Adipokine Levels in Men with Coronary Atherosclerosis on the Background of Abdominal Obesity
Abstract
:1. Introduction
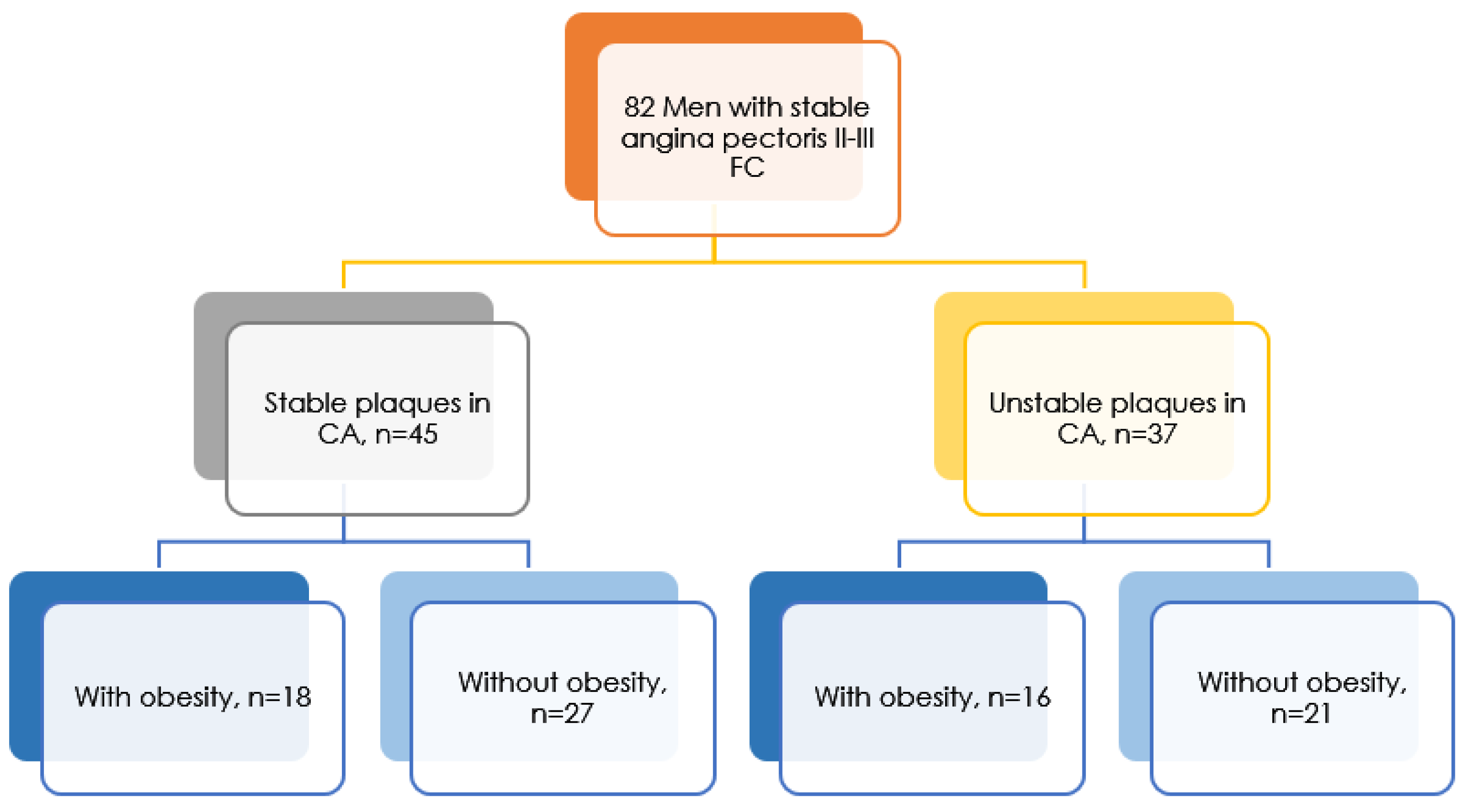
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Martinchik, A.N.; Laikam, K.E.; Kozyreva, N.A.; Keshabyants, E.E.; Mikhailov, N.A.; Baturin, A.K.; Smirnova, E.A. The prevalence of obesity in various socio-demographic groups of the population of Russia. Vopr. Pitan. 2021, 90, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Alferova, V.I.; Mustafina, S.V. The prevalence of obesity in the adult population of the Russian Federation (literature review). Obes. Metab. 2022, 19, 96–105. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Ambeba, E.J.; Styn, M.A.; Kuller, L.H.; Brooks, M.M.; Evans, R.W.; Burke, L.E. Longitudinal effects of weight loss and regain on cytokine concentration of obese adults. Metabolism 2013, 62, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Marx, N.; Walcher, D.; Raichle, C.; Aleksic, M.; Bach, H.; Grüb, M.; Hombach, V.; Libby, P.; Zieske, A.; Homma, S.; et al. C-Peptide Colocalizes with Macrophages in Early Arteriosclerotic Lesions of Diabetic Subjects and Induces Monocyte Chemotaxis In Vitro. Arter. Thromb. Vasc. Biol. 2004, 24, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kubota, N.; Kadowaki, T. The role of endothelial insulin signaling in the regulation of glucose metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Serruys, P.W.; Schaar, J. Handbook of the Vulnerable Plaque, 2nd ed.; CRC Press: London, UK, 2007; ISBN 978-1841846217. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardio-vascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Alves, M.T.; Ortiz, M.M.O.; dos Reis, G.V.O.P.; Dusse, L.M.S.A.; das Carvalho, M.G.; Fernandes, A.P.; Gomes, K.B. The dual effect of C-peptide on cellular activation and atherosclerosis: Protective or not? Diabetes Metab. Res. Rev. 2019, 35, e3071. [Google Scholar] [CrossRef] [Green Version]
- Wahren, J.; Larsson, C. C-peptide: New findings and therapeutic possibilities. Diabetes Res. Clin. Pr. 2015, 107, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Kunt, T.; Forst, T.; Closs, E.I.; Lehmann, R.; Flohr, T.; Gabriel, M.; Schäfer, D.; Göpfert, A.; Pfützner, A.; et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 2003, 9, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Haidet, J.; Cifarelli, V.; Trucco, M.; Luppi, P. C-peptide reduces pro-inflammatory cytokine secretion in LPS-stimulated U937 monocytes in condition of hyperglycemia. Inflamm. Res. 2012, 61, 27–35. [Google Scholar] [CrossRef]
- Walcher, D.; Babiak, C.; Poletek, P.; Rosenkranz, S.; Bach, H.; Betz, S.; Durst, R.; Grüb, M.; Hombach, V.; Strong, J.; et al. C-Peptide Induces Vascular Smooth Muscle Cell Proliferation: Involvement of Src-Kinase, Phosphatidylinositol 3-Kinase, and Extracellular Signal-Regulated Kinase 1/2. Circ. Res. 2006, 99, 1181–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, R.L.M.; Gigante, D.P.; De Oliveira, I.O.; Horta, B.L. C-Peptide and cardiovascular risk factors among young adults in a southern Brazilian cohort. BMC Endocr. Disord. 2018, 18, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Meng, L.; Li, Y.; Sato, Y. Associations of Serum C-Peptide Level with Body Fat Distribution and Ever Stroke in Nondiabetic Subjects. J. Stroke Cerebrovasc. Dis. 2014, 23, e163–e169. [Google Scholar] [CrossRef]
- McKellar, G.E.; McCarey, D.W.; Sattar, N.; McInnes, I.B. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 2009, 6, 410–417. [Google Scholar] [CrossRef]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin. Sci. 2005, 109, 143–159. [Google Scholar] [CrossRef]
- Virdis, A.; Colucci, R.; Bernardini, N.; Blandizzi, C.; Taddei, S.; Masi, S. Microvascular Endothelial Dysfunction in Human Obesity: Role of TNF-α. J. Clin. Endocrinol. Metab. 2019, 104, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J.S. Adipose tissue, insulin action and vascular disease: Inflammatory signals. Int. J. Obes. 2003, 27, S25–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and Haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schuett, H.; Luchtefeld, M.; Grothusen, C.; Grote, K.; Schieffer, B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 2009, 102, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Hartman, J.; Frishman, W.H. Inflammation and Atherosclerosis: A Review of the Role of Interleukin-6 in the Development of Atherosclerosis and the Potential for Targeted Drug Therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Woodward, M.; Samber, D.; Bucerius, J.; Tawakol, A.; Kallend, D.; Rudd, J.H.F.; Abt, M.; Fayad, Z.A. Predictors of change in carotid atherosclerotic plaque inflammation and burden as measured by 18-FDG-PET and MRI, respectively, in the dal-PLAQUE study. Int. J. Cardiovasc. Imaging 2014, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Sakaguchi, M.; Miwa, K.; Furukado, S.; Yamagami, H.; Yagita, Y.; Mochizuki, H.; Kitagawa, K. Association of Interleukin-6 With the Progression of Carotid Atherosclerosis: A 9-Year Follow-up Study. Stroke 2014, 45, 2924–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Zhao, T.; Wang, L.; Huang, X.; Jiao, J.; Gao, D.; Zhang, H.; Shen, T.; Man, Y.; Wang, S.; et al. miR-200s Contribute to Interleukin-6 (IL-6)-induced Insulin Resistance in Hepatocytes. J. Biol. Chem. 2013, 288, 22596–22606. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wen, J.G.; Feng, J.J.; Wang, Y.H.; Li, T.F.; Nurmi, K.; Eklund, K.K.; Xing, D. Forskolin attenuates the NLRP3 inflammasome activation and IL-1β secretion in human macrophages. Pediatr. Res. 2019, 86, 692–698. [Google Scholar] [CrossRef]
- Osaka, N.; Kushima, H.; Mori, Y.; Saito, T.; Hiromura, M.; Terasaki, M.; Yashima, H.; Ohara, M.; Fukui, T.; Matsui, T.; et al. Anti-inflammatory and atheroprotective properties of glucagon. Diabetes Vasc. Dis. Res. 2020, 17. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Maezono, K.; Osman, A.; Pendergrass, M.; Patti, M.E.; Pratipanawatr, T.; DeFronzo, R.A.; Kahn, C.R.; Mandarino, L.J. Insulin resistance differentially affects the PI 3-kinase– and MAP kinase–mediated signaling in human muscle. J. Clin. Investig. 2000, 105, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younk, L.M.; Lamos, E.M.; Davis, S.N. The cardiovascular effects of insulin. Expert Opin. Drug Saf. 2014, 13, 955–966. [Google Scholar] [CrossRef] [PubMed]

| Parameter | All Patients n = 82 | Patients with Stable Plaques in the Coronary Arteries | p | Patients with Unstable Plaques in the Coronary Arteries | p | ||
|---|---|---|---|---|---|---|---|
| The Presence of Obesity n = 18 | Absence of Obesity n = 27 | The Presence of Obesity n = 16 | Absence of Obesity n = 21 | ||||
| Average age (M ± SD) | 60.74 ± 7.16 | 62.20 ± 8.87 | 59.68 ± 5.41 | 0.530 | 59.13 ± 5.61 | 61.86 ± 8.20 | 0.182 |
| BMI, kg/m2 (M ± SD) | 29.18 ± 4.18 | 30.78 ± 3.85 | 27.27 ± 4.33 | <0.001 | 32.99 ± 2.49 | 27.01 ± 2.45 | <0.001 |
| WC, cm (M ± SD) | 92.83 ± 9.90 | 101.00 ± 8.16 | 85.05 ± 5.17 | 0.031 | 96.07 ± 10.00 | 91.58 ± 8.81 | 0.079 |
| SBP, mmHg (M ± SD) | 134.52 ± 13.36 | 130.90 ± 14.14 | 135.36 ± 14.65 | 0.972 | 137.88 ± 14.06 | 134.43 ± 10.19 | 0.397 |
| DBP, mmHg (M ± SD) | 82.09 ± 8.82 | 81.3 ± 8.44 | 81.68 ± 9.51 | 0.456 | 84.00 ± 9.00 | 81.86 ± 8.63 | 0.634 |
| Smoking status (absolute in %) | 62 (75.7%) | 72.2% | 66.7% | 0.753 | 75.0% | 95.2% | 0.144 |
| DM 2 type (absolute in %) | 17 (20.7%) | 11.1% | 18.5% | 0.684 | 37.5% | 19.0% | 0.274 |
| Parameter | Patients with Stable Plaques with Obesity n = 18 | Patients with Stable Plaques without Obesity n = 27 | p | Patients with Unstable Plaques with Obesity n = 16 | Patients with Unstable Plaques without Obesity n = 21 | p |
|---|---|---|---|---|---|---|
| C-peptide | 0.69 [0.14; 1.54] | 0.74 [0.36; 1.43] | 0.379 | 1.26 [0.77; 2.27] | 0.67 [0.33; 1.73] | 0.055 |
| Glucose-dependent insulinotropic polypeptide (GIP) | 34.01 [22.20; 63.85] | 27.51 [13.15; 49.51] | 0.276 | 38.30 [31.85; 54.56] | 16.92 [11.58; 39.80] | 0.023 |
| Glucagon-like peptide-1 (GLP-1) | 679.79 [305.23; 880.71] | 411.60 [215.67; 654.78] | 0.071 | 693.56 [420.36; 1430.44] | 444.74 [334.53; 681.92] | 0.044 |
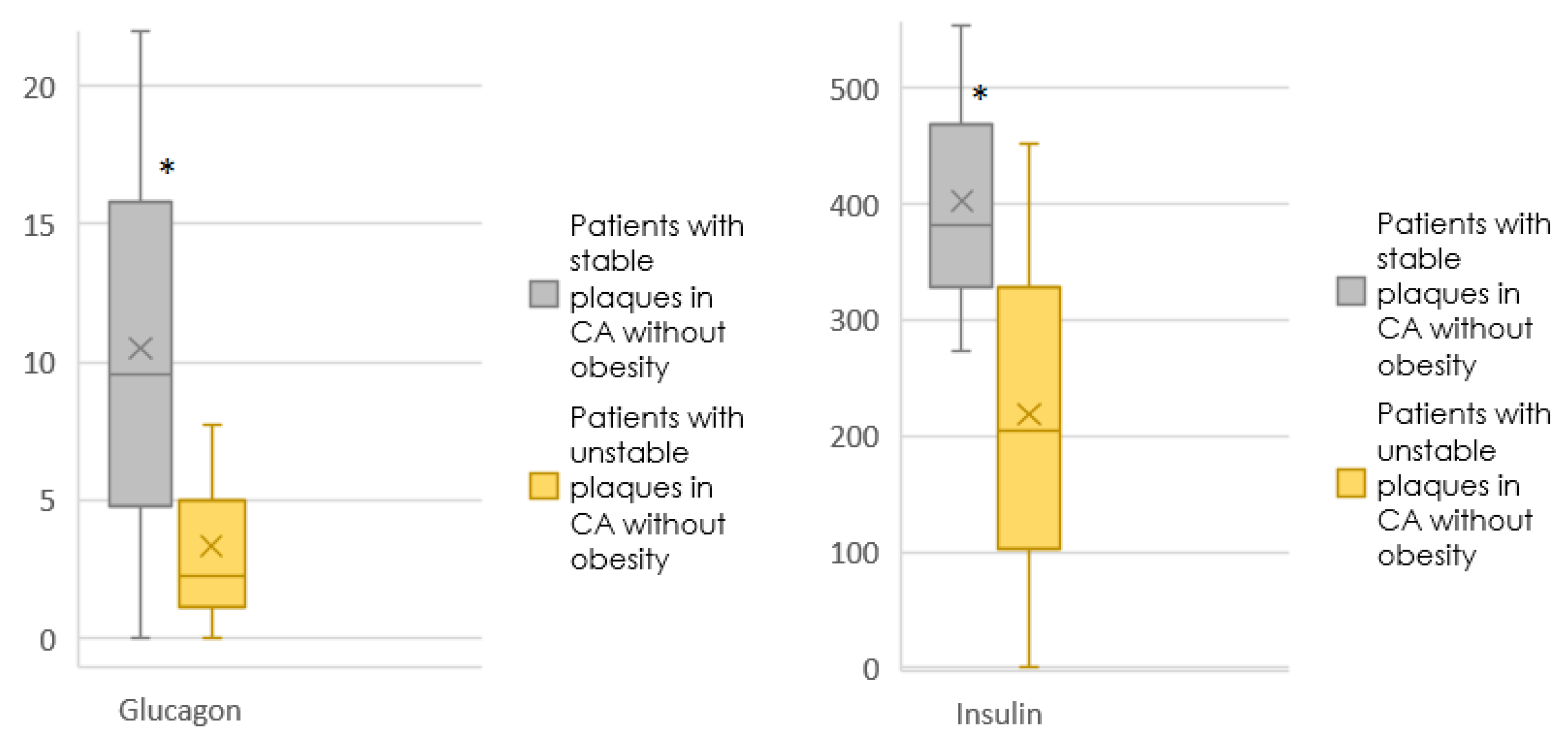
| Glucagon | 7.98 [0.00; 24.16] | 9.58 [0.001; 22.01] | 0.925 | 5.55 [0.00; 15.06] | 2.30 [0.001; 7.77] | 0.267 |
| Interleukin-6 (IL-6) | 2.86 [1.15; 7.05] | 7.01 [2.81; 13.80] | 0.095 | 7.98 [3.97; 18.20] | 8.81 [1.27; 9.92] | 0.055 |
| Insulin | 301.90 [272.95; 440.77] | 382.50 [272.95; 554.28] | 0.188 | 451.76 [272.95; 653.38] | 205.02 [0.001; 451.76] | 0.006 |
| Leptin | 6183.78 [4080.06; 12,751.17] | 5416.49 [1867.87; 8509.36] | 0.417 | 7491.86 [2167.75; 13,478.77] | 3062.16 [829.62; 6196.61] | 0.021 |
| Monocytic Chemoattractant Protein-1 (MCP-1) | 198.11 [128.49; 307.16] | 185.39 [120.45; 289.62] | 0.746 | 208.72 [160.27; 286.82] | 214.99 [155.57; 249.44] | 0.639 |
| Tumor necrosis factor α (TNFa) | 3.78 [2.43; 6.33] | 5.52 [2.69; 7.19] | 0.211 | 6.21 [4.02; 7.67] | 4.46 [2.83; 5.87] | 0.073 |
| Parameter | Model 1 | Model 2 (Age-Adjusted) |
|---|---|---|
| C-peptide, by 1 pg/mL | 1.394 (0.808–2.403), p = 0.232 | 0.994 (0.899–1.099), p = 0.906 |
| TNFa, by 1 pg/mL | 1.490 (1.034–2.149), p = 0.033 | 1.514 (1.041–2.201), p = 0.030 |
| IL-6, by 1 pg/mL | 0.996 (0.965–1.027), p = 0.784 | 0.996 (0.965–1.027), p = 0.795 |
| Glucagon, for 1 pg/mL | 0.951 (0.896–1.009), p = 0.094 | 0.951 (0.897–1.008), p = 0.092 |
| Insulin, by 1 pg/mL | 0.997 (0.994–1.000), p = 0.029 | 0.997 (0.994–1.000), p = 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Striukova, E.V.; Shramko, V.S.; Kashtanova, E.V.; Polonskaya, Y.V.; Stakhneva, E.M.; Kurguzov, A.V.; Chernyavsky, A.M.; Ragino, Y.I. Adipokine Levels in Men with Coronary Atherosclerosis on the Background of Abdominal Obesity. J. Pers. Med. 2022, 12, 1248. https://doi.org/10.3390/jpm12081248
Striukova EV, Shramko VS, Kashtanova EV, Polonskaya YV, Stakhneva EM, Kurguzov AV, Chernyavsky AM, Ragino YI. Adipokine Levels in Men with Coronary Atherosclerosis on the Background of Abdominal Obesity. Journal of Personalized Medicine. 2022; 12(8):1248. https://doi.org/10.3390/jpm12081248
Chicago/Turabian StyleStriukova, Evgeniia Vital’evna, Victoriya Sergeevna Shramko, Elena Vladimirovna Kashtanova, Yana Vladimirovna Polonskaya, Ekaterina Mikhailovna Stakhneva, Alexey Vitalievich Kurguzov, Alexander Mikhailovich Chernyavsky, and Yulia Igorevna Ragino. 2022. "Adipokine Levels in Men with Coronary Atherosclerosis on the Background of Abdominal Obesity" Journal of Personalized Medicine 12, no. 8: 1248. https://doi.org/10.3390/jpm12081248
APA StyleStriukova, E. V., Shramko, V. S., Kashtanova, E. V., Polonskaya, Y. V., Stakhneva, E. M., Kurguzov, A. V., Chernyavsky, A. M., & Ragino, Y. I. (2022). Adipokine Levels in Men with Coronary Atherosclerosis on the Background of Abdominal Obesity. Journal of Personalized Medicine, 12(8), 1248. https://doi.org/10.3390/jpm12081248







