Altered Cerebral Blood Flow in the Progression of Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
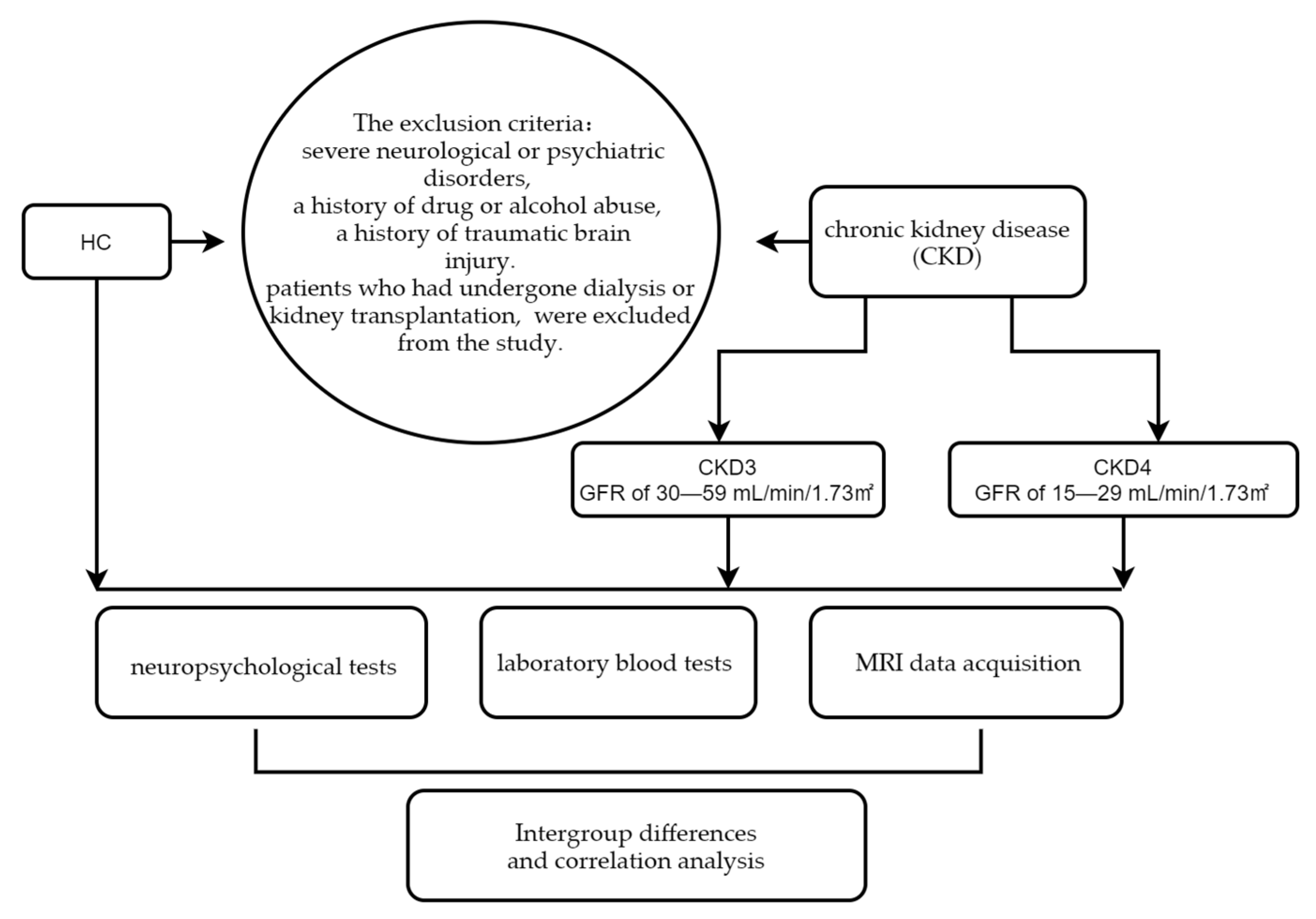
2.1. Participants
2.2. Neuropsychological Tests
2.3. Clinical Laboratory Tests
2.4. Acquisition of MRI Data
2.5. Processing of Cerebral Blood Flow Data
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
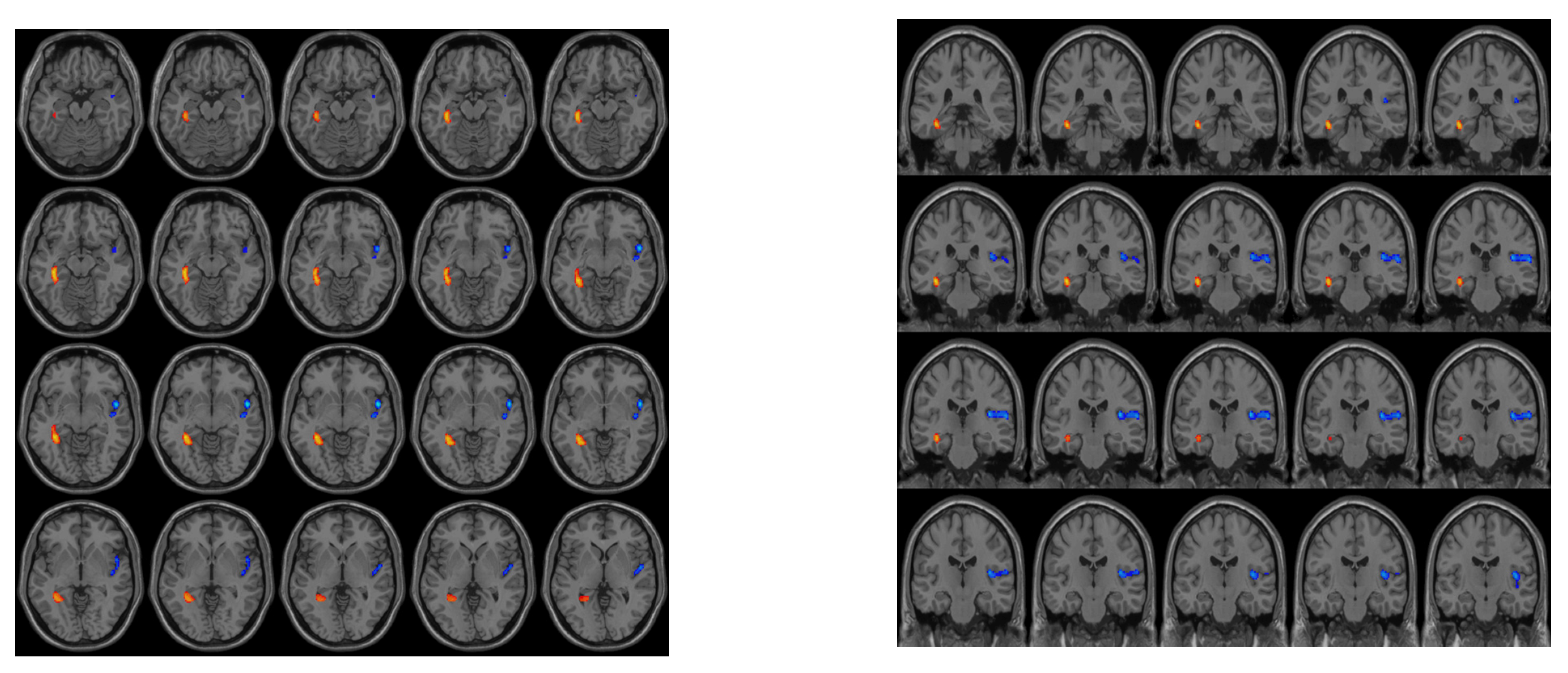
3.2. Differences in CBF between the Groups
3.3. Correlation of Clinical Biochemical Indicators and Neuropsychological Scores with CBF among Subgroups
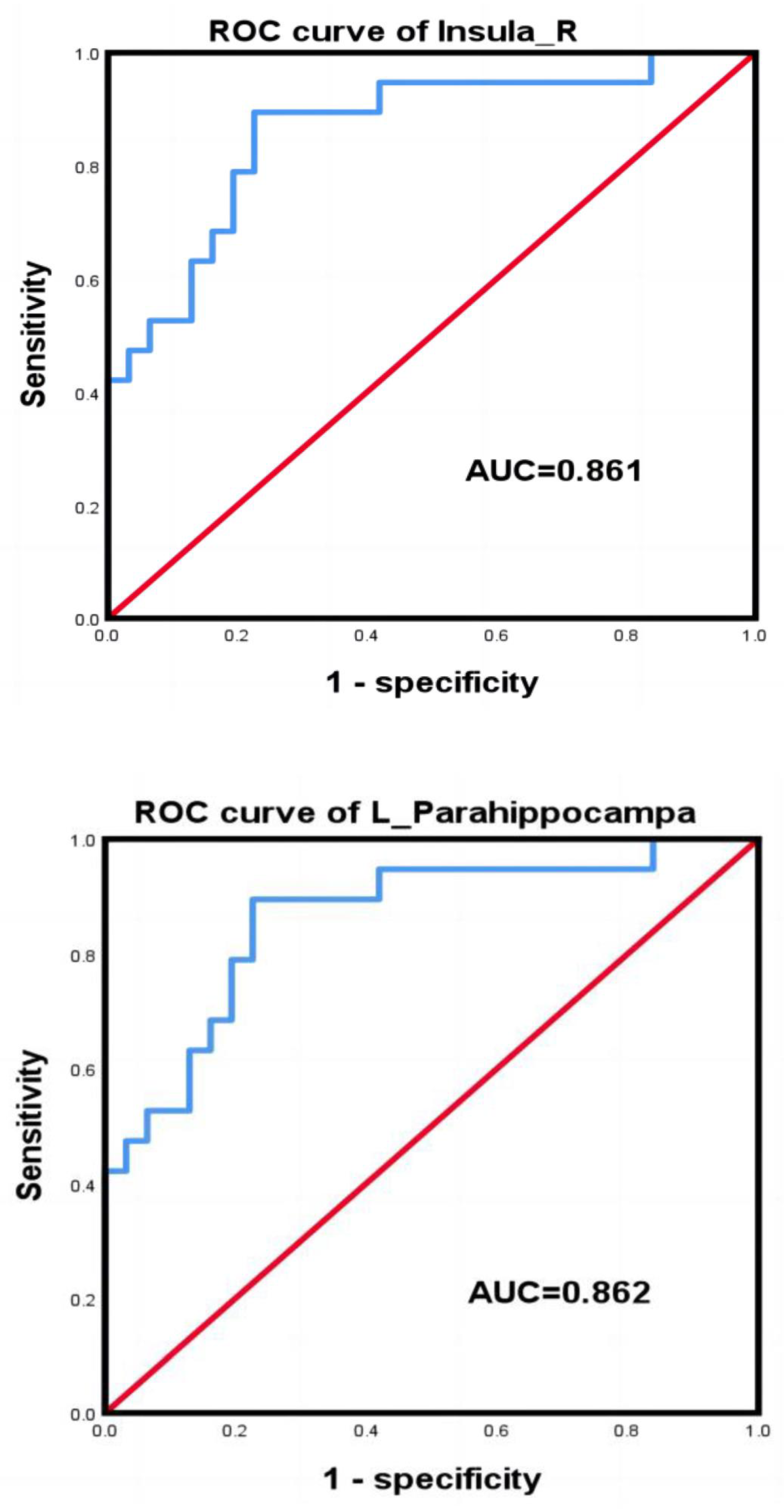
3.4. ROC Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Kurella, M.; Chertow, G.M.; Luan, J.; Yaffe, K. Cognitive impairment in chronic kidney disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.; Elias, M.F.; Robbins, M.A.; Seliger, S.L.; Dore, G.A. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol. Dial. Transpl. 2013, 28, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef]
- O’Lone, E.; Connors, M.; Masson, P.; Wu, S.; Kelly, P.J.; Gillespie, D.; Parker, D.; Whiteley, W.; Strippoli, G.F.; Palmer, S.C.; et al. Cognition in People With End-Stage Kidney Disease Treated with Hemodialysis: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016, 67, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Wu, S.; Masson, P.; Kelly, P.J.; Duthie, F.A.; Whiteley, W.; Parker, D.; Gillespie, D.; Webster, A.C. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Harrell, W.; Gipson, D.S.; Belger, A.; Matsuda-Abedini, M.; Bjornson, B.; Hooper, S.R. Functional Magnetic Resonance Imaging Findings in Children and Adolescents With Chronic Kidney Disease: Preliminary Findings. Semin. Nephrol. 2021, 41, 462–475. [Google Scholar] [CrossRef]
- Herrington, J.D.; Hartung, E.A.; Laney, N.C.; Hooper, S.R.; Furth, S.L. Decreased Neural Connectivity in the Default Mode Network Among Youth and Young Adults with Chronic Kidney Disease. Semin. Nephrol. 2021, 41, 455–461. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, W.; Ding, W.; Wang, Y.; Han, X.; Zhou, Y.; Xu, Q.; Zhang, Y.; Xu, J. Cerebral Blood Flow Alterations as Assessed by 3D ASL in Cognitive Impairment in Patients with Subcortical Vascular Cognitive Impairment: A Marker for Disease Severity. Front. Aging Neurosci. 2016, 8, 211. [Google Scholar] [CrossRef]
- Bangen, K.J.; Nation, D.A.; Clark, L.R.; Harmell, A.L.; Wierenga, C.E.; Dev, S.I.; Delano-Wood, L.; Zlatar, Z.Z.; Salmon, D.P.; Liu, T.T.; et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front. Aging Neurosci. 2014, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Ma, X.; Liebeskind, D.S.; Ma, N.; Tian, C.; Lyu, J.; Long, X.; Ma, L.; Wang, D.J. Collateral perfusion using arterial spin labeling in symptomatic versus asymptomatic middle cerebral artery stenosis. J. Cereb. Blood Flow Metab. 2019, 39, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Ma, N.; Tian, C.; Xu, F.; Shao, H.; Zhou, X.; Ma, L.; Lou, X. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc. Neurol. 2019, 4, 129–134. [Google Scholar] [CrossRef]
- Jiang, X.L.; Wen, J.Q.; Zhang, L.J.; Zheng, G.; Li, X.; Zhang, Z.; Liu, Y.; Zheng, L.J.; Wu, L.; Chen, H.J.; et al. Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: An arterial-spin labeling MR imaging. Metab. Brain Dis. 2016, 31, 929–936. [Google Scholar] [CrossRef]
- Liu, H.S.; Hartung, E.A.; Jawad, A.F.; Ware, J.B.; Laney, N.; Port, A.M.; Gur, R.C.; Hooper, S.R.; Radcliffe, J.; Furth, S.L.; et al. Regional Cerebral Blood Flow in Children and Young Adults with Chronic Kidney Disease. Radiology 2018, 288, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.L.; Kusek, J.; et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. 1), S1–S266. [Google Scholar]
- Barron, H.C.; Reeve, H.M.; Koolschijn, R.S.; Perestenko, P.V.; Shpektor, A.; Nili, H.; Rothaermel, R.; Campo-Urriza, N.; O’Reilly, J.X.; Bannerman, D.M.; et al. Neuronal Computation Underlying Inferential Reasoning in Humans and Mice. Cell 2020, 183, 228–243.e21. [Google Scholar] [CrossRef] [PubMed]
- Kessels, R.P.C.; Bergmann, H.C. What does the hippocampus do during working-memory tasks? A cognitive-neuropsychological perspective. Cogn. Neurosci. 2022, 13, 210–211. [Google Scholar] [CrossRef]
- Cheng, B.C.; Chen, P.C.; Chen, P.C.; Lu, C.H.; Huang, Y.C.; Chou, K.H.; Li, S.H.; Lin, A.N.; Lin, W.C. Decreased cerebral blood flow and improved cognitive function in patients with end-stage renal disease after peritoneal dialysis: An arterial spin-labelling study. Eur. Radiol. 2019, 29, 1415–1424. [Google Scholar] [CrossRef]
- Li, X.; Slinin, Y.X.; Zhang, L.; Dengel, D.R.; Tupper, D.; Metzger, G.J.; Murray, A.M. Cerebral blood flow characteristics following hemodialysis initiation in older adults: A prospective longitudinal pilot study using arterial spin labeling imaging. NeuroImage Clin. 2020, 28, 102434. [Google Scholar] [CrossRef]
- Seki, M.; Nakayama, M.; Sakoh, T.; Yoshitomi, R.; Fukui, A.; Katafuchi, E.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: A prospective observational study. BMC Nephrol. 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, Y.; Wang, C.; d’Oleire Uquillas, F.; He, Q.; Cheng, L.; Zou, Z. Negative Impact of Sadness on Response Inhibition in Females: An Explicit Emotional Stop Signal Task fMRI Study. Front. Behav. Neurosci. 2020, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Critchley, H.D.; Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009, 13, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, F.; Rodrigue, K.M.; Kennedy, K.M.; Cheng, Y.; Flicker, B.; Hebrank, A.C.; Uh, J.; Park, D.C. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb. Cortex 2011, 21, 1426–1434. [Google Scholar] [CrossRef]
- Esposito, C.; Grosjean, F.; Torreggiani, M.; Esposito, V.; Mangione, F.; Villa, L.; Sileno, G.; Rosso, R.; Serpieri, N.; Molinaro, M.; et al. Sirolimus prevents short-term renal changes induced by ischemia-reperfusion injury in rats. Am. J. Nephrol. 2011, 33, 239–249. [Google Scholar] [CrossRef]
- Lavi, S.; Gaitini, D.; Milloul, V.; Jacob, G. Impaired cerebral CO2 vasoreactivity: Association with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1856–H1861. [Google Scholar] [CrossRef]
- Akoudad, S.; Sedaghat, S.; Hofman, A.; Koudstaal, P.J.; van der Lugt, A.; Ikram, M.A.; Vernooij, M.W. Kidney function and cerebral small vessel disease in the general population. Int. J. Stroke 2015, 10, 603–608. [Google Scholar] [CrossRef]
- Potpara, T.S.; Ferro, C.J.; Lip, G.Y.H. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat. Rev. Nephrol. 2018, 14, 337–351. [Google Scholar] [CrossRef]
- Sedaghat, S.; Vernooij, M.W.; Loehrer, E.; Mattace-Raso, F.U.; Hofman, A.; van der Lugt, A.; Franco, O.H.; Dehghan, A.; Ikram, M.A. Kidney Function and Cerebral Blood Flow: The Rotterdam Study. J. Am. Soc. Nephrol. 2016, 27, 715–721. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.; Han, J.; Wang, Z.; Sun, D.; Ji, X.; Li, M.; Zhang, B. Montreal cognitive assessment and analysis of related factors for cognitive impairment in patients with chronic cerebral circulation insufficiency. Int. J. Psychiatry Med. 2015, 50, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, J.M.; Song, M.K.; Oh, Y.J.; Kim, C.J.; Kim, Y.J. The ameliorative effects of exercise on cognitive impairment and white matter injury from blood-brain barrier disruption induced by chronic cerebral hypoperfusion in adolescent rats. Neurosci. Lett. 2017, 638, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schiller, A.; Covic, A. Kidney and brain—A renal perspective of ‘Les Liaisons Dangereuses’. Nephrol. Dial. Transpl. 2010, 25, 1370–1373. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.L.; Chang, K.H.; Liao, H.W.; Chang, S.C.; Ovbiagele, B. Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ 2010, 341, c4249. [Google Scholar] [CrossRef]
- Koren-Morag, N.; Goldbourt, U.; Tanne, D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology 2006, 67, 224–228. [Google Scholar] [CrossRef]
- Sprick, J.D.; Nocera, J.R.; Hajjar, I.; O’Neill, W.C.; Bailey, J.; Park, J. Cerebral blood flow regulation in end-stage kidney disease. Am. J. Physiol. Ren. Physiol. 2020, 319, F782–F791. [Google Scholar] [CrossRef]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef]
- Górriz, J.L.; Molina, P.; Bover, J.; Barril, G.; Martín-de Francisco, A.L.; Caravaca, F.; Hervás, J.; Piñera, C.; Escudero, V.; Molinero, L.M. Characteristics of bone mineral metabolism in patients with stage 3–5 chronic kidney disease not on dialysis: Results of the OSERCE study. Nefrol. Publ. Of. De La Soc. Esp. Nefrol. 2013, 33, 46–60. [Google Scholar]
- Hurst, K. Primary hyperparathyroidism as a secondary cause of depression. J. Am. Board Fam. Med. JABFM 2010, 23, 677–680. [Google Scholar] [CrossRef]
- Lam, V.; Albrecht, M.A.; Takechi, R.; Prasopsang, P.; Lee, Y.P.; Foster, J.K.; Mamo, J.C. Serum 25-hydroxyvitamin D is associated with reduced verbal episodic memory in healthy, middle-aged and older adults. Eur. J. Nutr. 2016, 55, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Grützner, T.M.; Listunova, L.; Fabian, G.A.; Kramer, B.A.; Flach, D.; Weisbrod, M.; Roesch-Ely, D.; Sharma, A. Serum calcium levels and neuropsychological performance in depression and matched healthy controls: Reversal of correlation a marker of the aging cognitive clock? Psychoneuroendocrinology 2018, 91, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.K.; Heo, Y.J.; Lee, K.S.; Lee, B.L. Clinical Findings and Neurologic Outcome in Neonatal Encephalopathy with White Matter Injury Accompanied by Rotavirus. J. Child Neurol. 2018, 33, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Ma, S.; Li, P.; Ding, D.; Liu, H.; Liu, J.; Zhang, M. Association between abnormal thalamic metabolites and sleep disturbance in patients with end-stage renal disease. Metab. Brain Dis. 2018, 33, 1641–1648. [Google Scholar] [CrossRef]

| Clinical Measure | HC (n = 31) | CKD3 (n = 18) | CKD4 (n = 19) | p-Value | CKD3 vs. CKD4 | HC vs. CKD3 | HC vs. CKD4 |
|---|---|---|---|---|---|---|---|
| Age (Y) | 44.13 ± 10.63 | 44.61 ± 14.74 | 47.53 ± 11.35 | 0.609 | |||
| Sex (M/F) | 18/13 | 13/5 | 11/8 | 0.579 | |||
| Education (Y) | 9.71 ± 4.10 | 8.50 ± 3.03 | 8.00 ± 3.23 | 0.228 | |||
| Hemoglobin (g/dL) | 114.72 ± 20.72 | 101.42 ± 17.61 | 0.042 | ||||
| Urea nitrogen (mg/dL) | 10.88 ± 3.43 | 14.98 ± 5.43 | 0.010 | ||||
| Creatinine (mg/dL) | 262.27 ± 100.31 | 437.95 ± 168.44 | 0.001 | ||||
| Kalium (mg/dL) | 4.12 ± 0.42 | 4.58 ± 0.54 | 0.007 | ||||
| Calcium (mg/dL) | 2.25 ± 0.25 | 2.14 ± 0.22 | 0.169 | ||||
| eGFR (mL/min/1.73 m2) | 39.00 ± 7.84 | 21.04 ± 4.50 | <0.001 | ||||
| MoCA score | 27.00 (24.00,29.00) | 25.00 (22.00,28.00) | 24.00 (19.00,28.00) | 0.024 | 1.000 | 0.209 | 0.031 |
| MMSE score | 29.00 (28.00,30.00) | 29.00 (27.00,30.00) | 28.00 (26.00,29.00) | 0.020 | 0.281 | 1.000 | 0.016 |
| NCT-A score | 38.00 (33.00,47.00) | 45.00 (37.00,71.00) | 47.00 (32.00,83.00) | 0.163 | |||
| DST score | 45.00 (43.00,57.00) | 40.50 (20.25,56.00) | 34.00 (17.00,49.00) | 0.014 | 1.000 | 0.201 | 0.015 |
| Brain Area (AAL) | VOXEL | Peak MNI Coordinate | Peak | ||
|---|---|---|---|---|---|
| right insula | 257 | 50 | 6 | −6 | −5.0958 |
| left hippocampus | 70 | −36 | −40 | −8 | 4.8948 |
| Brain Regions | Sensitivity | Specificity | Youden Index | Cut-Off |
|---|---|---|---|---|
| right insula | 63.20% | 93.50% | 0.57 | 0.19 |
| left hippocampus | 89.5% | 77.4% | 0.67 | −0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Liu, M.; Wu, X.; Meng, S.; Yu, K.; Su, H.; Liang, Q.; Chen, F.; Li, J.; Xiao, W.; et al. Altered Cerebral Blood Flow in the Progression of Chronic Kidney Disease. J. Pers. Med. 2023, 13, 142. https://doi.org/10.3390/jpm13010142
Lin W, Liu M, Wu X, Meng S, Yu K, Su H, Liang Q, Chen F, Li J, Xiao W, et al. Altered Cerebral Blood Flow in the Progression of Chronic Kidney Disease. Journal of Personalized Medicine. 2023; 13(1):142. https://doi.org/10.3390/jpm13010142
Chicago/Turabian StyleLin, Weizhao, Mengchen Liu, Xixin Wu, Shandong Meng, Kanghui Yu, Huanhuan Su, Quanhai Liang, Feng Chen, Jincheng Li, Wenqin Xiao, and et al. 2023. "Altered Cerebral Blood Flow in the Progression of Chronic Kidney Disease" Journal of Personalized Medicine 13, no. 1: 142. https://doi.org/10.3390/jpm13010142
APA StyleLin, W., Liu, M., Wu, X., Meng, S., Yu, K., Su, H., Liang, Q., Chen, F., Li, J., Xiao, W., Ling, H., Wu, Y., & Jiang, G. (2023). Altered Cerebral Blood Flow in the Progression of Chronic Kidney Disease. Journal of Personalized Medicine, 13(1), 142. https://doi.org/10.3390/jpm13010142









