Clinicians’ Perceptions towards Precision Medicine Tools for Cardiovascular Disease Risk Stratification in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Questionnaire
2.3. Data Collection and Analyses
3. Results
3.1. Participant Characteristics and Exposure to Genetics and Cardiovascular Disease Screening
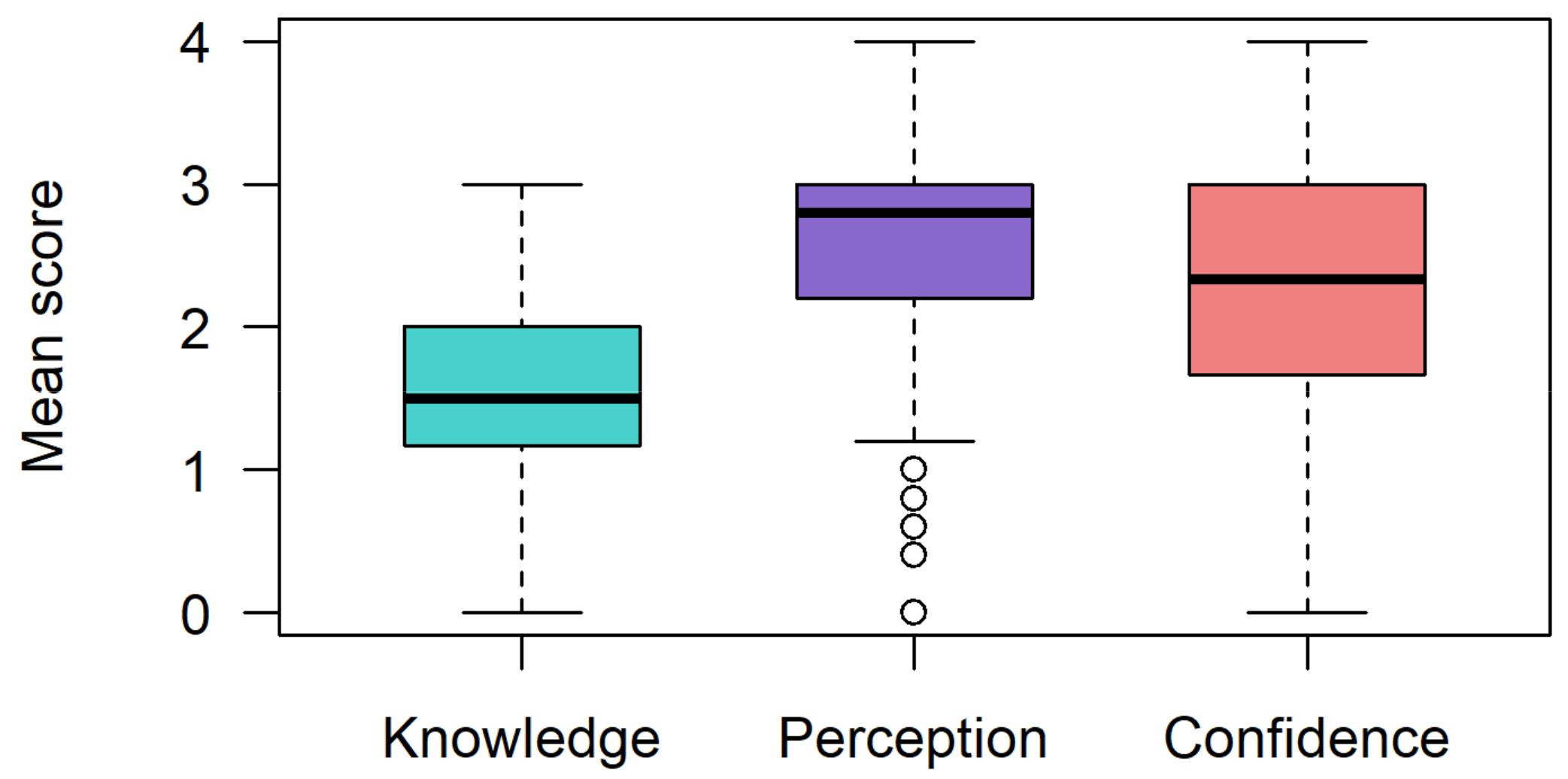
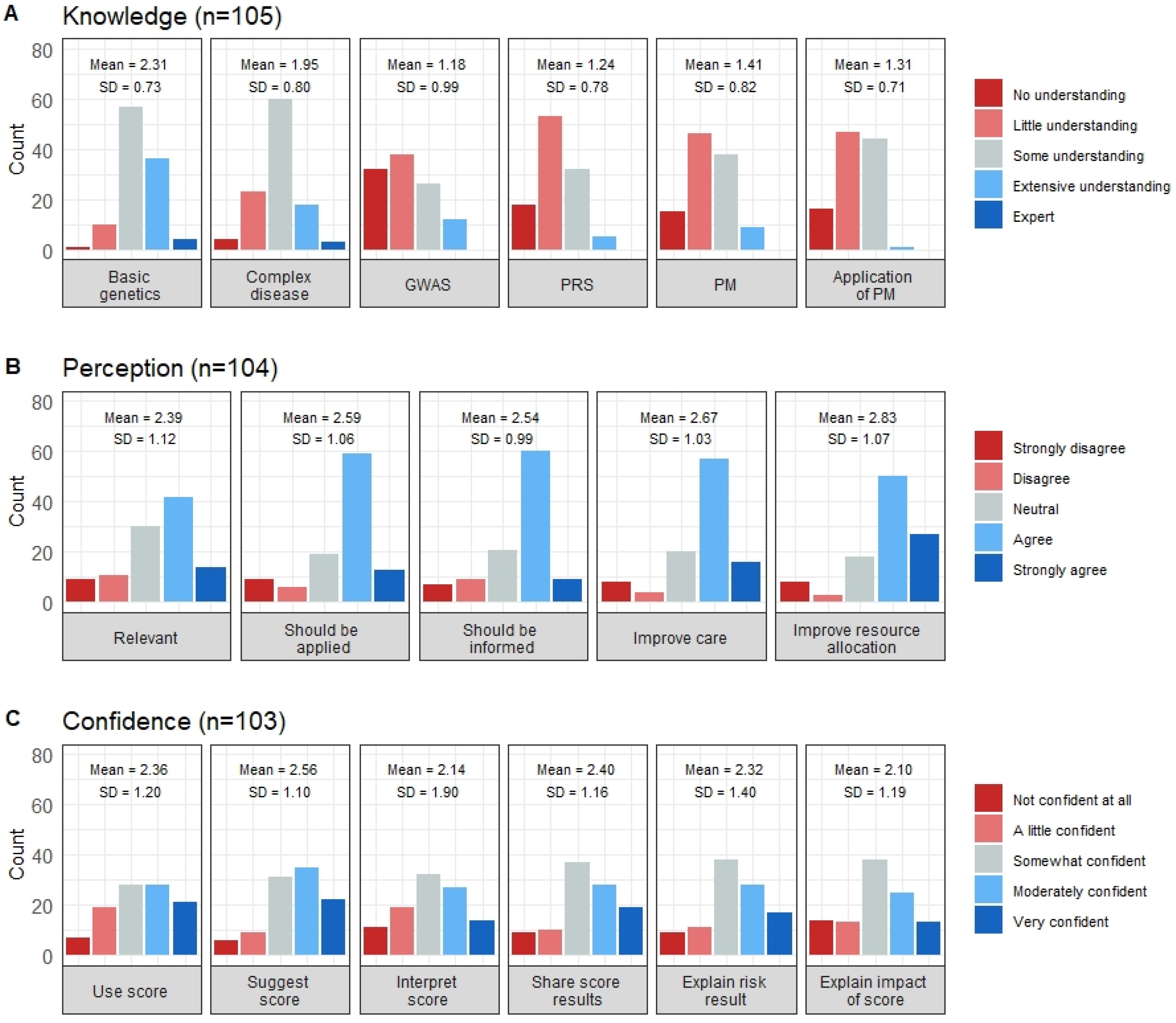
3.2. Genetics and Precision Medicine Knowledge
3.3. Perceptions toward Precision Medicine-Based CVD Risk Stratification
3.4. Confidence in Applying a Precision Medicine-Based CVD Risk Stratification Tool in Their Practice Settings
3.5. Factors Influencing Knowledge, Perceptions, and Confidence
3.6. Multivariable Analysis of the Factors Affecting Knowledge, Perceptions, and Confidence
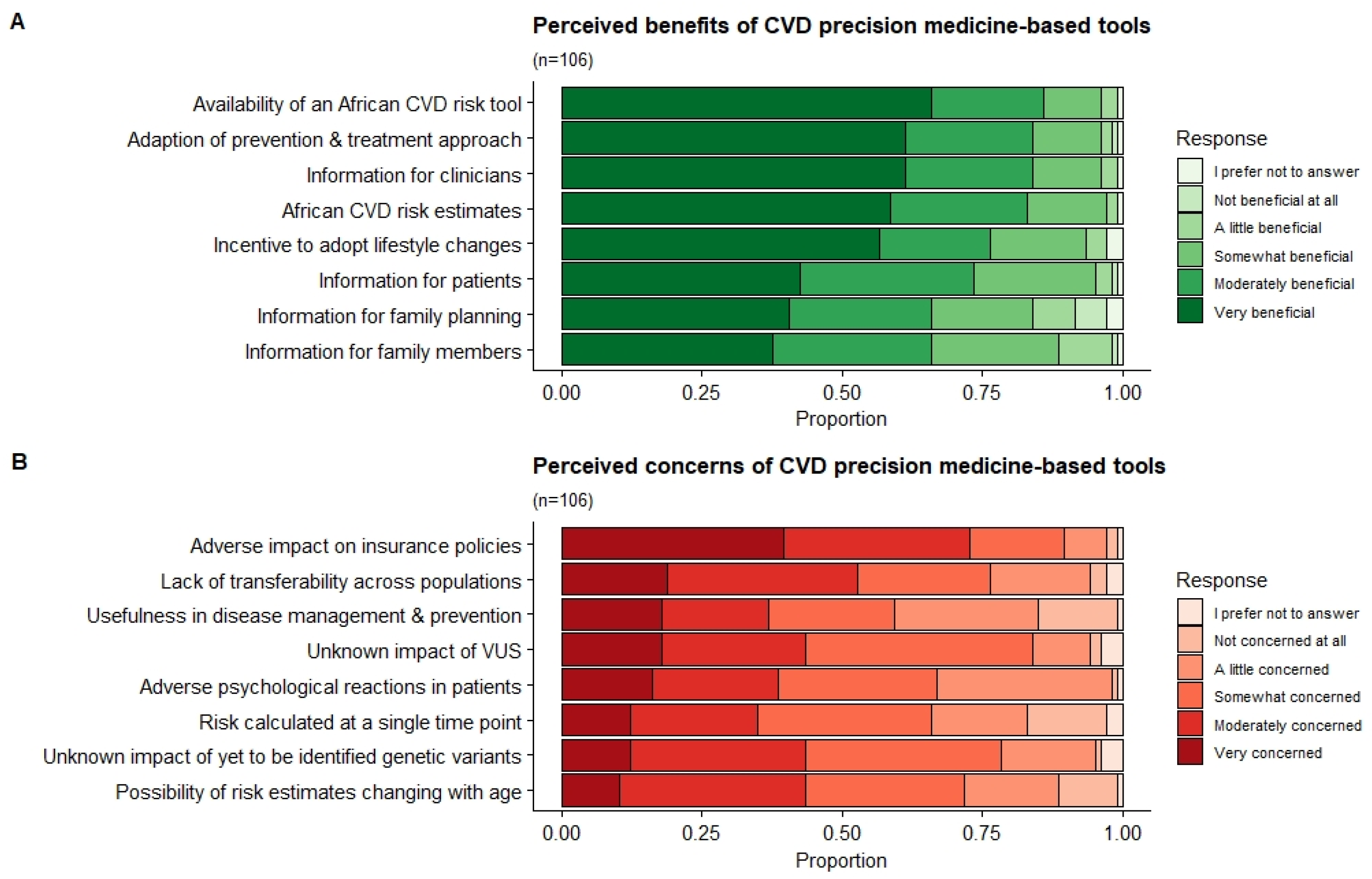
3.7. Benefits and Concerns of a PM-Based CVD Risk Stratification
3.8. Clinician’s Expectations: Time Horizon, Funding, and Expected Role
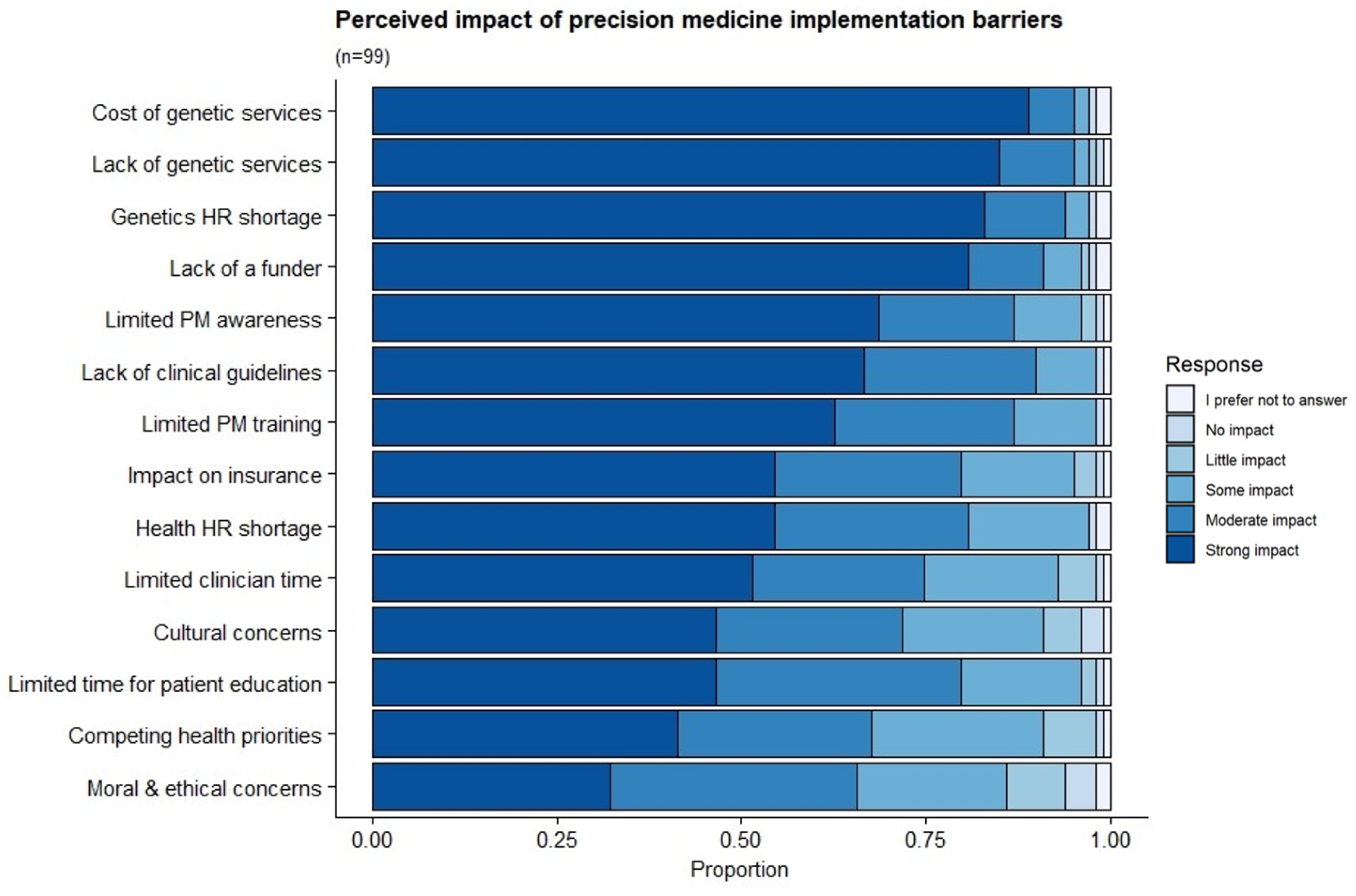
3.9. Perceived Barriers to the Implementation of PM-Based CVD Risk Stratification
4. Discussion
4.1. Positive Perceptions of PM-Based Tools despite Knowledge Gaps and Resource Constraints
4.2. Addressing the Genetics Education Gap Is Paramount to Successful Adoption
4.3. Integrated Models Compatible with Current Clinical Practices Are Needed
4.4. Funding Shortfalls and Skills Shortages Hinder PM Adoption in Resource-Scarce Settings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Olivé, F.X.; Ali, S.A.; Made, F.; Kyobutungi, C.; Nonterah, E.; Micklesfield, L.; Alberts, M.; Boua, R.; Hazelhurst, S.; Debpuur, C.; et al. Regional and Sex Differences in the Prevalence and Awareness of Hypertension: An H3Africa AWI-Gen Study Across 6 Sites in Sub-Saharan Africa. Glob. Heart 2017, 12, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Califf, R.M.; Armstrong, P.W.; Carver, J.R.; D’Agostino, R.B.; Strauss, W.E. Task Force 5. Stratification of Patients into High, Medium Risk Subgroups for Purposes of Risk Factor Management General Perspectives on Risk and Effect on Intervention. J. Am. Coll. Cardiol. 1996, 27, 964–1047. [Google Scholar] [CrossRef]
- Klug, E.Q.; Raal, F.J.; Marais, A.D.; Taskinen, M.R.; Dalby, A.J.; Schamroth, C.; Rapeport, N.; Jankelow, D.; Blom, D.J.; Catsicas, R.; et al. South African Dyslipidaemia Guideline Consensus Statement. S. Afr. Med J. 2012, 102, 178–188. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef]
- Lindbohm, J.V.; Sipilä, P.N.; Mars, N.J.; Pentti, J.; Ahmadi-Abhari, S.; Brunner, E.; Shipley, M.J.; Singh-Manoux, A.; Tabak, A.G.; Kivimaki, M. 5-year versus risk-category-specific screening intervals for cardiovascular disease prevention: A cohort study. Lancet Public Health 2019, 4, e189–e199. [Google Scholar] [CrossRef]
- Tamlander, M.; Mars, N.; Pirinen, M.; Palotie, A.; Daly, M.; Riley-Gills, B.; Jacob, H.; Paul, D.; Runz, H.; John, S.; et al. Integration of questionnaire-based risk factors improves polygenic risk scores for human coronary heart disease and type 2 diabetes. Commun. Biol. 2022, 5, 158. [Google Scholar] [CrossRef]
- Wagner, R.G.; Crowther, N.J.; Micklesfield, L.K.; Boua, P.R.; Nonterah, E.A.; Mashinya, F.; Mohamed, S.F.; Asiki, G.; Tollman, S.; Ramsay, M.; et al. Estimating the burden of cardiovascular risk in community dwellers over 40 years old in South Africa, Kenya, Burkina Faso and Ghana. BMJ Glob. Health 2021, 6, e003499. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Danish, S.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A Comprehensive 1000 Genomes-Based Genome-Wide Association Meta-Analysis of Coronary Artery Disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar] [PubMed]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Vassos, E. Prospects for using risk scores in polygenic medicine. Genome Med. 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Abraham, G.; Nelson, C.P.; Wood, A.M.; Sweeting, M.J.; Dudbridge, F.; Lai, F.Y.; Kaptoge, S.; Brozynska, M.; Wang, T.; et al. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults: Implications for Primary Prevention. J. Am. Coll. Cardiol. 2018, 72, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Riveros-Mckay, F.; Weale, M.E.; Moore, R.; Selzam, S.; Krapohl, E.; Sivley, R.M.; Tarran, W.A.; Sørensen, P.; Lachapelle, A.S.; Griffiths, J.A.; et al. Integrated Polygenic Tool Substantially Enhances Coronary Artery Disease Prediction. Circ. Genom. Precis. Med. 2021, 14, 192–200. [Google Scholar] [CrossRef]
- Kullo, I.J.; Jouni, H.; Austin, E.E.; Brown, S.-A.; Kruisselbrink, T.M.; Isseh, I.N.; Haddad, R.A.; Marroush, T.S.; Shameer, K.; Olson, J.E.; et al. Incorporating a Genetic Risk Score into Coronary Heart Disease Risk Estimates: Effect on Low-Density Lipoprotein Cholesterol Levels (the MI-GENES Clinical Trial). Circulation 2016, 133, 1181–1188. [Google Scholar] [CrossRef]
- Pereira, L.; Mutesa, L.; Tindana, P.; Ramsay, M. African genetic diversity and adaptation inform a precision medicine agenda. Nat. Rev. Genet. 2021, 22, 284–306. [Google Scholar] [CrossRef]
- Chikowore, T.; Kamiza, A.B.; Oduaran, O.H.; Machipisa, T.; Fatumo, S. Non-communicable diseases pandemic and precision medicine: Is Africa ready? eBioMedicine 2021, 65, 103260. [Google Scholar] [CrossRef]
- Kamp, M.; Krause, A.; Ramsay, M. Has translational genomics come of age in Africa? Hum. Mol. Genet. 2021, 30, R164–R173. [Google Scholar] [CrossRef]
- Tekola-Ayele, F.; Rotimi, C.N. Translational Genomics in Low- and Middle-Income Countries: Opportunities and Challenges. Public Health Genom. 2015, 18, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, C.; Abayomi, A.; Abimiku, A.; Adabayeri, V.M.; Adebamowo, C.; Adebiyi, E.; Ademola, A.D.; Adeyemo, A.; Adu, D.; Affolabi, D.; et al. Research capacity. Enabling the genomic revolution in Africa. Science 2014, 344, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.O.; Fei, K.; Levy, K.D.; Elsey, A.R.; Pollin, T.I.; Ramirez, A.H.; Weitzel, K.W.; Horowitz, C.R. Physician-Reported Benefits and Barriers to Clinical Implementation of Genomic Medicine: A Multi-Site IGNITE-Network Survey. J. Pers. Med. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Hartzler, A.; McCarty, C.A.; Rasmussen, L.V.; Williams, M.S.; Brilliant, M.; Bowton, E.A.; Clayton, E.W.; Faucett, W.A.; Ferryman, K.; Field, J.R.; et al. Stakeholder engagement: A key component of integrating genomic information into electronic health records. Genet. Med. 2013, 15, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Abdela, O.A.; Bhagavathula, A.S.; Gebreyohannes, E.A.; Tegegn, H.G. Ethiopian health care professionals’ knowledge, attitude, and interests toward pharmacogenomics. Pharm. Pers. Med. 2017, 10, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Muzoriana, N.; Gavi, S.; Nembaware, V.; Dhoro, M.; Matimba, A. Knowledge, Attitude, and Perceptions of Pharmacists and Pharmacy Students towards Pharmacogenomics in Zimbabwe. Pharmacy 2017, 5, 36. [Google Scholar] [CrossRef]
- Haga, S.; Burke, W.; Ginsburg, G.; Mills, R.; Agans, R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin. Genet. 2012, 82, 388–394. [Google Scholar] [CrossRef]
- Carroll, J.C.; Morrison, S.; Miller, F.A.; Wilson, B.J.; Permaul, J.A.; Allanson, J. Anticipating the primary care role in genomic medicine: Expectations of genetics health professionals. J. Community Genet. 2021, 12, 559–568. [Google Scholar] [CrossRef]
- Chambers, C.; Axell-House, D.; Mills, G.; Bittner-Fagan, H.; Rosenthal, M.; Johnson, M.; Stello, B. Primary Care Physicians’ Experience and Confidence with Genetic Testing and Perceived Barriers to Genomic Medicine. J. Fam. Med. 2015, 2, 1024. [Google Scholar]
- Yu, M.W.C.; Fung, J.L.F.; Ng, A.P.P.; Li, Z.; Lan, W.; Chung, C.C.Y.; Li, Y.; Liu, Y.; Chung, B.H.Y.; Wong, W.C.W. Preparing genomic revolution: Attitudes, clinical practice, and training needs in delivering genetic counseling in primary care in Hong Kong and Shenzhen, China. Mol. Genet. Genom. Med. 2021, 9, e1702. [Google Scholar] [CrossRef]
- Rubanovich, C.K.; Cheung, C.; Mandel, J.; Bloss, C.S. Physician preparedness for big genomic data: A review of genomic medicine education initiatives in the United States. Hum. Mol. Genet. 2018, 27, R250–R258. [Google Scholar] [CrossRef] [PubMed]
- Addis, A.; Trotta, F.; Tafuri, G.; de Fiore, L. Information Needs on Precision Medicine: A Survey of Italian Health Care Professionals. Ann. Ist. Super. Sanita 2018, 54, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.; Alshammari, S.; Ouda, G.; Koshy, S.; Awad, A. Knowledge, perceptions and confidence of physicians and pharmacists towards pharmacogenetics practice in Kuwait. PLoS ONE 2018, 13, e0203033. [Google Scholar] [CrossRef] [PubMed]
- Selkirk, C.G.; Weissman, S.M.; Anderson, A.; Hulick, P.J. Physicians’ Preparedness for Integration of Genomic and Pharmacogenetic Testing into Practice within a Major Healthcare System. Genet. Test. Mol. Biomark. 2013, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Chow-White, P.; Ha, D.; Laskin, J. Knowledge, attitudes, and values among physicians working with clinical genomics: A survey of medical oncologists. Hum. Resour. Health 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R.; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Revelle, W.R. Psych: Procedures for Personality and Psychological Research. Software; Northwestern University: Evanston, IL, USA, 2017. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Spiech, K.M.; Tripathy, P.R.; Woodcock, A.M.; Sheth, N.A.; Collins, K.S.; Kannegolla, K.; Sinha, A.D.; Sharfuddin, A.A.; Pratt, V.M.; Khalid, M.; et al. Implementation of a Renal Precision Medicine Program: Clinician Attitudes and Acceptance. Life 2020, 10, 32. [Google Scholar] [CrossRef]
- Jayasinghe, K.; Quinlan, C.; Mallett, A.J.; Kerr, P.G.; McClaren, B.; Nisselle, A.; Mallawaarachchi, A.; Polkinghorne, K.R.; Patel, C.; Best, S.; et al. Attitudes and Practices of Australian Nephrologists Toward Implementation of Clinical Genomics. Kidney Int. Rep. 2021, 6, 272–283. [Google Scholar] [CrossRef]
- Carroll, J.C.; Allanson, J.; Morrison, S.; Miller, F.A.; Wilson, B.J.; Permaul, J.A.; Telner, D. Informing Integration of Genomic Medicine Into Primary Care: An Assessment of Current Practice, Attitudes, and Desired Resources. Front. Genet. 2019, 10, 1189. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Shaqran, T.M.; Eltobgy, A.A.E.; Albalawi, A.R.; Alnawmasi, W.S. Physicians’ Perspective on Diabetes Mellitus Management within the Context of Personalized Medicine Era in Tabuk Governorate, Saudi Arabia. Open Access Maced. J. Med Sci. 2019, 7, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Albitar, L.; Alchamat, G.A. Pharmacogenetics: Knowledge assessment amongst Syrian pharmacists and physicians. BMC Health Serv. Res. 2021, 21, 1031. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Doll, B.; de Castro, M.J.; Fries, M.H.; Pock, A.R.; Seibert, D.; Yang, W. Precision Medicine—A Demand Signal for Genomics Education. Mil. Med. 2021, 187, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Crellin, E.; McClaren, B.; Nisselle, A.; Best, S.; Gaff, C.; Metcalfe, S. Preparing Medical Specialists to Practice Genomic Medicine: Education an Essential Part of a Broader Strategy. Front. Genet. 2019, 10, 789. [Google Scholar] [CrossRef]
- Cornel, M.C. Evidence-Based Genetic Education of Non-Genetic-Expert Physicians: Experiences Over Three Decades in Amsterdam. Front. Genet. 2019, 10, 712. [Google Scholar] [CrossRef]
- Nisselle, A.; King, E.A.; McClaren, B.; Janinski, M.; Metcalfe, S.; Gaff, C. Measuring physician practice, preparedness and preferences for genomic medicine: A national survey. BMJ Open 2021, 11, e044408. [Google Scholar] [CrossRef]
- Laplace, B.; Calvet, B.; Lacroix, A.; Mouchabac, S.; Picard, N.; Girard, M.; Charles, E. Acceptability of Pharmacogenetic Testing among French Psychiatrists, a National Survey. J. Pers. Med. 2021, 11, 446. [Google Scholar] [CrossRef]
- Wilcox, R.L.; Adem, P.V.; Afshinnekoo, E.; Atkinson, J.B.; Burke, L.W.; Cheung, H.; Dasgupta, S.; DeLaGarza, J.; Joseph, L.; LeGallo, R.; et al. The Undergraduate Training in Genomics (UTRIG) Initiative: Early & active training for physicians in the genomic medicine era. Pers. Med. 2018, 15, 199–208. [Google Scholar] [CrossRef]
- Reed, E.K.; Johansen Taber, K.A.; Ingram Nissen, T.; Schott, S.; Dowling, L.O.; O’Leary, J.C.; Scott, J.A. What works in genomics education: Outcomes of an evidenced-based instructional model for community-based physicians. Genet. Med. 2016, 18, 737–745. [Google Scholar] [CrossRef]
- Johnson, L.-M.; Valdez, J.M.; Quinn, E.A.; Sykes, A.D.; McGee, R.B.; Nuccio, R.; Hines-Dowell, S.J.; Baker, J.N.; Kesserwan, C.; Nichols, K.E.; et al. Integrating next-generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer 2017, 123, 2352–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moor, J.S.; Gray, S.W.; Mitchell, S.A.; Klabunde, C.N.; Freedman, A.N. Oncologist Confidence in Genomic Testing and Implications for Using Multimarker Tumor Panel Tests in Practice. JCO Precis. Oncol. 2020, 4, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Abramowicz, M.; Al-Mulla, F.; Anderson, W.; Balling, R.; Berger, A.C.; Bleyl, S.; Chakravarti, A.; Chantratita, W.; Chisholm, R.L.; et al. Global implementation of genomic medicine: We are not alone. Sci. Transl. Med. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.D. Sampling Methods for Web and E-Mail Surveys. In The SAGE Handbook of Online Research Methods; SAGE Publications Ltd.: London, UK, 2008. [Google Scholar]
- Tourangeau, R.; Rips, L.; Rasinski, K. The Psychology of Survey Response; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Ojji, D.B.; Lamont, K.; Ojji, O.I.; Egenti, B.N.; Sliwa, K. Primary care in the prevention, treatment and control of cardiovascular disease in sub-Saharan Africa. Cardiovasc. J. Afr. 2017, 28, 251–256. [Google Scholar] [CrossRef]
- Beaglehole, R.; Epping-Jordan, J.A.; Patel, V.; Chopra, M.; Ebrahim, S.; Kidd, M.; Haines, A. Improving the prevention and management of chronic disease in low-income and middle-income countries: A priority for primary health care. Lancet 2008, 372, 940–949. [Google Scholar] [CrossRef]
- Mash, R.; Almeida, M.; Wong, W.C.W.; Kumar, R.; Von Pressentin, K.B. The roles and training of primary care doctors: China, India, Brazil and South Africa. Hum. Resour. Health 2015, 13, 93. [Google Scholar] [CrossRef]
| Section | Theme | No. of Items | Scale |
|---|---|---|---|
| 1 | Demographic and professional | 10 | Not applicable |
| information | |||
| 2 | Exposure: CVD risk stratification, and | 21 | Not applicable |
| genetics training and testing | |||
| 3 | Knowledge: Genetics and PM, and | 15 | Understanding: None, Little, Some, Moderate, Expert |
| educational video | Agreement: True, False, I do not know | ||
| 4 | Perception toward PM-based CVD risk stratification | 5 | Agreement: Strongly disagree, Disagree, Neutral, Agree, Strongly agree |
| 5 | Benefits and concerns of PM-based CVD risk stratification | 19 | Benefit/concern: Not, A little, Somewhat, Moderately, Very |
| 6 | Confidence in applying PM-based CVD risk stratification | 8 | Confidence: None, A little, Somewhat, Moderately, Very |
| 7 | Expectations in applying PM-based CVD risk stratification | 9 | Role: None, Supporting, Primary, Not sure |
| Adoption likelihood: Very unlikely, Unlikely, No difference, Likely, Very likely | |||
| 8 | Barriers to implementing PM-based CVD risk stratification | 15 | Impact: None, Little, Some, Moderate, Strong |
| Variable | Description | Type (Range) | Categories | |
|---|---|---|---|---|
| Outcome variables | ||||
| Mean knowledge score (MKS) | Average self-reported knowledge relating to genetics and PM | Continuous (0–4) | Not applicable | |
| Mean perception score (MPS) | Average self-reported perception relating to value of PM-based CVD risk stratification tool | Continuous (0–4) | Not applicable | |
| Mean confidence score (MCS) | Average self-reported confidence relating to implementation of PM-based CVD risk stratification tool | Continuous (0–4) | Not applicable | |
| Predictor variable | ||||
| Sex | Reported sex | Categorical | Female | |
| Male | ||||
| Medical group | Practitioner type based on self-reported practice level and years of experience | Categorical | Consultant | |
| Trainee | ||||
| Clinical experience | Number of years conducting clinical duties | Categorical | <8 years | |
| ≥8 years | ||||
| Postgraduate qualifications | Have a postgraduate qualification in addition to a medical degree | Categorical | Yes | |
| No | ||||
| Involvement in research | Are involved in medical research activities | Categorical | Yes | |
| No | ||||
| Genetics or PM training | Have training specifically relating to genetics and/or PM | Categorical | Yes | |
| No | ||||
| CVD stratification in clinical practice | Conducts CVD screening in their clinical practice | Categorical | Yes | |
| Sometimes | ||||
| No | ||||
| Variable | Detail | N (%) |
|---|---|---|
| Hospital affiliation | Chris Hani Baragwanath Academic Hospital | 23 (21.1) |
| Charlotte Maxeke Johannesburg Academic Hospital | 36 (33.0) | |
| Helen Joseph Hospital | 13 (11.9) | |
| Rahima Moosa Mother and Child Hospital | 32 (29.4) | |
| I prefer not to answer | 5 (4.6) | |
| Medical specialty | Internal medicine | 21 (19.3) |
| Non-internal medicine | 57 (52.3) | |
| Not yet specialized (trainee) | 27 (24.8) | |
| I prefer not to answer | 4 (3.7) | |
| Sex | Female | 67 (61.5) |
| Male | 42 (38.5) | |
| Age | <33 years | 57 (51.4) |
| ≥33 years | 51 (46.8) | |
| I prefer not to answer | 1 (0.9) | |
| Medical group | Consultant | 82 (75.2) |
| Trainee | 27 (24.8) | |
| Clinical experience | <8 years | 55 (50.5) |
| ≥8 years | 50 (45.9) | |
| Postgraduate qualifications | Yes | 66 (60.6) |
| No | 41 (37.6) | |
| I prefer not to answer | 2 (1.8) | |
| Involvement in medical research | Yes | 59 (54.1) |
| No | 50 (45.9) | |
| CVD stratification in clinical practice | Yes | 41 (37.6) |
| Sometimes | 31 (28.4) | |
| No | 36 (33.0) | |
| I prefer not to answer | 1 (0.9) | |
| CVD stratification using a risk score | Yes | 25 (34.7) |
| (n = 72) | Sometimes | 19 (26.4) |
| No | 28 (38.9) | |
| Genetics training | Yes | 17 (15.6) |
| No | 91 (83.5) | |
| I prefer not to answer | 1 (0.9) | |
| Genetics testing in clinical practice | Yes | 31 (28.4) |
| No | 78 (71.6) | |
| Conditions genetics testing discussed * (n = 31) | Monogenic disorders | 30 (96.8) |
| Cancers | 16 (51.6) | |
| CVD | 6 (19.4) | |
| Nutrigenomics | 1 (3.2) | |
| Pharmacogenomics | 2 (6.5) | |
| Genetic testing frequency (n = 31) | 1 patient every 2 to 3 months | 13 (41.9) |
| 1 patient per month | 2 (6.5) | |
| 2 to 5 patients per month | 6 (19.4) | |
| 6 to 10 patients per month | 2 (6.5) | |
| 11 to 20 patients per month | 3 (9.7) | |
| 20+ patients per month | 3 (9.7) | |
| I prefer not to answer | 2 (6.5) |
| Variable | Mean (SD) Knowledge Score (n = 107) | p Value | Mean (SD) Confidence Score (n = 104) | p Value | Mean (SD) Perception Score (n = 106) | p Value |
|---|---|---|---|---|---|---|
| Medical specialty | ||||||
| Internal medicine | 1.67 (0.67) | 2.70 (0.94) | 2.95 (0.64) | 0.052 | ||
| Non-internal medicine | 1.47 (0.62) | 0.310 | 2.12 (1.09) | 0.098 | 2.40 (0.98) | |
| Not yet specialized | 1.66 (0.62) | 2.30 (0.92) | 2.58 (0.85) | |||
| Sex | ||||||
| Female | 1.59 (0.67) | 0.682 | 2.41 (0.98) | 0.404 | 2.45 (1.11) | 0.632 |
| Male | 1.54 (0.61) | 2.26 (1.08) | 2.64 (0.74) | |||
| Medical group | ||||||
| Clinician | 1.52 (0.64) | 0.318 | 2.26 (1.08) | 0.933 | 2.54 (0.93) | 0.969 |
| Trainee | 1.66 (0.62) | 2.30 (0.92) | 2.58 (0.85) | |||
| Practice level | ||||||
| Specialist | 1.61 (0.66) | 0.470 | 2.25 (1.27) | 0.897 | 2.48 (0.90) | 0.526 |
| Registrar | 1.41 (0.65) | 2.32 (0.81) | 2.71 (1.05) | |||
| Medical officer | 1.48 (0.53) | 2.56 (0.86) | 2.60 (0.82) | |||
| Intern | 1.66 (0.62) | 2.30 (0.92) | 2.58 (0.85) | |||
| Clinical experience | ||||||
| <8 years | 1.59 (0.60) | 0.580 | 2.32 (0.84) | 0.651 | 2.59 (0.86) | 0.945 |
| ≥8 years | 1.53 (0.67) | 2.31 (1.23) | 2.55 (0.95) | |||
| Postgrad. qualifications | ||||||
| Yes | 1.62 (0.68) | 0.195 | 2.32 (1.17) | 0.490 | 2.62 (0.890) | 0.317 |
| No | 1.45 (0.53) | 2.27 (0.78) | 2.48 (0.93) | |||
| Medical research involvement | ||||||
| Yes | 1.71 (0.61) | 0.007 ** | 2.57 (0.96) | 0.007 ** | 2.69 (0.87) | 0.249 |
| No | 1.38 (0.61) | 2.01 (1.06) | 2.43 (0.92) | |||
| Genetics training | ||||||
| Yes | 2.26 (0.62) | 3.518 × 10−5 *** | 2.46 (0.86) | 0.720 | 2.96 (0.61) | 0.146 |
| No | 1.42 (0.54) | 2.29 (1.17) | 2.50 (0.93) | |||
| CVD screening | ||||||
| Yes | 1.75 (0.59) | 0.074 | 2.65 (1.10) | 0.010 * | 2.89 (0.63) | 4.162 × 10−4 *** |
| No | 1.46 (0.63) | 1.93 (0.96) | 2.11 (1.03) | |||
| Sometimes | 1.45 (0.65) | 2.33 (0.94) | 2.65 (0.88) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamp, M.; Pain, O.; May, A.; Lewis, C.M.; Ramsay, M. Clinicians’ Perceptions towards Precision Medicine Tools for Cardiovascular Disease Risk Stratification in South Africa. J. Pers. Med. 2022, 12, 1360. https://doi.org/10.3390/jpm12091360
Kamp M, Pain O, May A, Lewis CM, Ramsay M. Clinicians’ Perceptions towards Precision Medicine Tools for Cardiovascular Disease Risk Stratification in South Africa. Journal of Personalized Medicine. 2022; 12(9):1360. https://doi.org/10.3390/jpm12091360
Chicago/Turabian StyleKamp, Michelle, Oliver Pain, Andrew May, Cathryn M. Lewis, and Michèle Ramsay. 2022. "Clinicians’ Perceptions towards Precision Medicine Tools for Cardiovascular Disease Risk Stratification in South Africa" Journal of Personalized Medicine 12, no. 9: 1360. https://doi.org/10.3390/jpm12091360
APA StyleKamp, M., Pain, O., May, A., Lewis, C. M., & Ramsay, M. (2022). Clinicians’ Perceptions towards Precision Medicine Tools for Cardiovascular Disease Risk Stratification in South Africa. Journal of Personalized Medicine, 12(9), 1360. https://doi.org/10.3390/jpm12091360







