Impact of TRAF3IP2, IL10 and HCP5 Genetic Polymorphisms in the Response to TNF-i Treatment in Patients with Psoriatic Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Extraction and Genotyping
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
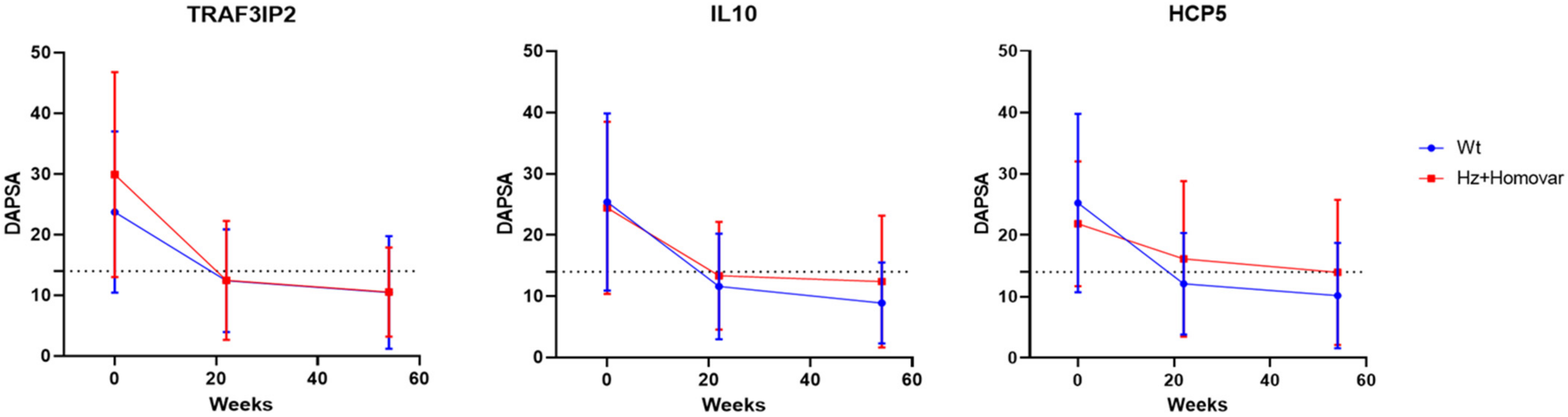
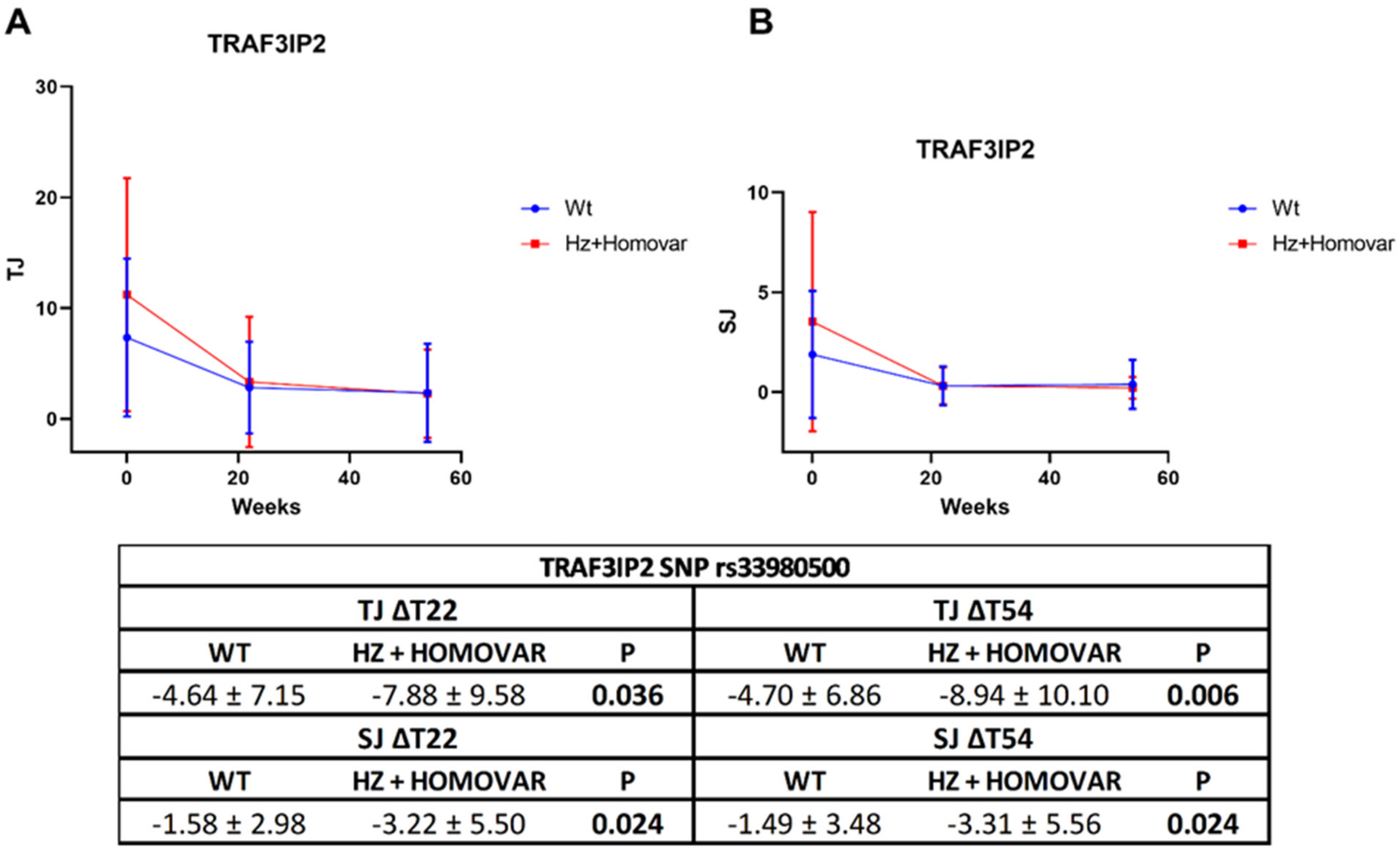
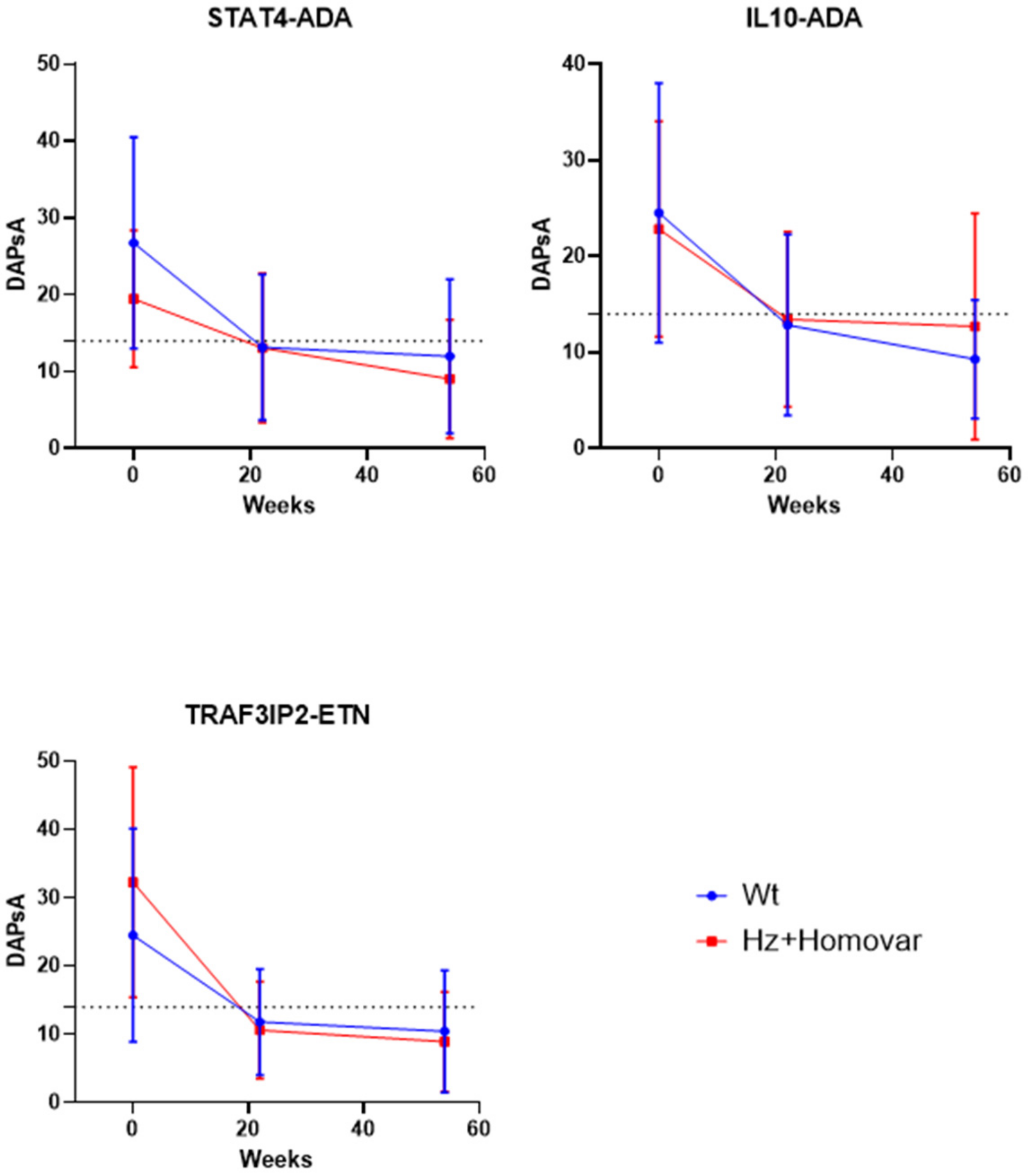
3.2. Associations of Genetic Variants with Response to TNF-i Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calabresi, E.; Monti, S.; Terenzi, R.; Zanframundo, G.; Perniola, S.; Carli, L. One year in review 2019: Psoriatic arthritis. Clin. Exp. Rheumatol. 2020, 38, 1046–1055. [Google Scholar] [PubMed]
- Rahmati, S.; Tsoi, L.; O’Rielly, D.; Chandran, V.; Rahman, P. Complexities in Genetics of Psoriatic Arthritis. Curr. Rheumatol. Rep. 2020, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Chimenti, M.S.; D’Antonio, A.; Conigliaro, P.; Ferrigno, S.; Vendola, A.; Ferraioli, M.; Triggianese, P.; Costa, L.; Caso, F.; Perricone, R. An Update for the Clinician on Biologics for the Treatment of Psoriatic Arthritis. Biologics 2020, 14, 53–75. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Triggianese, P.; Conigliaro, P.; Tonelli, M.; Gigliucci, G.; Novelli, L.; Teoli, M.; Perricone, R. A 2-year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin. Rheumatol. 2017, 36, 2253–2260. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, S.; Ogdie, A.; Kraft, W.K. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert. Rev. Clin. Pharmacol. 2017, 10, 899–910. [Google Scholar] [CrossRef]
- Noviani, M.; Feletar, M.; Nash, P.; Leung, Y.Y. Choosing the right treatment for patients with psoriatic arthritis. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A. Advances in the management of psoriatic arthritis. Nat. Rev. Rheumatol. 2014, 10, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T.; Ilan, Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: A Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front. Immunol. 2019, 10, 2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovejero-Benito, M.C.; Muñoz-Aceituno, E.; Reolid, A.; Fisas, L.H.; Llamas-Velasco, M.; Prieto-Pérez, R.; Abad-Santos, F.; Daudén, E. Polymorphisms associated with anti-TNF drugs response in patients with psoriasis and psoriatic arthritis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e175–e177. [Google Scholar] [CrossRef]
- Antonatos, C.; Stavrou, E.F.; Evangelou, E.; Vasilopoulos, Y. Exploring pharmacogenetic variants for predicting response to anti-TNF therapy in autoimmune diseases: A meta-analysis. Pharmacogenomics 2021, 22, 435–445. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Darvishi, M.; Ghasemiadl, M. Pharmacogenetics: A strategy for personalized medicine for autoimmune diseases. Clin. Genet. 2018, 93, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdaca, G.; Gulli, R.; Spanò, F.; Lantieri, F.; Burlando, M.; Parodi, A.; Mandich, P.; Puppo, F. TNF-α gene polymorphisms: Association with disease susceptibility and response to anti-TNF-α treatment in psoriatic arthritis. J. Investig. Dermatol. 2014, 134, 2503–2509. [Google Scholar] [CrossRef] [Green Version]
- Iwaszko, M.; Wielińska, J.; Świerkot, J.; Kolossa, K.; Sokolik, R.; Bugaj, B.; Chaszczewska-Markowska, M.; Jeka, S.; Bogunia-Kubik, K. IL-33 Gene Polymorphisms as Potential Biomarkers of Disease Susceptibility and Response to TNF Inhibitors in Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients. Front. Immunol. 2021, 12, 631603. [Google Scholar] [CrossRef]
- David, T.; Jani, M.; OUTPASS; Bowes, J.; Chinoy, H.; Barton, A. Is HLA-B27 a predictor of treatment response to biologics in psoriatic arthritis? Rheumatology 2019, 58, iii128. [Google Scholar] [CrossRef] [Green Version]
- Fabris, M.; Quartuccio, L.; Fabro, C.; Sacco, S.; Lombardi, S.; Ramonda, R.; Biasi, D.; Punzi, D.; Adami, S.; Olivieri, I.; et al. The -308 TNFα and the -174 IL-6 promoter polymorphisms associate with effective anti-TNFα treatment in seronegative spondyloarthritis. Pharm. J. 2016, 16, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Strafella, C.; Longo, G.; Maccarone, M.; Borgiani, P.; Sangiuolo, F.; Novelli, G.; Giardina, E. Pharmacogenomics of multifactorial diseases: A focus on psoriatic arthritis. Pharmacogenomics 2016, 17, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Magee, C.; Jethwa, H.; FitzGerald, O.M.; Jadon, D.R. Biomarkers predictive of treatment response in psoriasis and psoriatic arthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, G.; Latini, A.; Conigliaro, P.; Triggianese, P.; Bergamini, A.; Novelli, L.; Ciccacci, C.; Chimenti, M.S.; Borgiani, P. A multilocus genetic study evidences the association of autoimmune-related genes with Psoriatic Arthritis in Italian patients. Immunobiology 2022, 227, 152232. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Smolen, J.S.; Ramiro, S.; de Wit, M.; Cutolo, M.; Dougados, M.; Emery, P.; Landewé, R.; Oliver, S.; Aletaha, D.; et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheum. Dis. 2016, 75, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Soriano, E.; Corp, N.; Bertheussen, H.; Callis-Duffin, K.; Barbosa, C.C.; Chau, J.; Eder, L.; Fernandez, D.; Fitzgerald, O.; et al. OP0229 The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Treatment Recommendations 2021. Ann. Rheum. Dis. 2021, 80, 139–140. [Google Scholar] [CrossRef]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease activity in psoriatic arthritis (PsA): Defining remission and treatment success using the DAPSA score. Ann. Rheum. Dis. 2016, 75, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, L.; Bulek, K.; Martin, B.N.; Zepp, J.A.; Kang, Z.; Liu, C.; Herjan, T.; Misra, S.; Carman, J.A.; et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat. Immunol. 2013, 14, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conigliaro, P.; Ciccacci, C.; Politi, C.; Triggianese, P.; Rufini, S.; Kroegler, B.; Perricone, C.; Latini, A.; Novelli, G.; Borgiani, P.; et al. Polymorphisms in STAT4, PTPN2, PSORS1C1 and TRAF3IP2 Genes Are Associated with the Response to TNF Inhibitors in Patients with Rheumatoid Arthritis. PLoS ONE 2017, 12, e0169956. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Padyukov, L.; Lampa, J.; Heimbürger, M.; Ernestam, S.; Cederholm, T.; Lundkvist, I.; Andersson, P.; Hermansson, Y.; Harju, A.; Klareskog, L.; et al. Genetic markers for the efficacy of tumour necrosis factor blocking therapy in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 526–529. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Batliwalla, F.; Li, W.; Lee, A.; Roubenoff, R.; Beckman, E.; Khalili, H.; Damle, A.; Kern, M.; Furie, R.; et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol. Med. 2008, 14, 575–581. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; Bossacoma, F.; Pujol-Muncunill, G.; Navas-López, V.M.; Gallego-Fernández, C.; Viada, J.; Muñoz-Codoceo, R.; Magallares, L.; Martínez-Ojinaga, E.; Moreno-Álvarez, A.; et al. Genetic Predictors of Long-term Response to Antitumor Necrosis Factor Agents in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 508–515. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Burguete-García, A.I.; Cruz, M.; Martínez-Nava, G.A.; Bahena-Román, M.; Ortíz-Flores, E.; Ramírez-González, A.; López-Estrada, G.; Delgado-Romero, K.; Madrid-Marina, V. The SNP at -592 of human IL-10 gene is associated with serum IL-10 levels and increased risk for human papillomavirus cervical lesion development. Infect. Agent Cancer 2012, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Schotte, H.; Schlüter, B.; Schmidt, H.; Gaubitz, M.; Drynda, S.; Kekow, J.; Willeke, P. Putative IL-10 Low Producer Genotypes Are Associated with a Favourable Etanercept Response in Patients with Rheumatoid Arthritis. PLoS ONE 2015, 10, e0130907. [Google Scholar]
- Ciccacci, C.; Perricone, C.; Ceccarelli, F.; Rufini, S.; Di Fusco, D.; Alessandri, C.; Spinelli, F.R.; Cipriano, E.; Novelli, G.; Valesini, G.; et al. A multilocus genetic study in a cohort of Italian SLE patients confirms the association with STAT4 gene and describes a new association with HCP5 gene. PLoS ONE 2014, 9, e111991. [Google Scholar] [CrossRef] [PubMed]
- Colafrancesco, S.; Ciccacci, C.; Priori, R.; Latini, A.; Picarelli, G.; Arienzo, F.; Novelli, G.; Valesini, G.; Perricone, C.; Borgiani, P. STAT4, TRAF3IP2, IL10, and HCP5 Polymorphisms in Sjögren’s Syndrome: Association with Disease Susceptibility and Clinical Aspects. J. Immunol. Res. 2019, 2019, 7682827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, D.; Nagraj, S.; Kumar, K.S.P.; Kutty, A.V.M.; Balakrishna, S. Evaluation of HCP5 and Chemokine C Receptor type 5 Gene Polymorphisms in Indian Psoriatic Patients. Indian J. Dermatol. 2019, 64, 182–186. [Google Scholar]
- Remmers, E.F.; Plenge, R.M.; Lee, A.T.; Graham, R.R.; Hom, G.; Behrens, T.W.; de Bakker, P.I.; Le, J.M.; Lee, H.S.; Batliwalla, F.; et al. P.K. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 2007, 357, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamana, A.; López-Santalla, M.; Castillo-González, R.; Ortiz, A.M.; Martín, J.; García-Vicuña, R.; González-Álvaro, I. The Minor Allele of rs7574865 in the STAT4 Gene Is Associated with Increased mRNA and Protein Expression. PLoS ONE 2015, 10, e0142683. [Google Scholar] [CrossRef]
- Korman, B.D.; Alba, M.I.; Le, J.M.; Alevizos, I.; Smith, J.A.; Nikolov, N.P.; Kastner, D.L.; Remmers, E.F.; Illei, G.G. Variant form of STAT4 is associated with primary Sjögren’s syndrome. Genes Immun. 2008, 9, 267–270. [Google Scholar] [CrossRef]
- Silvagni, E.; Missiroli, S.; Perrone, M.; Patergnani, S.; Boncompagni, C.; Bortoluzzi, A.; Govoni, M.; Giorgi, C.; Alivernini, S.; Pinton, P.; et al. From Bed to Bench and Back: TNF-α, IL-23/IL-17A, and JAK-Dependent Inflammation in the Pathogenesis of Psoriatic Synovitis. Front. Pharmacol. 2021, 12, 672515. [Google Scholar] [CrossRef]
| Sex (% of males) | 50.9 |
| Age (mean ± SD) | 59.9 ± 12.48 |
| Age at diagnosis (mean ± SD) | 44.7 ± 12.47 |
| TJ (mean ± SD) | 8.04 ± 7.91 |
| SJ (mean ± SD) | 2.23 ± 3.84 |
| CRP (mean ± SD) | 1.97 ± 5.23 |
| pVAS (mean ± SD) | 7.12 ± 6.44 |
| gVAS (mean ± SD) | 6.79 ± 5.19 |
| DAPsA (mean ± SD) | 24.95 ± 14.24 |
| Enthesitis (%) | 17.3 |
| Dactylitis (%) | 11.7 |
| Erosions (%) | 11.1 |
| Psoriasis (%) | 51.3 |
| ΔT22 | ΔT54 | |||||
|---|---|---|---|---|---|---|
| STAT4 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs7574865 | −13.50 ± 14.34 | −11.52 ± 14.27 | 0.39 | −14.46 ± 14.53 | −14.54 ± 14.82 | 0.97 |
| TRAF3IP2 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs33980500 | −11.36 ± 13.08 | −17.42 ± 17.53 | 0.032 | −13.11 ± 13.35 | −19.97 ± 17.73 | 0.019 |
| TNFAIP3 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs6920220 | −12.93 ± 13.69 | −12.12 ± 15.29 | 0.73 | −14.81 ± 14.84 | −14.26 ± 14.33 | 0.82 |
| rs2230926 | −12.76 ± 14.51 | −10.21 ± 9.83 | 0.58 | −14.77 ± 14.80 | −10.81 ± 10.10 | 0.43 |
| MIR146A SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs2910164 | −12.28 ± 15.09 | −13.01 ± 13.18 | 0.75 | −14.90 ± 14.06 | −14.06 ± 15.28 | 0.73 |
| PSORS1C1 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs2233945 | −12.43 ± 13.33 | −12.94 ± 16.24 | 0.84 | −14.12 ± 13.24 | −15.27 ± 17.19 | 0.65 |
| IL10 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs1800872 | −13.89 ± 14.74 | −11.09 ± 13.59 | 0.22 | −16.92 ± 14.04 | −11.79 ± 14.78 | 0.031 |
| HCP5 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs3099844 | −13.22 ± 14.61 | −5.69 ± 6.47 | 0.068 | −15.15 ± 14.77 | −7.90 ± 10.43 | 0.086 |
| ERAP1 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs27524 | −13.25 ± 15.54 | −12.26 ± 13.91 | 0.71 | −14.78 ± 13.42 | −14.38 ± 15.15 | 0.88 |
| (a) | ||||
| Independent Variables | Beta Standardized Coefficient | t-Statistics | p | R2 |
| TRAF3IP2 rs33980500 | −0.196 | −2.445 | 0.016 | 0.061 |
| HCP5 rs3099844 | 0.171 | 2.134 | 0.035 | |
| (b) | ||||
| Independent Variables | Beta Standardized Coefficient | t-Statistics | p | R2 |
| TRAF3IP2 rs33980500 | −0.220 | −2.727 | 0.007 | 0.110 |
| HCP5 rs3099844 | 0.186 | 2.320 | 0.022 | |
| IL10 rs1800872 | 0.171 | 2.123 | 0.036 | |
| ETANERCEPT | DAPsA ΔT22 | DAPsA ΔT54 | ||||
| TRAF3IP2 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs33980500 | −12.59 ± 14.84 | −21.67 ± 19.22 | 0.043 | −12.84 ± 16.34 | −23.84 ± 18.54 | 0.029 |
| ADALIMUMAB | DAPsA ΔT22 | DAPsA ΔT54 | ||||
| STAT4 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs7574865 | −13.58 ± 12.17 | −6.45 ± 10.61 | 0.006 | −14.73 ± 12.94 | −11.81 ± 9.99 | 0.274 |
| IL10 SNP | WT | HZ + HOMOVAR | p | WT | HZ + HOMOVAR | p |
| rs1800872 | −11.95 ± 13.22 | −9.37 ± 10.51 | 0.319 | −16.50 ± 12.76 | −10.33 ± 10.04 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Benedittis, G.; Latini, A.; Ciccacci, C.; Conigliaro, P.; Triggianese, P.; Fatica, M.; Novelli, L.; Chimenti, M.S.; Borgiani, P. Impact of TRAF3IP2, IL10 and HCP5 Genetic Polymorphisms in the Response to TNF-i Treatment in Patients with Psoriatic Arthritis. J. Pers. Med. 2022, 12, 1094. https://doi.org/10.3390/jpm12071094
De Benedittis G, Latini A, Ciccacci C, Conigliaro P, Triggianese P, Fatica M, Novelli L, Chimenti MS, Borgiani P. Impact of TRAF3IP2, IL10 and HCP5 Genetic Polymorphisms in the Response to TNF-i Treatment in Patients with Psoriatic Arthritis. Journal of Personalized Medicine. 2022; 12(7):1094. https://doi.org/10.3390/jpm12071094
Chicago/Turabian StyleDe Benedittis, Giada, Andrea Latini, Cinzia Ciccacci, Paola Conigliaro, Paola Triggianese, Mauro Fatica, Lucia Novelli, Maria Sole Chimenti, and Paola Borgiani. 2022. "Impact of TRAF3IP2, IL10 and HCP5 Genetic Polymorphisms in the Response to TNF-i Treatment in Patients with Psoriatic Arthritis" Journal of Personalized Medicine 12, no. 7: 1094. https://doi.org/10.3390/jpm12071094
APA StyleDe Benedittis, G., Latini, A., Ciccacci, C., Conigliaro, P., Triggianese, P., Fatica, M., Novelli, L., Chimenti, M. S., & Borgiani, P. (2022). Impact of TRAF3IP2, IL10 and HCP5 Genetic Polymorphisms in the Response to TNF-i Treatment in Patients with Psoriatic Arthritis. Journal of Personalized Medicine, 12(7), 1094. https://doi.org/10.3390/jpm12071094






