Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Unmatched Series
3.2. Recurrence Patients
3.3. Matched Series
3.4. Survival Analysis
4. Discussion
Principal Findings
5. Strengths and Weaknesses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Querleu, D.; Morrow, C.P. Classification of Radical Hysterectomy. Lancet Oncol. 2008, 9, 297–303. Available online: https://pubmed.ncbi.nlm.nih.gov/18308255/ (accessed on 4 January 2022). [CrossRef]
- Sant, M.; Lopez, M.D.C.; Agresti, R.; Pérez, M.J.S.; Holleczek, B.; Bielska-Lasota, M.; Dimitrova, N.; Innos, K.; Katalinic, A.; Langseth, H.; et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 64–84. Available online: https://pubmed.ncbi.nlm.nih.gov/30659131/ (accessed on 5 January 2022). [CrossRef] [PubMed]
- Novak, F. Radical abdominal subcorporeal extirpation of the cervix with bilateral pelvic lymph nodes dissection in cancer in situ of the cervix uteri. Acta Med. Iugosl. 1952, 6, 59–71. [Google Scholar] [PubMed]
- Aburel, E. Sub-corporeal extended colpohysterectomy in therapy of incipient cancer of cervix. Comptes R. Soc. Fr. Gyncol. 1957, 27, 237–243. [Google Scholar]
- Dargent, D.; Martin, X.; Sacchetoni, A.; Mathevet, P. Laparoscopic vaginal radical trachelectomy: A treatment to preserve the fertility of cervical carcinoma patients. Cancer 2000, 88, 1877–1882. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Salvo, G.; Ramirez, P.T.; Leitao, M.; Cibula, D.; Fotopoulou, C.; Kucukmetin, A.; Rendon, G.; Perrotta, M.; Ribeiro, R.; Vieira, M.; et al. International Radical Trachelectomy Assessment: IRTA Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, 29, 635–638. Available online: https://pubmed.ncbi.nlm.nih.gov/30765489/ (accessed on 14 January 2022). [CrossRef]
- Pecorelli, S.; Zigliani, L.; Odicino, F. Revised FIGO staging for carcinoma of the cervix. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2009, 105, 107–108. Available online: https://pubmed.ncbi.nlm.nih.gov/19342051/ (accessed on 14 January 2022). [CrossRef] [PubMed]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar] [PubMed]
- Anderson-Cook, C.M. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. J. Am. Stat. Assoc. 2005, 100, 708. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L. Verifying assumptions of the Cox proportional hazards model. In Proceedings of the Eleventh Annual SAS Users Group International Conference, Atlanta, GA, USA, 9–12 February 1986; pp. 823–828. [Google Scholar]
- Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of Laparoscopic-Assisted Resection vs. Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015, 314, 1346–1355. Available online: https://pubmed.ncbi.nlm.nih.gov/26441179/ (accessed on 14 January 2022). [CrossRef] [PubMed]
- Stevenson, A.R.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.; Hague, W.; Simes, J.; et al. Effect of Laparoscopic-Assisted Resection vs. Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015, 314, 1356–1363. Available online: https://pubmed.ncbi.nlm.nih.gov/26441180/ (accessed on 14 January 2022). [CrossRef]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs. Total Abdominal Hysterectomy on Disease-Free Survival among Women with Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. Available online: https://pubmed.ncbi.nlm.nih.gov/28350928/ (accessed on 14 January 2022). [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. Available online: https://www.jstatsoft.org/index.php/jss/article/view/v042i08 (accessed on 16 January 2022). [CrossRef]
- Noah Greifer. Covariate Balance Tables and Plots. R Package Version 4.3.1. 2021. Available online: https://CRAN.R-project.org/package=cobalt (accessed on 1 May 2022).
- Therneau, T.M.; Grambsch, P.M. A Package for Survival Analysis in S, Version 2.38. Modeling Survival Data: Extending the Cox Model. 2000. Available online: http://cran.r-project.org/package=survival (accessed on 16 January 2022).
- Möllenhoff, K.; Tresch, A. Survival Analysis under Non-Proportional Hazards: Investigating Non-Inferiority or Equivalence in Time-to-Event Data. 2020. Available online: http://arxiv.org/abs/2009.06699 (accessed on 16 January 2022).
- Kassambara, A. Drawing Survival Curves Using “ggplot2” [R Package Survminer Version 0.4.9]. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 16 January 2022).
- Tseng, J.H.; Aloisi, A.; Sonoda, Y.; Gardner, G.J.; Zivanovic, O.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Less Versus More Radical Surgery in Stage IB1 Cervical Cancer: A Population-Based Study of Long-Term Survival. Gynecol. Oncol. 2018, 150, 44–49. Available online: https://pubmed.ncbi.nlm.nih.gov/29776598/ (accessed on 21 January 2022). [CrossRef] [PubMed]
- Gorostidi, M.; Gil-Ibañez, B.; Alonso, S.; Gil-Moreno, A.; Hernandez, A.; Torné, A.; Zapardiel, I. Fertility Preservation Treatment of Gynecological Cancer Patients in Spain: A National Survey (GOFER Study). Arch. Gynecol. Obstet. 2020, 301, 793–800. Available online: https://pubmed.ncbi.nlm.nih.gov/32124016/ (accessed on 21 January 2022). [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 641–655. Available online: https://pubmed.ncbi.nlm.nih.gov/29688967/ (accessed on 5 January 2022). [CrossRef]
- Bentivegna, E.; Gouy, S.; Maulard, A.; Chargari, C.; Leary, A.; Morice, P. Oncological Outcomes after Fertility-Sparing Surgery for Cervical Cancer: A Systematic Review. Lancet Oncol. 2016, 17, e240–e253. Available online: https://pubmed.ncbi.nlm.nih.gov/27299280/ (accessed on 5 January 2022). [CrossRef]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Outcomes after Radical Hysterectomy according to Tumor Size Divided by 2-cm Interval in Patients with Early Cervical Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 59–67. Available online: https://pubmed.ncbi.nlm.nih.gov/20595451/ (accessed on 5 January 2022). [CrossRef]
- Cao, D.Y.; Yang, J.X.; Wu, X.H.; Chen, Y.L.; Li, L.; Liu, K.J.; Cui, M.H.; Xie, X.; Wu, Y.M.; Kong, B.H.; et al. Comparisons of Vaginal and Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer: Preliminary Results of A Multi-Center Research in China. Br. J. Cancer 2013, 109, 2778–2782. Available online: https://pubmed.ncbi.nlm.nih.gov/24169350/ (accessed on 5 January 2022). [CrossRef] [PubMed][Green Version]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, iv72–iv83. Available online: https://pubmed.ncbi.nlm.nih.gov/28881916/ (accessed on 5 January 2022). [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Leitao, M.M.; Cibula, D.; Wu, X.; Falconer, H.; Persson, J.; Perrotta, M.; Mosgaard, B.J.; Kucukmetin, A.; et al. Open vs minimally invasive radical trachelectomy in early-stage cervical cancer: International Radical Trachelectomy Assessment Study. Am. J. Obstet. Gynecol. 2022, 226, 97.e1–97.e16. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and Negative Predictive Value for Sentinel Lymph Node Biopsy in Women with Early-Stage Cervical Cancer. Gynecol. Oncol. 2017, 145, 96–101. Available online: https://pubmed.ncbi.nlm.nih.gov/28188015/ (accessed on 21 January 2022). [CrossRef] [PubMed]

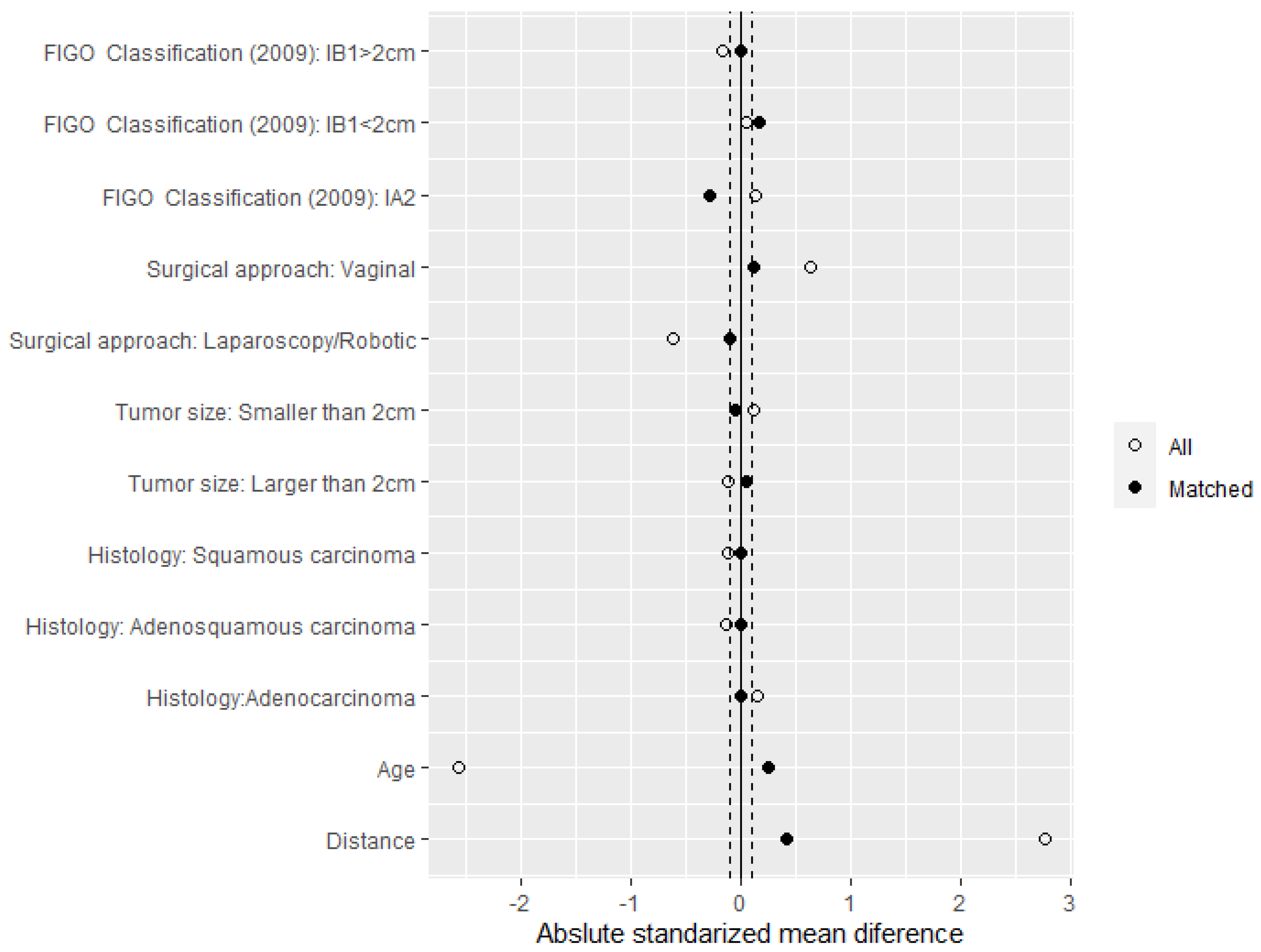

| Total n = 222 | Fertility-Sparing Surgery n = 111 | Radical Hysterectomy n = 111 | p Value | |
|---|---|---|---|---|
| Age | 39.5 ± 10.6 years | 46.2 ± 10 years | 32.7 ± 5.3 years | 0.001 |
| Follow up | 62.8 ± 45.1 months | 65.9 ± 46.7 months | 59.6 ± 46.4 months | 0.299 |
| FIGO Classification (2009) | 0.294 | |||
| IA1 plus LVI+ | 7 | 5 (4.5%) | 2 (1.8%) | |
| IA2 | 25 | 15 (13.5%) | 10 (9%) | |
| IB1 < 2 cm | 133 | 67 (60.4%) | 66 (59.4%) | |
| IB1 ≥ 2 cm | 57 | 24 (21.6%) | 33 (29.7%) | |
| Histology | 0.4469 | |||
| Adenocarcinoma | 84 (37.8%) | 45 (40.5%) | 39 (35.1%) | |
| Squamous carcinoma | 137 (61.7%) | 66 (59.5%) | 71 (64%) | |
| Adenosquamous carcinoma | 1 (0.5%) | 0 | 1 (0.9%) | |
| LVI | 0.8444 | |||
| Positive | 30 (13.5%) | 16 (14.4%) | 14 (12.6%) | |
| Negative | 192 (86.5%) | 95 (85.6%) | 97 (87.4%) | |
| Tumour size | 0.3803 | |||
| Less than 2 cm | 155 (69.8%) | 81 (73.0%) | 74 (66.7%) | |
| Greater or equal than 2 cm | 67 (30.2%) | 30 (27.0%) | 37 (33.3%) | |
| Nodal assessment | 0.6971 | |||
| PLD + SLN | 165 | 80 (72.1%) | 85 (76.6%) | |
| Only SLN | 55 | 29 (26.1%) | 26 (23.4%) | |
| Missing values | 2 | 2 (1.8%) | 0 | |
| Intraoperative complications | 0.48 | |||
| Yes | 9 (4.1%) | 6 (5.4%) | 3 (2.7%) | |
| No | 213 (95.9%) | 105 (94.6%) | 108 (97.3%) | |
| Recurrence localizations | ||||
| Distance | 2 (12.5%) | 0 (0.0%) | 2 (40.0%) | 0.1532 |
| Adnexal | 2 (12.5%) | 2 (18.2%) | 0 (0.0%) | |
| Cervical | 3 (18.8%) | 3 (27.3%) | 0 (0.0%) | |
| Lymph nodes | 3 (18.8%) | 2 (18.2%) | 1 (20.0%) | |
| Local | 6 (37.5%) | 4 (36.4%) | 2 (40.0%) | |
| Surgical approach | <0.001 | |||
| Laparoscopy/Robotic | 109 (49.1%) | 39 (35.1%) | 70 (63.1%) | |
| Laparotomy | 4 (1.8%) | 0 (0.0%) | 4 (3.6%) | |
| Vaginal | 109 (49.1%) | 72 (64.9%) | 37 (33.3%) | |
| Postoperative complications | 0.001 | |||
| Yes | 7 | 0 (0%) | 7 (6.3%) | |
| No | 215 | 111 (100%) | 104 (93.7%) | |
| Recurrence | 0.1944 | |||
| Yes | 16 (7.2%) | 11 (9.9%) | 5 (4.5%) | |
| No | 205 (92.8%) | 100 (90.1%) | 106 (95.5%) |
| Patients with Recurrence | |||||
|---|---|---|---|---|---|
| Patients with Recurrence (Recurrence/Total) | p Value | Fertility-Sparing Surgery n = 11 | Radical Hysterectomy n = 5 | p Value | |
| Age | 38.2 ± 12.3 years | 31.18 ± 5.7 years | 53.6 ± 7.5 years | 0.0008 | |
| Follow-up | 66.6 ± 46.8 months | 77.94 ± 51.6 months | 41.8 ± 21.2 months | 0.067 | |
| Time to recurrence | 23.11 ± 20.38 months | 21.45 ± 19.29 months | 26.75 ± 24.54 months | 0.68 | |
| FIGO Classification (2009) | 0.0343 | 0.294 | |||
| IA1 y LVI + | 0/7 | 0 | 0 | ||
| IA2 | 1/25 (4%) | 1/11 (9.1%) | 0 | ||
| IB1 < 2 cm | 6/133 (4.5%) | 5/11 (45.5%) | 1/5 (20.0%) | ||
| IB1 ≥ 2 cm | 9/57 (15.8%) | 5/11 (45.5%) | 4/5 (80.0%) | ||
| Histology | 0.5669 | 1 | |||
| Adenocarcinoma | 8/84 (9.5%) | 6/11 (54.5%) | 2/5 (40.0%) | ||
| Squamous carcinoma | 8/137 (5.8%) | 5/11 (45.5%) | 3/5 (60.0%) | ||
| Adenosquamous carcinoma | 0/1 | 0 | 0 | ||
| LVI | 0.3098 | 1 | |||
| Positive | 4/30 (13.3%) | 3/11 (27.3%) | 1/5 (20.0%) | ||
| Negative | 12/192 (6.2%) | 8/11 (72.7%) | 4/5 (80.0%) | ||
| Tumour size | |||||
| Smaller than 2 cm | 6/155 (3.9%) | 0.0083 | 5/11 (45.5%) | 1/5 (20.0%) | 0.3803 |
| Larger or equal than 2 cm | 10/67 (14.9%) | 6/11 (54.5%) | 4/5 (80.0%) | ||
| Intraoperative complications | |||||
| Yes | 0/9 | 0 | 0 | ||
| No | 16/212 (8.3%) | 11/11 (100%) | 5/5 (100%) | ||
| Recurrence localizations | |||||
| Distance | 2 | 0 (0.0%) | 2 (40.0%) | 0.1532 | |
| Adnexal | 2 | 2 (18.2%) | 0 (0.0%) | ||
| Cervical | 3 | 3 (27.3%) | 0 (0.0%) | ||
| Lymph nodes | 3 | 2 (18.2%) | 1 (20.0%) | ||
| Local | 6 | 4 (36.4%) | 2 (40.0%) | ||
| Surgical approach | |||||
| Laparoscopy/Robotic | 10/109 (9.2%) | 6 (54.5%) | 4 (80.0%) | 0.5879 | |
| Laparotomy | 0 | 0 (0.0%) | 0 (0.0%) | ||
| Vaginal | 6/109 (5.5%) | 5 (45.5%) | 1 (20.0%) | ||
| Postoperative complications | |||||
| Yes | 0 | 0 | 0 | ||
| No | 16/206 | 11(100%) | 5(100%) | ||
| Matched Subsample n = 76 | Fertility-Sparing Surgery n = 38 | Radical Hysterectomy n = 38 | p-Value | |
|---|---|---|---|---|
| Age | 35.9 ± 7.1 years | 34.2 ± 5.5 years | 37.7 ± 8.1 years | 0.189 |
| Follow-up | 65.7 ± 43.1 months | 61.3 ± 40.5 months | 70.1 ± 45.6 months | 0.3749 |
| FIGO Classification (2009) IA1 and LVI+ | 0 | 0 | 0 | 0.5435 |
| IA2 | 9 (11.8%) | 3 (7.9%) | 6 (15.8%) | |
| IB1 < 2 cm | 41 (53.9%) | 22 (57.9%) | 19 (50.0%) | |
| IB1 > 2 cm | 26 (34.2%) | 13 (34.2%) | 13 (34.2%) | |
| Histology | ||||
| Adenocarcinoma | 28 (36.8%) | 14 (36.8%) | 14 (36.8%) | 1 |
| Squamous carcinoma | 48 (63.2%) | 24 (63.2%) | 24 (63.2%) | |
| Adenosquamous carcinoma | 0 | 0 | 0 | |
| LVI | ||||
| Positive | 14 (18.4%) | 8 (21.1%) | 6 (15.8%) | 0.7673 |
| Negative | 62 (81.6%) | 30 (78.9%) | 32 (84.2%) | |
| Tumour size | ||||
| Smaller than 2 cm | 45 (59.2%) | 22 (57.9%) | 23 (60.5%) | 1 |
| Larger than 2 cm | 31 (40.8%) | 16 (42.1%) | 15 (39.5%) | |
| Intraoperative complications | ||||
| Yes | 4 (5.3%) | 3 (7.9%) | 1 (2.6%) | 0.6075 |
| No | 72 (94.7%) | 35 (92.1%) | 37 (97.4%) | |
| Recurrence localizations | ||||
| Distance | 2 (12.5%) | 0 (0.0%) | 2 (40.0%) | 0.1532 |
| Adnexal | 2 (12.5%) | 2 (18.2%) | 0 (0.0%) | |
| Cervical | 3 (18.8%) | 3 (27.3%) | 0 (0.0%) | |
| Lymph nodes | 3 (18.8%) | 2 (18.2%) | 1 (20.0%) | |
| Local | 6 (37.5%) | 4 (36.4%) | 2 (40.0%) | |
| Surgical approach | ||||
| Laparoscopy/robotic | 48 (63.2%) | 23 (60.5%) | 25 (65.8%) | 0.812 |
| Laparotomy | 0 | 0 | 0 | |
| Vaginal | 28 (36.8%) | 15 (39.5%) | 13 (34.2%) | |
| Recurrence | ||||
| Yes | 16 (21.1%) | 11 (28.9%) | 5 (13.2%) | 0.1595 |
| No | 60 (78.9%) | 27 (71.1%) | 33 (86.8%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Age | 1.06 (0.98–1.13) | 0.101 | 1.05 (0.99–1.12) | 0.06 |
| Tumour size larger than 2 cm vs. tumour size smaller than 2 cm | 2.091 (1.02–4.28) | 0.04 | 1.99 (0.94–4.19) | 0.07 |
| Squamous carcinoma vs. adenocarcinoma | 0.59 (0.22–1.57) | 0.288 | 0.59 (0.20–1.76) | 0.34 |
| Vaginal surgical approach vs. laparoscopy/robotic | 0.82 (0.29–2.27) | 0.702 | 0.91 (0.31–2.66) | 0.87 |
| LVI-positive vs. LVI-negative | 2.101 (0.67–6.54) | 0.2 | 1.83 (0.47–7.01) | 0.37 |
| Fertility-Sparing Surgery n = 38 | Hysterectomy n = 38 | HR (Fertility-Sparing Surgery vs. Hysterectomy) (95% CI) | |
|---|---|---|---|
| Matched subsample: | 11/38 (28.9%) | 5/38 (13.2%) | 2.5 (0.89; 7.41) |
| Tumour size: smaller than 2 cm | 5/22 (22.72%) | 1/23 (4.35%) | 5.90 (0.69; 50.63) |
| Tumour size: greater than 2 cm | 6/16 (37.5%) | 4/15 (26.67%) | 1.71 (0.48; 6.11) |
| Histology: adenocarcinoma | 6/14 (42.86%) | 2/14 (14.29%) | 3.84 (0.77; 19.20) |
| Histology: squamous carcinoma | 5/24 (20.83%) | 3/24 (12.5%) | 1.87 (0.44; 7.85) |
| Surgical approach: laparoscopic/robotic | 6/23 (26.09%) | 4/25 (16%) | 1.85 (0.52; 6.61) |
| Surgical approach: vaginal | 5/15 (33.33%) | 1/13 (7.69%) | 6.33 (0.73; 54.99) |
| Figo Classification (2009): IB1 < 2 cm | 5/22 (22.73%) | 1/19 (5.26%) | 5.35 (0.62; 45.99) |
| Figo Classification (2009): IB1 > 2 cm | 5/13 (38.46%) | 4/13 (30.77%) | 1.50 (0.40; 5.67) |
| LVI: Negative | 8/30 (26.67%) | 4/32 (12.5%) | 2.55 (0.77; 8.53) |
| LVI: Positive | 3/8 (37.5%) | 1/6 (16.67%) | 2.74 (0.28; 26.68) |
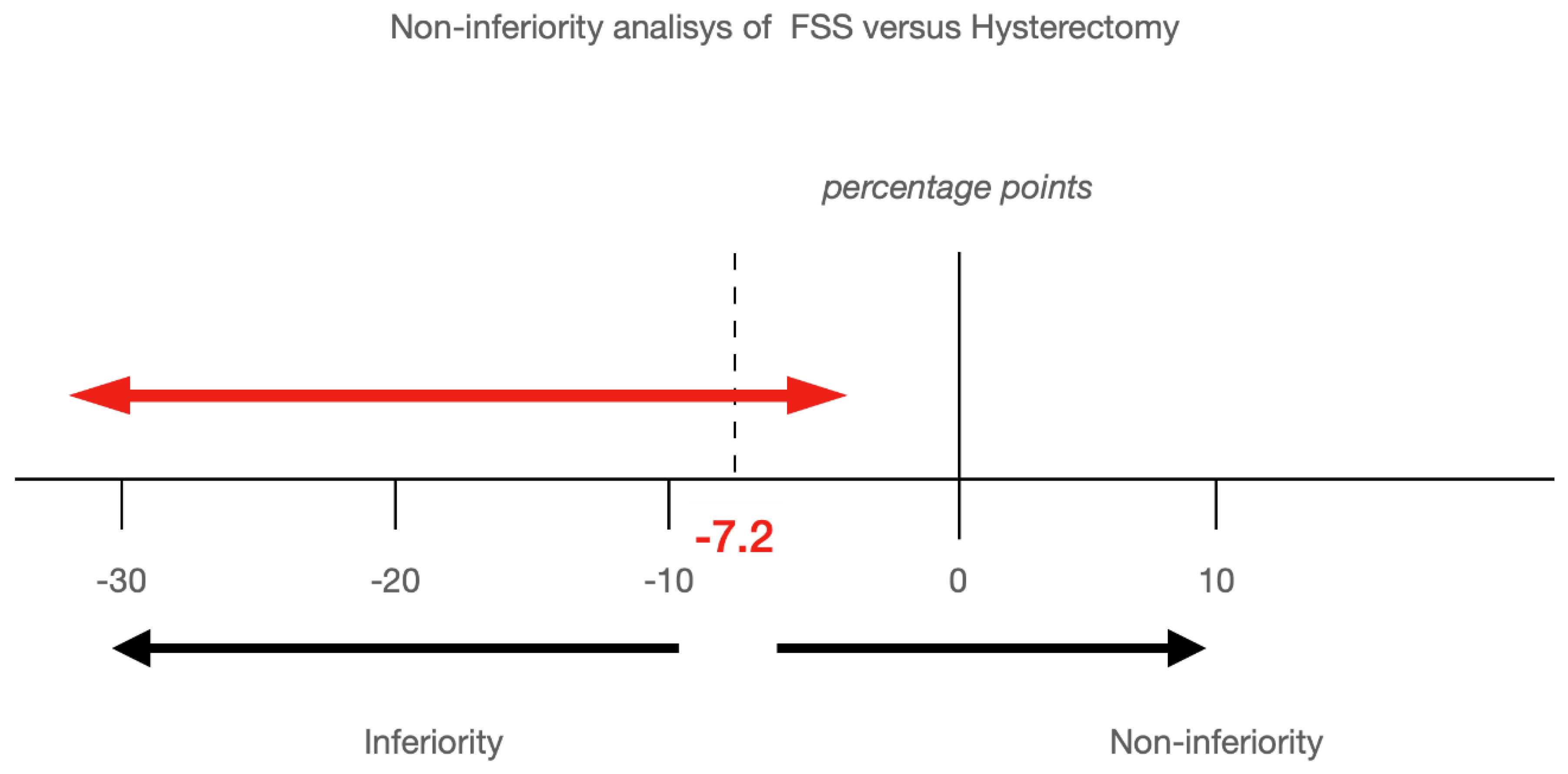
| Years from Surgery | Disease-Free Recurrence Rate (Rate (95% CI)) | Difference (95% CI) | |
|---|---|---|---|
| Fertility-Sparing Surgery | Hysterectomy | ||
| 2.5 years | 77.46 (64.79–92.60) | 91.40 (82.53–100) | −13.94 (−24.84, −3.03) |
| 5 years | 68.99 (54.22–87.77) | 88.01 (77.59–99.83) | −19.02 (−32.08, −5.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llueca, A.; Ibañez, M.V.; Torne, A.; Gil-Moreno, A.; Martin-Jimenez, A.; Diaz-Feijoo, B.; Serra, A.; Climent, M.T.; Gil-Ibañez, B.; on behalf of the Spain-GOG Cervical Cancer Working Group. Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study. J. Pers. Med. 2022, 12, 1081. https://doi.org/10.3390/jpm12071081
Llueca A, Ibañez MV, Torne A, Gil-Moreno A, Martin-Jimenez A, Diaz-Feijoo B, Serra A, Climent MT, Gil-Ibañez B, on behalf of the Spain-GOG Cervical Cancer Working Group. Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study. Journal of Personalized Medicine. 2022; 12(7):1081. https://doi.org/10.3390/jpm12071081
Chicago/Turabian StyleLlueca, Antoni, Maria Victoria Ibañez, Aureli Torne, Antonio Gil-Moreno, Angel Martin-Jimenez, Berta Diaz-Feijoo, Anna Serra, Maria Teresa Climent, Blanca Gil-Ibañez, and on behalf of the Spain-GOG Cervical Cancer Working Group. 2022. "Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study" Journal of Personalized Medicine 12, no. 7: 1081. https://doi.org/10.3390/jpm12071081
APA StyleLlueca, A., Ibañez, M. V., Torne, A., Gil-Moreno, A., Martin-Jimenez, A., Diaz-Feijoo, B., Serra, A., Climent, M. T., Gil-Ibañez, B., & on behalf of the Spain-GOG Cervical Cancer Working Group. (2022). Fertility-Sparing Surgery versus Radical Hysterectomy in Early Cervical Cancer: A Propensity Score Matching Analysis and Noninferiority Study. Journal of Personalized Medicine, 12(7), 1081. https://doi.org/10.3390/jpm12071081







