A Contemporary Approach to Non-Invasive 3D Determination of Individual Masticatory Muscle Forces: A Proof of Concept
Abstract
:1. Introduction
2. Materials and Methods
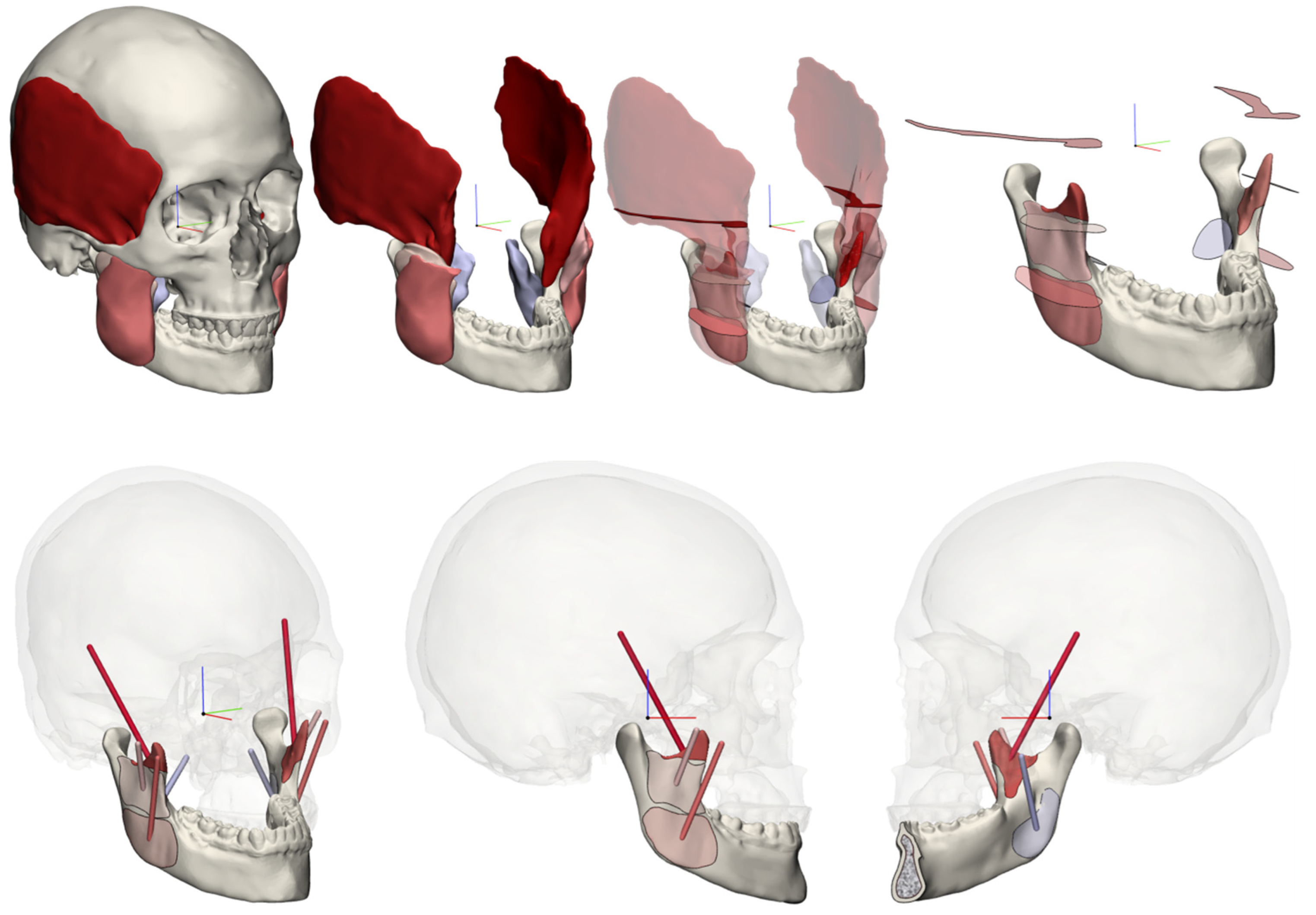
2.1. 3D Muscular Model
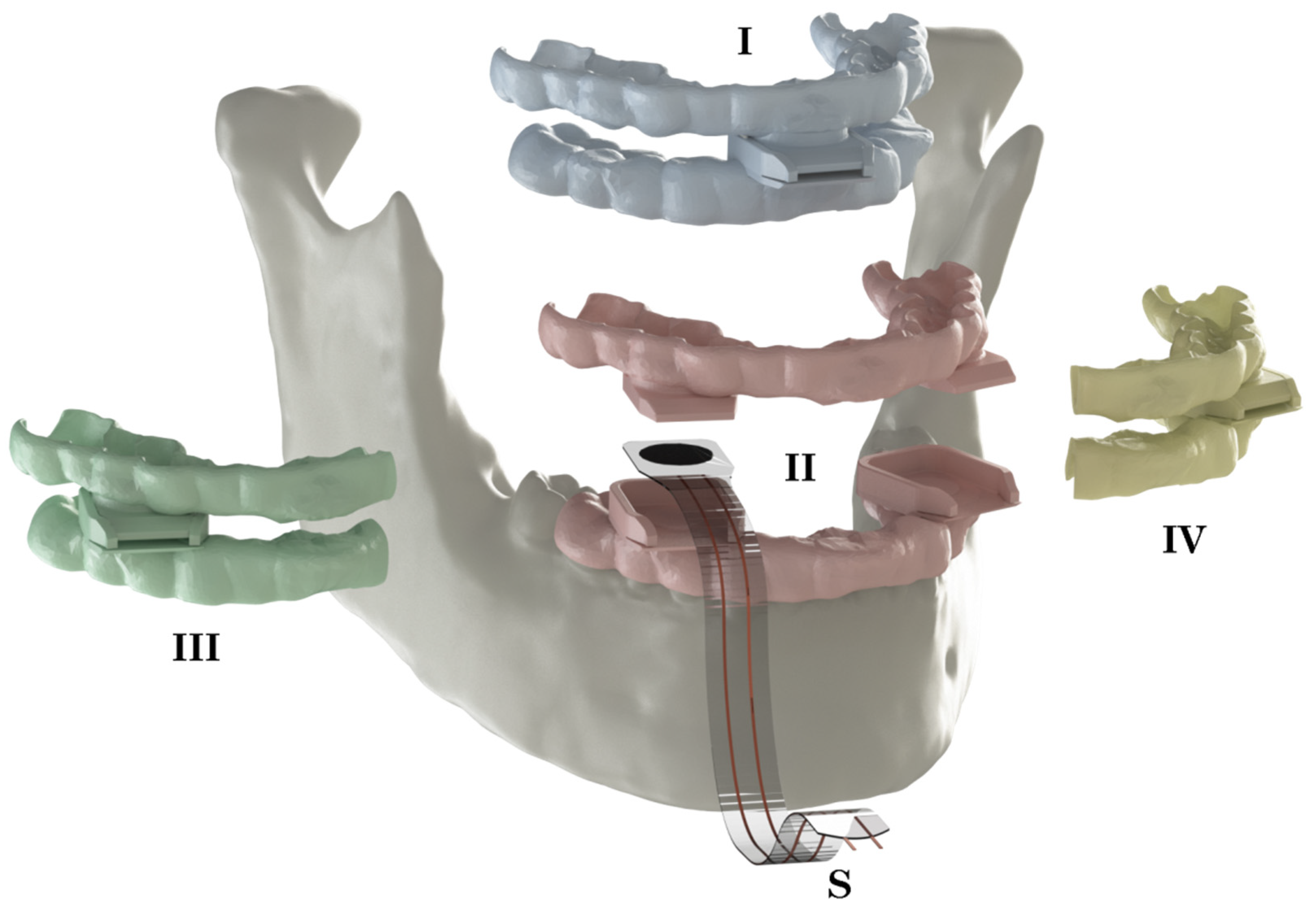
2.2. Bite Force Measurements
2.3. Finite Element Model
- (1)
- Use the subject’s muscle CSA and calculate the muscle forces with the intrinsic strength (P) value of 37 [N/cm], as suggested by Weijs et al. [19], and analyse the resulting bite forces.
- (2)
- Use the subject’s muscle CSA and matching measured bite forces and calculate the patient-specific intrinsic strength value.
2.4. Pre-Processing/Model Preparation
2.5. Subjects
3. Results
3.1. Muscular Model
| P = 37 [N/cm²] | P = 40.6 [N/cm²] | ||||||||||
| Muscle | Laterality | CSA [cm²] | ∑ Force [N] | Force Components [N] | ∑ Force [N] | Force Components [N] | |||||
| Subject 1, 29 y.o. | x | y | z | x | y | z | |||||
| Masseter superficialis | Right | 4.64 | 171.76 | 53.22 | 24.07 | 161.52 | 188.27 | 58.34 | 26.39 | 177.05 | |
| Left | 3.97 | 146.89 | 26.65 | 32.16 | 140.83 | 161.01 | 29.21 | 35.26 | 154.37 | ||
| Masseter profunda | Right | 3.14 | 116.31 | 14.70 | 33.41 | 110.44 | 127.49 | 16.11 | 36.62 | 121.05 | |
| Left | 2.57 | 95.07 | 6.12 | 30.67 | 89.78 | 104.21 | 6.71 | 33.62 | 98.42 | ||
| Pterygoideus medialis | Right | 3.34 | 123.53 | 7.04 | 57.22 | 109.25 | 135.40 | 7.71 | 62.72 | 119.75 | |
| Left | 3.40 | 125.71 | 11.28 | 61.27 | 109.19 | 137.80 | 12.37 | 67.16 | 119.69 | ||
| Temporalis | Right | 7.49 | 277.18 | 139.94 | 55.13 | 232.82 | 303.83 | 153.40 | 60.43 | 255.20 | |
| Left | 6.34 | 234.64 | 113.21 | 47.02 | 200.07 | 257.20 | 124.10 | 51.54 | 219.30 | ||
| P = 37 [N/cm²] | P = 25.6 [N/cm²] | ||||||||||
| Subject 2, 56 y.o. | Masseter superficialis | Right | 5.17 | 191.15 | 57.11 | 33.41 | 179.33 | 126.83 | 37.89 | 22.17 | 118.99 |
| Left | 5.76 | 213.30 | 67.44 | 49.60 | 196.18 | 141.52 | 44.75 | 32.91 | 130.17 | ||
| Masseter profunda | Right | 2.78 | 102.81 | 17.79 | 31.55 | 96.22 | 68.21 | 11.80 | 20.93 | 63.84 | |
| Left | 2.77 | 102.48 | 14.36 | 39.58 | 93.43 | 67.99 | 9.53 | 26.26 | 61.99 | ||
| Pterygoideus medialis | Right | 4.02 | 148.91 | 40.65 | 67.95 | 126.12 | 98.80 | 26.97 | 45.08 | 83.68 | |
| Left | 3.93 | 145.27 | 35.12 | 60.36 | 127.39 | 96.39 | 23.30 | 40.05 | 84.52 | ||
| Temporalis | Right | 6.55 | 242.46 | 82.58 | 53.03 | 221.71 | 160.87 | 54.79 | 35.19 | 147.10 | |
| Left | 7.72 | 285.46 | 105.95 | 67.88 | 256.23 | 189.40 | 70.30 | 45.04 | 170.01 | ||
3.2. Bite Force Experiments
3.3. Finite Element Analyses
3.4. Maximum Mandibular Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraeima, J.; Merema, B.J.; Witjes, M.J.H.; Spijkervet, F.K.L. Development of a patient-specific temporomandibular joint prosthesis according to the Groningen principle through a cadaver test series. J. Cranio-Maxillofac. Surg. 2018, 46, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Merema, B.B.J.; Kraeima, J.; Visscher, S.A.H.J.; Minnen, B.; Spijkervet, F.K.L.; Schepman, K.; Witjes, M.J.H. Novel finite element-based plate design for bridging mandibular defects: Reducing mechanical failure. Oral Dis. 2020, 26, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Kraeima, J.; Glas, H.H.; Merema, B.B.J.; Vissink, A.; Spijkervet, F.K.L.; Witjes, M.J.H. Three-dimensional virtual surgical planning in the oncologic treatment of the mandible. Oral Dis. 2021, 27, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.M.; Garcia-Aznar, J.M.; Dominguez, J.; Doblare, M. Numerical estimation of bone density and elastic constants distribution in a human mandible. J. Biomech. 2007, 40, 828–836. [Google Scholar] [CrossRef]
- Narra, N.; Valášek, J.; Hannula, M.; Marcián, P.; Sándor, G.K.; Hyttinen, J.; Wolff, J. Finite element analysis of customized reconstruction plates for mandibular continuity defect therapy. J. Biomech. 2014, 47, 264–268. [Google Scholar] [CrossRef]
- Pinheiro, M.; Willaert, R.; Khan, A.; Krairi, A.; Van Paepegem, W. Biomechanical evaluation of the human mandible after temporomandibular joint replacement under different biting conditions. Sci. Rep. 2021, 11, 14034. [Google Scholar] [CrossRef]
- Oenning, A.C.; Freire, A.R.; Rossi, A.C.; Prado, F.B.; Caria, P.H.F.; Correr-Sobrinho, L.; Haiter-Neto, F. Resorptive potential of impacted mandibular third molars: 3D simulation by finite element analysis. Clin. Oral Investig. 2018, 22, 3195–3203. [Google Scholar] [CrossRef]
- Merema, B.B.J.; Kraeima, J.; Glas, H.H.; Spijkervet, F.K.L.; Witjes, M.J.H. Patient-specific finite element models of the human mandible: Lack of consensus on current set-ups. Oral Dis. 2021, 27, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Weijs, W.A.; Hillen, B. Correlations between the cross-sectional area of the jaw muscles and craniofacial size and shape. Am. J. Phys. Anthropol. 1986, 70, 423–431. [Google Scholar] [CrossRef]
- Abdi, A.H.; Sagl, B.; Srungarapu, V.P.; Stavness, I.; Prisman, E.; Abolmaesumi, P.; Fels, S. Characterizing Motor Control of Mastication With Soft Actor-Critic. Front. Hum. Neurosci. 2020, 14, 188. [Google Scholar] [CrossRef]
- Wood, W.W.; Takada, K.; Hannam, A.G. The electromyographic activity of the inferior part of the human lateral pterygoid muscle during clenching and chewing. Arch. Oral Biol. 1986, 31, 245–253. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Indino, B.; Graven-Nielsen, T. Surface EMG crosstalk evaluated from experimental recordings and simulated signals. Reflections on crosstalk interpretation, quantification and reduction. Methods Inf. Med. 2004, 43, 30–35. [Google Scholar] [PubMed]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, E. Muskelbewegung. In Wagner, Handwӧrterbuch der Physiologie; Bieweg: Braunschweig, Germany, 1846; pp. 1–122. [Google Scholar]
- Nygaard, E.; Houston, M.; Suzuki, Y.; Jorgensen, K.; Saltin, B. Morphology of the brachial biceps muscle and elbow flexion in man. Acta Physiol. Scand. 1983, 117, 287–292. [Google Scholar] [CrossRef]
- Morris, C.B. The measurement of the strength of muscle relative to the cross section. Res. Q. 1948, 19, 295–303. [Google Scholar] [CrossRef]
- Haxton, H.A. Absolute muscle force in the ankle flexors of man. J. Physiol. 1944, 103, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Franke, F. Die Kraftkurve menschlicher Muskeln bei willkiirlicher Innerration und die Frage der absoluten Muskelkraft. Pflüg. Arch. Ges. Physiol. 1920, 184, 300–323. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Cross-sectional areas and estimated intrinsic strength of the human jaw muscles. Acta Morphol. Neerl. Scand. 1985, 23, 267–274. [Google Scholar]
- Toro-Ibacache, V.; Zapata Munoz, V.; O’Higgins, P. The relationship between skull morphology, masticatory muscle force and cranial skeletal deformation during biting. Ann. Anat. 2016, 203, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Koolstra, J.H.; van Eijden, T.M.G.J.; Weijs, W.A.; Naeije, M. A three-dimensional mathematical model of the human masticatory system predicting maximum possible bite forces. J. Biomech. 1988, 21, 563–576. [Google Scholar] [CrossRef]
- Peck, C.C.; Langenbach, G.E.; Hannam, A.G. Dynamic simulation of muscle and articular properties during human wide jaw opening. Arch. Oral Biol. 2000, 45, 963–982. [Google Scholar] [CrossRef]
- Hannam, A.G.; Stavness, I.; Lloyd, J.E.; Fels, S. A dynamic model of jaw and hyoid biomechanics during chewing. J. Biomech. 2008, 41, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Sagl, B.; Schmid-Schwap, M.; Piehslinger, E.; Kundi, M.; Stavness, I. A Dynamic Jaw Model With a Finite-Element Temporomandibular Joint. Front. Physiol. 2019, 10, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannam, A.G.; Wood, W.W. Relationships between the size and spatial morphology of human masseter and medial pterygoid muscles, the craniofacial skeleton, and jaw biomechanics. Am. J. Phys. Anthropol. 1989, 80, 429–445. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, P.H.; Weijs, W.A.; Valk, J.; Prahl-Andersen, B.; van Ginkel, F.C. A comparison of jaw muscle cross-sections of long-face and normal adults. J. Dent. Res. 1992, 71, 1279–1285. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Relationship between the physiological cross-section of the human jaw muscles and their cross-sectional area in computer tomograms. Acta Anat. 1984, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instrument in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Mackenna, B.R.; Turker, K.S. Jaw separation and maximum incising force. J. Prosthet. Dent. 1983, 49, 726–730. [Google Scholar] [CrossRef]
- Fields, H.W.; Proffit, W.R.; Case, J.C.; Vig, K.W. Variables affecting measurements of vertical occlusal force. J. Dent. Res. 1986, 65, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Manns, A.; Miralles, R.; Palazzi, C. EMG, bite force, and elongation of the masseter muscle under isometric voluntary contractions and variations of vertical dimension. J. Prosthet. Dent. 1979, 42, 674–682. [Google Scholar] [CrossRef]
- van Steenberghe, D.; de Vries, J.H. The influence of local anaesthesia and occlusal surface area on the forces developed during repetitive maximal clenching efforts. J. Periodontal. Res. 1978, 13, 270–274. [Google Scholar] [CrossRef] [PubMed]
- van der Bilt, A.; Tekamp, A.; van der Glas, H.; Abbink, J. Bite force and electromyograpy during maximum unilateral and bilateral clenching. Eur. J. Oral Sci. 2008, 116, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Draf, W.; Lang, J. Surgery of the Skull Base; Springer: Berlin/Heidelberg, Germany, 1989; pp. 41–42. [Google Scholar] [CrossRef]
- Ramos, A.; Mesnard, M. A new condyle implant design concept for an alloplastic temporomandibular joint in bone resorption cases. J. Cranio-Maxillofac. Surg. 2016, 44, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; del Pozo, R.; Tanaka, M.; Asai, D.; Hirose, M.; Iwabe, T.; Tanne, K. Three-dimensional finite element analysis of human temporomandibular joint with and without disc displacement during jaw opening. Med. Eng. Phys. 2004, 26, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Toro-Ibacache, V.; Zapata MuNoz, V.; O’Higgins, P. The Predictability from Skull Morphology of Temporalis and Masseter Muscle Cross-Sectional Areas in Humans. Anat. Rec. 2015, 298, 1261–1270. [Google Scholar] [CrossRef]
- Langenbach, G.E.; Hannam, A.G. The role of passive muscle tensions in a three-dimensional dynamic model of the human jaw. Arch. Oral Biol. 1999, 44, 557–573. [Google Scholar] [CrossRef]
- Koolstra, J.H.; van Eijden, T.M.G.J.; Weijs, W.A. Three-Dimensional performance of the human masticatory system: The influence of the orientation and physiological cross-section of the masticatory muscles. In International Series on Biomechanics 7-A; Free University Press: Amsterdam, The Netherlands, 1988; pp. 101–106. [Google Scholar]
- Tortopidis, D.; Lyons, M.F.; Baxendale, R.H.; Gilmour, W.H. The variability of bite force measurement between sessions, in different positions within the dental arch. J. Oral Rehabil. 1998, 25, 681–686. [Google Scholar] [CrossRef]
- Bakke, M.; Michler, L.; Han, K.; Moller, E. Clinical significance of isometric bite force versus electrical activity in temporal and masseter muscles. Scand. J. Dent. Res. 1989, 97, 539–551. [Google Scholar] [CrossRef]
- Hagberg, C. Assessment of bite force: A review. J. Cranio-Maxillofac. Disord. 1987, 1, 162–169. [Google Scholar]
- Bakke, M. Mandibular elevator muscles: Physiology, action, and effect of dental occlusion. Scand. J. Dent. Res. 1993, 101, 314–331. [Google Scholar] [CrossRef]
- van Loon, J.P.; Otten, E.; Falkenstrom, C.H.; de Bont, L.G.; Verkerke, G.J. Loading of a unilateral temporomandibular joint prosthesis: A three-dimensional mathematical study. J. Dent. Res. 1998, 77, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Orchardson, R.; MacFarlane, S.H. The effect of local periodontal anaesthesia on the maximum biting force achieved by human subjects. Arch. Oral Biol. 1980, 25, 799–804. [Google Scholar] [CrossRef]
- Fintova, S.; Dlhý, P.; Mertová, K.; Chlup, Z.; Duchek, M.; Procházka, R.; Hutař, P. Fatigue properties of UFG Ti grade 2 dental implant vs. conventionally tested smooth specimens. J. Mech. Behav. Biomed. Mater. 2021, 123, 104715. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.P.; Verkerke, G.J.; de Vries, M.P.; de Bont, L.G. Design and wear testing of a temporomandibular joint prosthesis articulation. J. Dent. Res. 2000, 79, 715–721. [Google Scholar] [CrossRef] [PubMed]

| Subject 1, 29 y.o. | Subject 2, 56 y.o. | |||||
| Muscle | CSA [cm2] | |||||
| Right | Left | Mean | Right | Left | Mean | |
| Masseter superficialis | 4.64 | 3.97 | 4.31 | 5.17 | 5.76 | 5.47 |
| Masseter profunda | 3.14 | 2.57 | 2.86 | 2.78 | 2.77 | 2.77 |
| Pterygoideus medialis | 3.34 | 3.40 | 3.37 | 4.02 | 3.93 | 3.98 |
| Temporalis | 7.49 | 6.34 | 6.92 | 6.55 | 7.72 | 7.13 |
| Subject 1, 29 y.o. | ||||||||
| Premolar Laterality | Condyle | |||||||
| Bite Position | ∑ F. Bite | Right | Left | Incisal | Right | Left | ||
| In-vivo | ||||||||
| Bilat. premolar | 474 | 256 | 218 | - | - | - | ||
| Premolar R | 318 | 318 | - | - | - | - | ||
| Premolar L | 345 | - | 345 | - | - | - | ||
| Incisal | 189 | - | - | 189 | - | - | ||
| P = 37 [N/cm²] | In-silico | |||||||
| Bilat. premolar | 432 | 241 | 181 | - | 392 | 330 | ||
| Premolar R | 426 | 426 | - | - | 326 | 402 | ||
| Premolar L | 425 | - | 425 | - | 482 | 247 | ||
| Incisal | 339 | - | - | 339 | 445 | 370 | ||
| P = 40.6 [N/cm²] | Bilat. premolar | 474 (0%) | 264 (+3%) | 210 (−4%) | - | 429 | 361 | |
| Premolar R | 467 | 467 | - | - | 357 | 440 | ||
| Premolar L | 466 | - | 466 | - | 528 | 270 | ||
| Incisal | 371 | - | - | 371 | 488 | 405 | ||
| Subject 2, 56 y.o. | ||||||||
| Premolar Laterality | Condyle | |||||||
| Bite Position | ∑ F.Bite | Right | Left | Incisal | Right | Left | ||
| In-vivo | ||||||||
| Bilat. premolar | 342 | 195 | 147 | - | - | - | ||
| Premolar R | 197 | 197 | - | - | - | - | ||
| Premolar L | 248 | 248 | - | - | - | |||
| Incisal | 79 | - | - | 79 | - | - | ||
| P = 37 [N/cm²] | In-silico | |||||||
| Bilat. premolar | 520 | 257 | 263 | - | 360 | 416 | ||
| Premolar R | 502 | 502 | - | - | 280 | 515 | ||
| Premolar L | 510 | - | 510 | - | 453 | 333 | ||
| Incisal | 409 | - | - | 409 | 415 | 473 | ||
| P = 25.6 [N/cm²] | Bilat. premolar | 342 (0%) | 168 (−14%) | 174 (+18%) | - | 241 | 276 | |
| Premolar R | 333 | 333 | - | - | 186 | 341 | ||
| Premolar L | 338 | - | 338 | - | 301 | 222 | ||
| Incisal | 271 | - | - | 271 | 275 | 314 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merema, B.B.J.; Sieswerda, J.J.; Spijkervet, F.K.L.; Kraeima, J.; Witjes, M.J.H. A Contemporary Approach to Non-Invasive 3D Determination of Individual Masticatory Muscle Forces: A Proof of Concept. J. Pers. Med. 2022, 12, 1273. https://doi.org/10.3390/jpm12081273
Merema BBJ, Sieswerda JJ, Spijkervet FKL, Kraeima J, Witjes MJH. A Contemporary Approach to Non-Invasive 3D Determination of Individual Masticatory Muscle Forces: A Proof of Concept. Journal of Personalized Medicine. 2022; 12(8):1273. https://doi.org/10.3390/jpm12081273
Chicago/Turabian StyleMerema, Bram B. J., Jelbrich J. Sieswerda, Frederik K. L. Spijkervet, Joep Kraeima, and Max J. H. Witjes. 2022. "A Contemporary Approach to Non-Invasive 3D Determination of Individual Masticatory Muscle Forces: A Proof of Concept" Journal of Personalized Medicine 12, no. 8: 1273. https://doi.org/10.3390/jpm12081273
APA StyleMerema, B. B. J., Sieswerda, J. J., Spijkervet, F. K. L., Kraeima, J., & Witjes, M. J. H. (2022). A Contemporary Approach to Non-Invasive 3D Determination of Individual Masticatory Muscle Forces: A Proof of Concept. Journal of Personalized Medicine, 12(8), 1273. https://doi.org/10.3390/jpm12081273







