Risk Factors for Chronic Non-Communicable Diseases and Osteoporotic Fractures in a Middle and Elderly-Aged Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Questionnaire
2.3. Biochemical Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Eastern European & Central Asian Regional Audit: Epidemiology, Costs & Burden of Osteoporosis in 2010 [Electronic re-source]/International Osteoporosis Foundation. 2011. Available online: https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Eastern%20Europe_Central%20Asia/Eastern_European_Central_Asian_Audit_2010.pdf (accessed on 26 May 2020).
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Mazurenko, E.S.; Malyutina, S.K.; Shcherbakova, L.V.; Mustafina, S.V.; Nikitenko, T.M.; Bobak, M.; Rymar, O.D. The forearm fractures in patients with diabetes and without diabetes in population sample aged over 50 years (Novosibirsk). Probl. Endocrinol. 2019, 65, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, U.; Melhus, H.; Gedeborg, R.; Byberg, L.; Garmo, H.; Ahlbom, A.; Pedersen, N.L.; Michaëlsson, K. Cardiovascular Diseases and Risk of Hip Fracture. JAMA 2009, 302, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Marcovitz, P.A.; Tran, H.H.; Franklin, B.A.; O’Neill, W.W.; Yerkey, M.; Boura, J.; Kleerekoper, M.; Dickinson, C.Z. Usefulness of Bone Mineral Density to Predict Significant Coronary Artery Disease. Am. J. Cardiol. 2005, 96, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Demer, L.L. Effects of bioactive lipids and lipoproteins on bone. Trends Endocrinol. Metab. 2013, 25, 53–59. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Hansen, S.; Frost, M.; Jørgensen, N.R.; Hermann, A.P.; Henriksen, J.E.; Brixen, K. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur. J. Endocrinol. 2016, 174, 115–124. [Google Scholar] [CrossRef]
- Mazurenko, E.S.; Malutina, S.K.; Shcherbakova, L.V.; Hrapova, Y.V.; Isaeva, M.P.; Rymar, O.D. 10-year risk of fractures (FRAX) in people with diabetes type 2. Ter. Arkhiv 2019, 91, 76–81. [Google Scholar] [CrossRef]
- Mazurenko, E.S.; Rymar, O.D.; Scherbakova, L.V.; Khrapova, Y.V.; Isayeva, M.P.; Munts, I.V.; Hubacek, J.; Bobak, M.; Malutina, S.K. 10-year fracture risk (FRAX®), mineral bone density and trabecular bone index in women with type 2 diabetes mellitus. Med Counc. 2019, 4, 62–68. [Google Scholar] [CrossRef]
- Munz, I.V.; Direev, A.O.; Verevkin, E.G.; Mesenzev, E.M.; Shapkina, M.Y.; Avdeeva, E.M.; Mazurenko, E.S.; Bobak, M.; Malyutina, S.K.; Ryabikov, A.N. The comorbidity of cardiovascular and non-communicable diseases with oph-thalmologic diseases in population. Klin. Med. 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Peasey, A.; Bobak, M.; Kubinova, R.; Malyutina, S.; Pajak, A.; Tamosiunas, A.; Pikhart, H.; Nicholson, A.; Marmot, M. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: Rationale and design of the HAPIEE study. BMC Public Health 2006, 6, 255. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J. Measuring Quantity, Frequency, and Volume of Drinking. Alcohol. Clin. Exp. Res. 1998, 22, 4s–14s. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Department of Noncommunicable Disease Management. Screening for Type 2 Diabetes: Report of a World Health Organization and International Diabetes Federation meeting; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/handle/10665/68614 (accessed on 8 September 2022).
- Hiligsmann, M.; Kanis, J.A.; Compston, J.; Cooper, C.; Flamion, B.; Bergmann, P.; Body, J.-J.; Boonen, S.; Bruyere, O.; Devogelaer, J.-P.; et al. Health Technology Assessment in Osteoporosis. Calcif. Tissue Res. 2013, 93, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, A.; Khodyrev, A.; Lesnyak, O.M. AN EPIDEMIOLOGICAL SURVEY OF OSTEOPOROTIC FRACTURES IN OLDER RESIDENTS FROM THE MIDDLE URALS. Rheumatol. Sci. Pract. 2014, 52, 643–649. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, W.W.; Yang, L.; Chen, W.W.; Zhang, H.X. Association between lipid profiles and osteoporosis in postmenopausal women: A me-ta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghorabi, S.; Shab-Bidar, S.; Sadeghi, O.; Nasiri, M.; Khatibi, S.R.; Djafarian, K. Lipid Profile and Risk of Bone Fracture: A Systematic Review and Meta-Analysis of Observational Studies. Endocr. Res. 2019, 44, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Wang, X.; Cao, H.; Liu, S.; Wang, Y. Impact of Cardiovascular Risk Factors on Osteoporosis among Different Age Patients with Coronary Artery Disease. J. Res. Cardiol. 2016, 307728. [Google Scholar] [CrossRef]
- Dennison, E.M.; Syddall, H.E.; Sayer, A.A.; Martin, H.J.; Cooper, C. for the Hertfordshire Cohort Study Group Lipid profile, obesity and bone mineral density: The Hertfordshire Cohort Study. QJM Int. J. Med. 2007, 100, 297–303. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Schirmer, H.; Berntsen, G.K.; Fønnebø, V.; Joakimsen, R.M. Features of the metabolic syndrome and the risk of non-vertebral fractures: The Tromsø study. Osteoporos. Int. 2005, 17, 426–432. [Google Scholar] [CrossRef]
- Kha, H.T.; Basseri, B.; Shouhed, D.; Richardson, J.; Tetradis, S.; Hahn, T.J.; Parhami, F. Oxysterols Regulate Differentiation of Mesenchymal Stem Cells: Pro-Bone and Anti-Fat. J. Bone Miner. Res. 2004, 19, 830–840. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid Oxidation Products Have Opposite Effects on Calcifying Vascular Cell and Bone Cell Differentiation. Arter. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Papachristou, N.I.; Blair, H.C.; Kypreos, K.; Papachristou, D.J. High-density lipoprotein (HDL) metabolism and bone mass. J. Endocrinol. 2017, 233, R95–R107. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Qin, L.-Q.; Ye, J.-K. Leptin levels and risk of type 2 diabetes: Gender-specific meta-analysis. Obes. Rev. 2013, 15, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Heil, A.L.; Hendriks, H.F.; Mukamal, K.J.; Beulens, J.W. The Effect of Alcohol Consumption on Insulin Sensitivity and Glycemic Status: A Systematic Review and Meta-analysis of Intervention Studies. Diabetes Care 2015, 38, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2004, 16, 155–162. [Google Scholar] [CrossRef]
- Yang, S.; Nguyen, N.D.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Association between hypertension and fragility fracture: A longitudinal study. Osteoporos. Int. 2013, 25, 97–103. [Google Scholar] [CrossRef]
- Yazici, S.; Yazici, M.; Korkmaz, U.; Erkan, M.E.; Baki, A.E.; Erden, I.; Özhan, H.; Ataoglu, S. Relationship between blood pressure levels and bone mineral density in postmenopausal Turkish women. Arch. Med. Sci. 2011, 2, 264–270. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [Green Version]
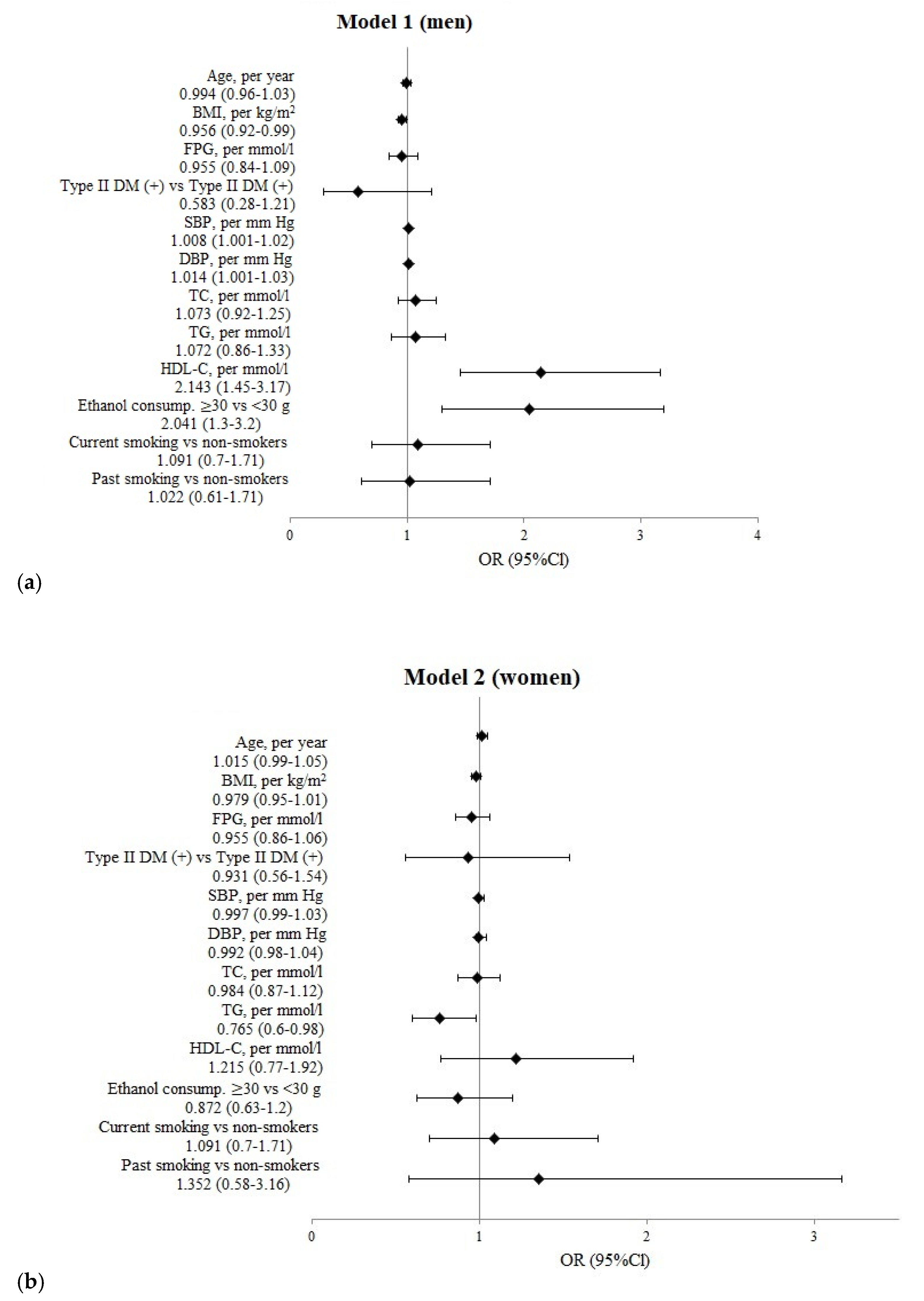
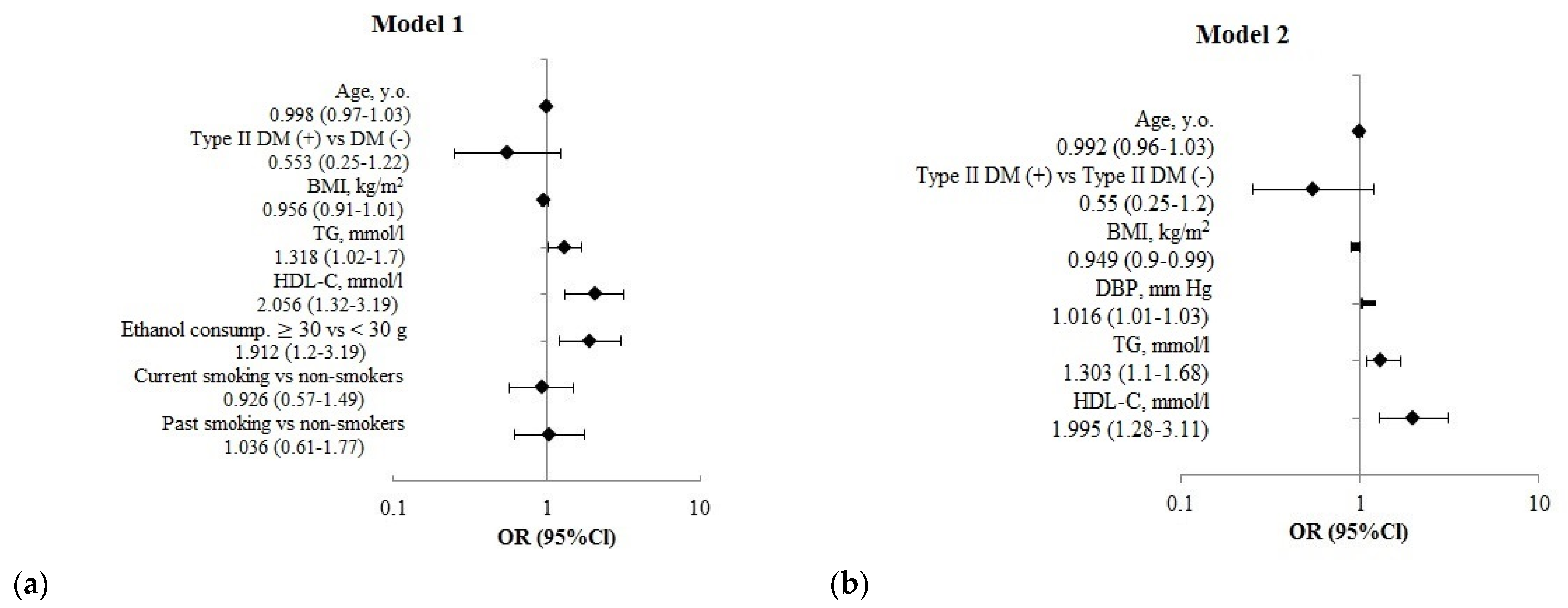

| Indicators | Fractures+ | Fractures− | p1 |
|---|---|---|---|
| (n = 113) | (n = 3398) | ||
| Age, y.o. | 60.1 ± 5.6 | 60.3 ± 5.8 | 0.689 |
| Height, cm | 170.4 ± 5.6 | 170.7 ± 6.3 | 0.623 |
| Weight, kg | 75.0 ± 13.4 | 77.9 ± 14.4 | 0.038 |
| BMI, kg/m2 | 25.8 ± 4.0 | 26.7 ± 4.4 | 0.030 |
| BMI ≥ 30 kg/m2, (n/%) | 20/17.7% | 725/21.3% | 0.352 |
| WC, cm | 92.2 ± 12.3 | 94.5 ± 12.2 | 0.052 |
| SBP, mm Hg | 150.0 ± 25.4 | 145.9 ± 23.7 | 0.110 |
| DBP, mm Hg | 93.6 ± 15.0 | 91.1 ± 13.4 | 0.050 |
| FPG, mmol/L | 5.9 ± 1.8 | 6.1 ± 1.7 | 0.477 |
| TC, mmol/L | 6.1 ± 1.3 | 6.0 ± 1.2 | 0.713 |
| TG, mmol/L | 1.5 ± 0.9 | 1.5 ± 0.8 | 0.904 |
| HDL-C, mmol/L | 1.6 ± 0.5 | 1.5 ± 0.4 | 0.001 |
| LDL-C, mmol/L | 3.7 ± 1.1 | 3.8 ± 1.1 | 0.317 |
| Tobacco smoking, (n/%) | |||
| Non-smokers | 28/24.8% | 909/26.8% | 0.641 |
| Past smokers | 28/24.8% | 896/26.4% | 0.706 |
| Current smokers | 57/50.4% | 1593/46.9% | 0.456 |
| Alcohol consumption: average ethanol consumption (g) | |||
| ≥30 g per session, (n/%) | 89/78.8% | 2190/64.4% | 0.002 |
| ≥30 g per day, (n/%) | 16/14.2% | 400/11.8% | 0.441 |
| Education (n/%) | |||
| Basic general education | 9/8.0% | 442/13.0% | 0.115 |
| Secondary (complete) education | 72/63.7% | 1862/54.8% | 0.061 |
| Higher education | 32/28.3% | 1094/32.2% | 0.385 |
| T2DM (n/%) | 8/7.1% | 394/11.6% | 0.138 |
| HT, (n/%) | 78/69.6% | 2279/67.1% | 0.663 |
| Indicators | Fractures+ | Fractures− | p1 |
|---|---|---|---|
| (n = 154) | (n = 3704) | ||
| Age, y.o. | 61.2 ± 5.9 | 60.8 ± 5.6 | 0.331 |
| Height, cm | 158.1 ± 6.5 | 157.4 ± 5.8 | 0.165 |
| Weight, kg | 74.4 ± 13.5 | 75.5 ± 14.6 | 0.332 |
| BMI, kg/m2 | 29.8 ± 5.2 | 30.5 ± 5.6 | 0.123 |
| BMI ≥ 30 kg/m2, (n/%) | 72/46.8% | 1812/49.0% | 0.585 |
| WC, cm | 91.9 ± 12.4 | 92.9 ± 13.2 | 0.343 |
| SBP, mm Hg | 146.7 ± 27.9 | 148.2 ± 26.3 | 0.494 |
| DBP, mm Hg | 90.0 ± 14.5 | 91.4 ± 13.4 | 0.213 |
| FPG, mmol/L | 6.0 ± 1.4 | 6.1 ± 1.8 | 0.436 |
| TC, mmol/L | 6.6 ± 1.3 | 6.7 ± 1.3 | 0.911 |
| TG, mmol/L | 1.5 ± 0.6 | 1.6 ± 0.9 | 0.044 |
| HDL-C, mmol/L | 1.6 ± 0.4 | 1.6 ± 0.3 | 0.400 |
| LDL-C, mmol/L | 4.4 ± 1.2 | 4.3 ± 1.2 | 0.752 |
| Tobacco smoking, (n/%) | |||
| Non-smokers | 131/85.1% | 3289/88.9% | 0.136 |
| Past smokers | 6/3.9 | 121/3.3% | 0.670 |
| Current smokers | 17/11.0% | 288/7.8% | 0.143 |
| Alcohol consumption: average ethanol consumption (g) | |||
| ≥ 20 g per session, (n/%) | 72/46.8% | 1855/50.2% | 0.407 |
| ≥ 20 g per day, (n/%) | 1/0.6% | 14/0.4% | 0.597 |
| Education, (n/%) | |||
| Basic general education | 21/13.6% | 442/12.0% | 0.528 |
| Secondary (complete) education | 95/61.7% | 2343/63.4% | 0.674 |
| Higher education | 38/24.7% | 913/24.7% | 0.997 |
| Menopause duration, years | 13.2 ± 7.6 | 12.2 ± 7.2 | 0.093 |
| Receive HRT, (n/%) | 9/5.8% | 251/6.8% | 0.647 |
| T2DM (n/%) | 18/11.7% | 451/12.2% | 0.850 |
| HT, (n/%) | 108/70.1% | 2705/73.1% | 0.408 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurenko, E.; Rymar, O.; Rerikh, V.; Khrapova, Y.; Direev, A.; Shcherbakova, L.; Malyutina, S. Risk Factors for Chronic Non-Communicable Diseases and Osteoporotic Fractures in a Middle and Elderly-Aged Population. J. Pers. Med. 2022, 12, 1475. https://doi.org/10.3390/jpm12091475
Mazurenko E, Rymar O, Rerikh V, Khrapova Y, Direev A, Shcherbakova L, Malyutina S. Risk Factors for Chronic Non-Communicable Diseases and Osteoporotic Fractures in a Middle and Elderly-Aged Population. Journal of Personalized Medicine. 2022; 12(9):1475. https://doi.org/10.3390/jpm12091475
Chicago/Turabian StyleMazurenko, Elena, Oksana Rymar, Victor Rerikh, Yuliya Khrapova, Artem Direev, Liliya Shcherbakova, and Sofia Malyutina. 2022. "Risk Factors for Chronic Non-Communicable Diseases and Osteoporotic Fractures in a Middle and Elderly-Aged Population" Journal of Personalized Medicine 12, no. 9: 1475. https://doi.org/10.3390/jpm12091475
APA StyleMazurenko, E., Rymar, O., Rerikh, V., Khrapova, Y., Direev, A., Shcherbakova, L., & Malyutina, S. (2022). Risk Factors for Chronic Non-Communicable Diseases and Osteoporotic Fractures in a Middle and Elderly-Aged Population. Journal of Personalized Medicine, 12(9), 1475. https://doi.org/10.3390/jpm12091475







