Enhancing Anticoagulation Monitoring and Therapy in Patients Undergoing Microvascular Reconstruction in Maxillofacial Surgery: A Prospective Observational Trial
Abstract
:1. Introduction
- (1)
- Identifying patients at high risk for ATE/VTE using VET to detect hypercoagulability and/or impaired fibrinolytic capacity before and after surgery (Powered analysis);
- (2)
- Exploratory evaluation of POC testing for the management of anticoagulant and—if applicable—antiplatelet treatment regimens (Pilot study).
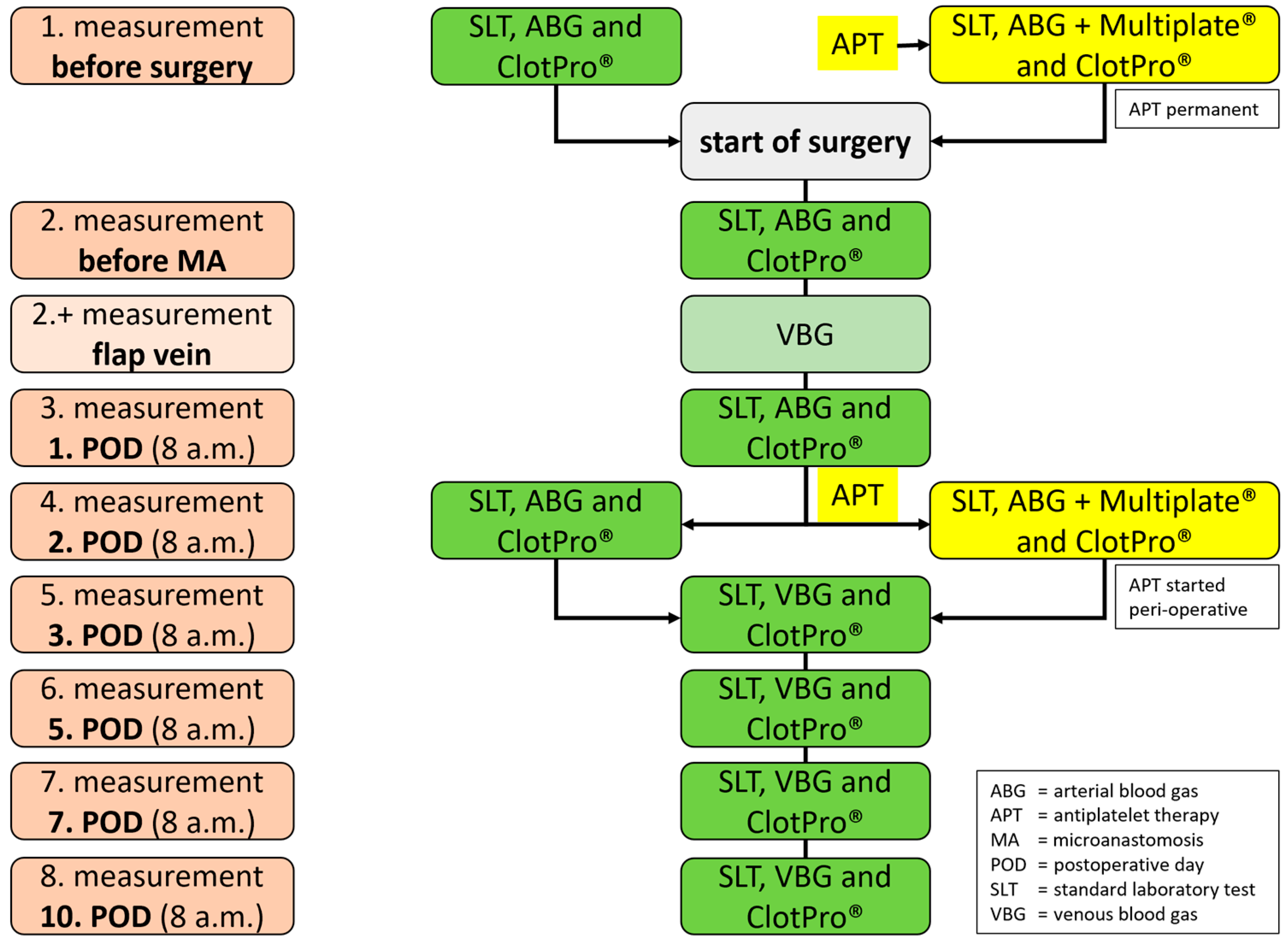
2. Methods: Study Setting, Participants and Outcome
2.1. Study Setting
2.2. Eligibility Criteria
- Patients who receive microvascular reconstruction after ablative tumor surgery;
- Patients who receive microvascular reconstruction for secondary reconstruction;
- Patients who receive microvascular reconstruction in the context of bone necrosis or osteomyelitis.
2.3. Exclusion Criteria
- Patients who receive therapeutic anticoagulation before surgery;
- Patients with hereditary coagulopathies (von Willebrand disease, hemophilia A, B and C, factor VII deficiency, congenital thrombocytopenia);
- Patients who do not give their consent to participate in this study;
- Patients who receive dual platelet inhibition.
2.4. Test Methods
2.5. Viscoelastic Testing (VET)
2.6. Platelet Monitoring (PM)
2.7. Standard Laboratory Tests (SLT)
2.8. Anticoagulation Management
2.9. Assessments of Flap Complications
3. Methods: Statistical Analysis
3.1. Primary Outcome (Powered Analysis)
- MCF parameter in the INtest at baseline and occurrence of free flap failure;
- CFT parameter in the INtest at baseline and occurrence of free flap failure;
- A10 parameter in the INtest at baseline and occurrence of free flap failure.
3.2. Sample Size
3.3. Statistical Analysis
3.4. Power
3.5. Secondary Outcomes (Exploratory Study)
3.6. Statistical Analysis
3.7. Supportive Analyses
3.8. Data Exclusion
4. Methods: Recruitment, Data Collection and Management
4.1. Recruitment
4.2. Data Collection Methods
4.3. Data Management
4.4. Data Monitoring
4.5. Harms
4.6. Auditing
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABG | arterial blood gas |
| ADP | adenosine diphosphate |
| APT | antiplatelet therapy |
| aPTT | activated partial clotting time |
| ASPI | arachidonic acid |
| ATE | arterial thromboembolic complications |
| AU | aggregation units |
| CFT | clot formation time |
| COL | collagen |
| CRP | C-reactive protein |
| CT | clotting time |
| EX | extrinsically activated |
| FIB | fibrin clot polymerization |
| GFR | glomerular filtration rate |
| ICU | intensive care unit |
| IN | intrinsically activated |
| IRB | institutional review board |
| IU | international units |
| LT | lysis time |
| MA | micro anastomosis |
| MCF | maximum clot firmness |
| ML | maximum lysis |
| PCT | procalcitonin |
| PM | Platelet monitoring |
| POC | point-of-care |
| POD | postoperative day |
| PT | prothrombin time |
| RCT | randomized controlled trial |
| Risto | ristocetin |
| r-tPA | recombinant tissue plasminogen activator |
| RVV | Russell’s viper venom |
| SAP | Statistical Analysis Plan |
| SLT | standard laboratory test |
| TPA | texture profile analysis |
| TRAP | thrombin receptor-activating peptide |
| UFH | unfractionated heparin |
| VBG | venous blood gas |
| VET | viscoelastic testing |
| VTE | venous thromboembolic complications |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Friel, M.T.; Klem, C.; Hsu, P.W.; Robb, G.L.; Weber, R.S.; Roberts, D.B.; Chang, D.W. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck 2009, 31, 1289–1296. [Google Scholar] [CrossRef]
- Hidalgo, D.A.; Disa, J.J.; Cordeiro, P.G.; Hu, Q.Y. A review of 716 consecutive free flaps for oncologic surgical defects: Refinement in donor-site selection and technique. Plast. Reconstr. Surg. 1998, 102, 722–732; discussion 733–734. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.T.; Butler, J.S.; Murphy, S.M.; Cronin, K.J. Health-related quality of life, surgical and aesthetic outcomes following microvascular free flap reconstructions: An 8-year institutional review. Ann. R. Coll. Surg. Engl. 2012, 94, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Pohlenz, P.; Klatt, J.; Schön, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int J Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kansy, K.; Mueller, A.A.; Mücke, T.; Kopp, J.B.; Koersgen, F.; Wolff, K.D.; Zeilhofer, H.F.; Hölzle, F.; Pradel, W.; Schneider, M.; et al. Microsurgical reconstruction of the head and neck--current concepts of maxillofacial surgery in Europe. J. Craniomaxillofac. Surg. 2014, 42, 1610–1613. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, Z.H.; Chickooree, D.; Wu, H.J.; Tan, H.Y.; Wang, K.; He, Z.J.; Gong, C.J.; Ram, V.; Zhang, S. The effect of early detection of anterolateral thigh free flap crisis on the salvage success rate, based on 10 years of experience and 1072 flaps. Int. J. Oral. Maxillofac. Surg. 2014, 43, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Carlsen, B.T.; Festekjian, J.H.; Da Lio, A.L.; Crisera, C.A. Salvage rates of compromised free flap breast reconstruction after recurrent thrombosis. Ann. Plast. Surg. 2013, 71, 68–71. [Google Scholar] [CrossRef]
- Chae, M.P.; Rozen, W.M.; Whitaker, I.S.; Chubb, D.; Grinsell, D.; Ashton, M.W.; Hunter-Smith, D.J.; Lineaweaver, W.C. Current evidence for postoperative monitoring of microvascular free flaps: A systematic review. Ann. Plast. Surg. 2015, 74, 621–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selber, J.C.; Angel Soto-Miranda, M.; Liu, J.; Robb, G. The survival curve: Factors impacting the outcome of free flap take-backs. Plast. Reconstr. Surg. 2012, 130, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, W.B.; Yu, Y.; Wang, Y.; Mao, C.; Guo, C.B.; Yu, G.Y.; Peng, X. Risk factors for free flap failure: A retrospective analysis of 881 free flaps for head and neck defect reconstruction. Int. J. Oral. Maxillofac. Surg. 2017, 46, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Kawabata, K.; Mitani, H. Factors involved in free flap thrombosis after reconstructive surgery for head and neck cancer. Auris Nasus Larynx 2010, 37, 212–216. [Google Scholar] [CrossRef]
- Biben, J.A.; Atmodiwirjo, P. Free flap thrombosis in patients with hypercoagulability: A systematic review. Arch. Plast. Surg. 2019, 46, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Lin, P.Y.; Chew, K.Y.; Kuo, Y.R. Free tissue transfers in head and neck reconstruction: Complications, outcomes and strategies for management of flap failure: Analysis of 2019 flaps in single institute. Microsurgery 2014, 34, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wang, T.H.; Chiu, Y.H.; Chang, D.H. Postoperative Hematoma in Microvascular Reconstruction of the Head and Neck. Ann. Plast. Surg. 2018, 80, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Levy, J.H. COVID-19–associated Coagulopathy: Less Fibrinolysis Can Be More Harmful! Anesthesiology 2021, 134, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Moore, H.B.; Moore, E.E.; Liras, I.N.; Gonzalez, E.; Harvin, J.A.; Holcomb, J.B.; Sauaia, A.; Cotton, B.A. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J. Am. Coll. Surg. 2016, 222, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, H.B.; Moore, B.A.; Sauaia, A.; Moore, E.E. Clinical relevance and practical assessment of fibrinolysis shutdown. ANZ J. Surg. 2020, 90, 413–414. [Google Scholar] [CrossRef] [Green Version]
- Michelson, A.D.; Frelinger, A.L.; Furman, M.I. Current Options in Platelet Function Testing. Am. J. Cardiol. 2006, 98, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Moore, E.E.M. Trauma Induced Coagulopathy, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 157–171. [Google Scholar]
- Heubner, L.; Mirus, M.; Vicent, O.; Güldner, A.; Tiebel, O.; Beyer-Westendorf, J.; Fries, D.; Spieth, P.M. Point of care coagulation management in anesthesiology and critical care. Minerva Anestesiol. 2022, 88, 615–628. [Google Scholar] [CrossRef]
- Calatzis, A.; Leitner, M.; Panzer, S. Monitoring anticoagulation of primary haemostasis--estimation of platelet function in whole blood assays. Hamostaseologie 2009, 29, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Görlinger, K.; Almutawah, H.; Almutawaa, F.; Alwabari, M.; Alsultan, Z.; Almajed, J.; Alwabari, M.; Alsultan, M.; Shahwar, D.I.; Yassen, K.A. The Role of Rotational Thromboelastometry during the COVID-19 Pandemic: A Narrative Review. Korean J. Anesth. 2021, 74, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Scarlatescu, E.; Juffermans, N.P.; Thachil, J. The current status of viscoelastic testing in septic coagulopathy. Thromb. Res. 2019, 183, 146–152. [Google Scholar] [CrossRef]
- Spielbauer, K.K.; Sunde, J.; Buchakjian, M.; Casper, K.A.; Malloy, K.M.; Stucken, C.L.; Prince, M.E.; Rosko, A.J.; Schechtman, S.; Chinn, S.B.; et al. Use of rotational thromboelastometry (ROTEM®) to predict thrombotic complications of microvascular head and neck reconstruction. Oral. Oncol. 2021, 124, 105515. [Google Scholar] [CrossRef]
- Malapati, H.; Hanwright, P.J.; Tuffaha, S.H. Utility of Viscoelastic Tests to Predict Flap Thrombosis: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3769. [Google Scholar] [CrossRef]
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M.; et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth. 2020, 126, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Groene, P.; Wagner, D.; Kammerer, T.; Kellert, L.; Giebl, A.; Massberg, S.; Schäfer, S.T. Viscoelastometry for detecting oral anticoagulants. Thromb. J. 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Braun, S.; Morath, T.; Mehilli, J.; Vogt, W.; Schömig, A.; Kastrati, A.; von Beckerath, N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 2009, 53, 849–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, J.; Murphy, M.; Dias, J.D. Viscoelastic Hemostatic Assays: Moving from the Laboratory to the Site of Care-A Review of Established and Emerging Technologies. Diagnostics 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Calatzis, A.W.M.; Leyser, H.; Hipp, Q.; Spannagl, M. ClotPro-a new generation viscoelastic whole blood coagulation analyzer. Hämostaseologie 2018, 4, A32. [Google Scholar]
- Hartert, H. Die Thrombelastographie. Z. Für. Die Gesamte Exp. Med. 1951, 117, 189–203. [Google Scholar] [CrossRef]
- Sedghi, A.; Heubner, L.; Klimova, A.; Tiebel, O.; Pietsch, J.; Mirus, M.; Barlinn, K.; Minx, T.; Beyer-Westendorf, J.; Puetz, V.; et al. Point of Care Assessment of Direct Oral Anticoagulation in Acute Ischemic Stroke: Protocol for a Prospective Observational Diagnostic Accuracy Study. Thromb. Haemost. 2022. [Google Scholar] [CrossRef]
- Seyfert, U.T.; Haubelt, H.; Vogt, A.; Hellstern, P. Variables influencing Multiplate™ whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets 2007, 18, 199–206. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, T.A.; Leonhardt, H.; Haim, D.; Bräuer, C.; Papadopoulos, K.K.; Vicent, O.; Güldner, A.; Mirus, M.; Schmidt, J.; Held, H.C.; et al. Enhancing Anticoagulation Monitoring and Therapy in Patients Undergoing Microvascular Reconstruction in Maxillofacial Surgery: A Prospective Observational Trial. J. Pers. Med. 2022, 12, 1229. https://doi.org/10.3390/jpm12081229
Schröder TA, Leonhardt H, Haim D, Bräuer C, Papadopoulos KK, Vicent O, Güldner A, Mirus M, Schmidt J, Held HC, et al. Enhancing Anticoagulation Monitoring and Therapy in Patients Undergoing Microvascular Reconstruction in Maxillofacial Surgery: A Prospective Observational Trial. Journal of Personalized Medicine. 2022; 12(8):1229. https://doi.org/10.3390/jpm12081229
Chicago/Turabian StyleSchröder, Tom A., Henry Leonhardt, Dominik Haim, Christian Bräuer, Kiriaki K. Papadopoulos, Oliver Vicent, Andreas Güldner, Martin Mirus, Jürgen Schmidt, Hanns C. Held, and et al. 2022. "Enhancing Anticoagulation Monitoring and Therapy in Patients Undergoing Microvascular Reconstruction in Maxillofacial Surgery: A Prospective Observational Trial" Journal of Personalized Medicine 12, no. 8: 1229. https://doi.org/10.3390/jpm12081229
APA StyleSchröder, T. A., Leonhardt, H., Haim, D., Bräuer, C., Papadopoulos, K. K., Vicent, O., Güldner, A., Mirus, M., Schmidt, J., Held, H. C., Tiebel, O., Birkner, T., Beyer-Westendorf, J., Lauer, G., Spieth, P. M., Koch, T., & Heubner, L. (2022). Enhancing Anticoagulation Monitoring and Therapy in Patients Undergoing Microvascular Reconstruction in Maxillofacial Surgery: A Prospective Observational Trial. Journal of Personalized Medicine, 12(8), 1229. https://doi.org/10.3390/jpm12081229





