 12.9 | 0.99 | -- |
| Creatinine(mg/dL) | 1.12 ± 0.92 | 1.12 ± 0.57 | 1.15 ± 0.96 | 0.99 | -- |
| Blood urea nitrogen/Creatinine ratio | 20.5 ± 5.4 | 19.3 ± 5.1 | 18.9 ± 3.3 | 0.48 | -- |
| Sodium (mmol/L) | 136.5 ± 3.4 | 142.9 ± 2.9 | 137.4 ± 3.8 | <0.001 | D2 versus D0 |
| Potassium (mmol/L) | 3.73 ± 0.35 | 3.70 ± 0.31 | 3.75 ± 0.43 | 0.88 | -- |
| C-reactive protein (mg/dL) | 0.70 ± 1.51 | 7.42 ± 8.00 | 5.76 ± 6.30 | <0.001 | D5 versus D0, D2 versus D0 |
| Fibrinogen (mg/dL) | 411.3 ± 108.4 | 509.3 ± 159.2 | 601.7 ± 145.7 | <0.001 | D5 versus D0, D2 versus D0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-S.; Chen, J.-L.; Sung, S.-Y.; Tsai, Y.-T.; Lin, C.-Y.; Wu, Y.-F.; Tsai, C.-S. Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery. J. Pers. Med. 2023, 13, 1434. https://doi.org/10.3390/jpm13101434
Hsu P-S, Chen J-L, Sung S-Y, Tsai Y-T, Lin C-Y, Wu Y-F, Tsai C-S. Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery. Journal of Personalized Medicine. 2023; 13(10):1434. https://doi.org/10.3390/jpm13101434
Chicago/Turabian StyleHsu, Po-Shun, Jia-Lin Chen, Shih-Ying Sung, Yi-Ting Tsai, Chih-Yuan Lin, Yi-Fan Wu, and Chien-Sung Tsai. 2023. "Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery" Journal of Personalized Medicine 13, no. 10: 1434. https://doi.org/10.3390/jpm13101434
APA StyleHsu, P.-S., Chen, J.-L., Sung, S.-Y., Tsai, Y.-T., Lin, C.-Y., Wu, Y.-F., & Tsai, C.-S. (2023). Inflammatory Biomarkers and Blood Physical Property Transformations Following On-Pump Coronary Artery Bypass Graft Surgery. Journal of Personalized Medicine, 13(10), 1434. https://doi.org/10.3390/jpm13101434






