Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer
Abstract
:1. Introduction
2. Materials and Methods
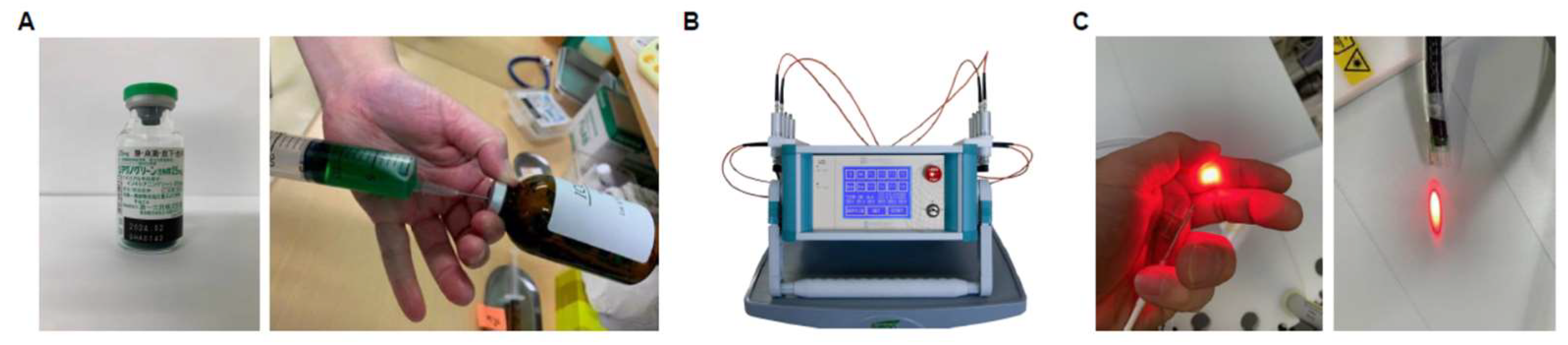
2.1. Preparation of ICG Liposomes and PDT
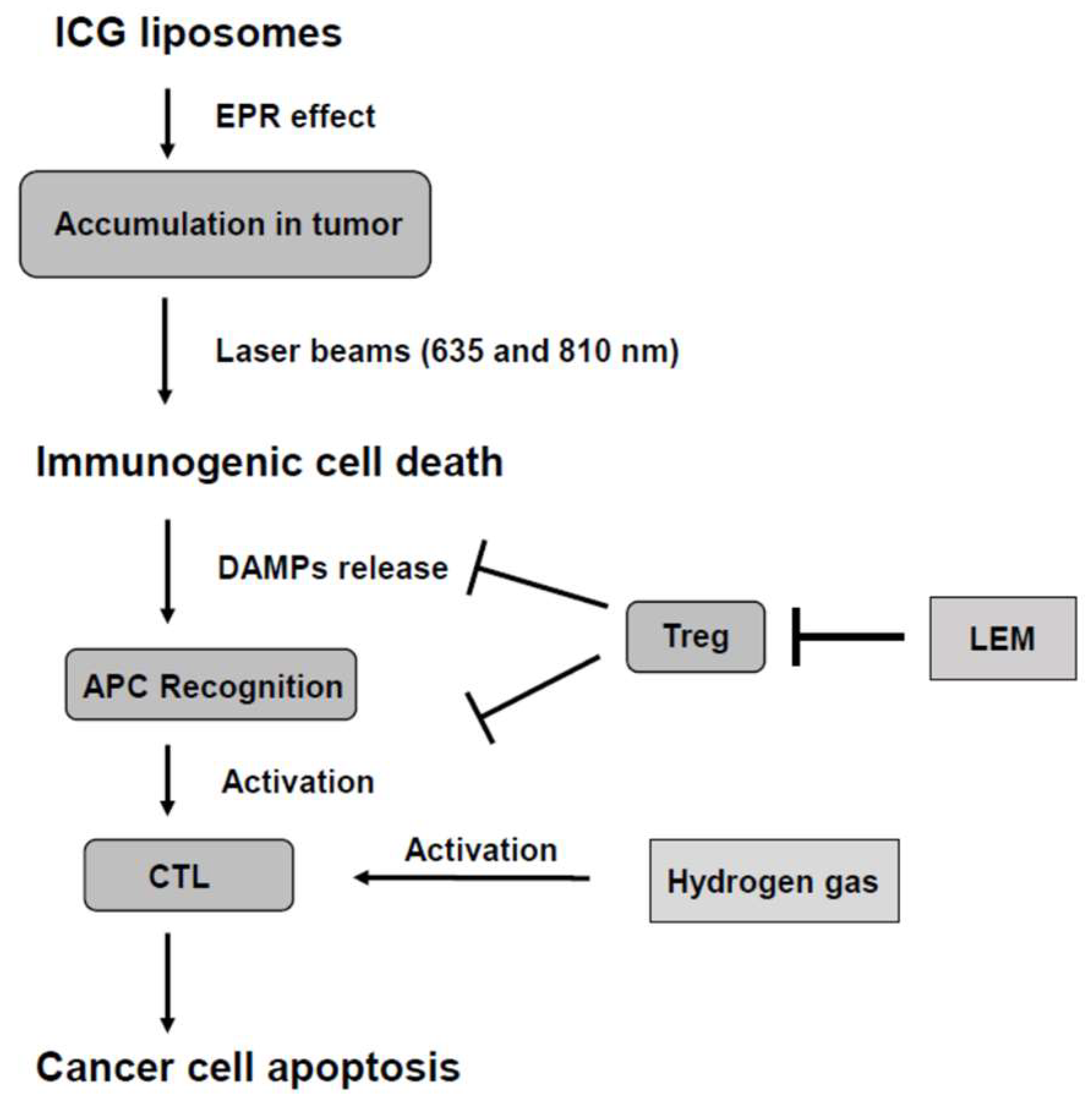
2.2. Combination of LEM and Hydrogen Gas Therapies
3. Results
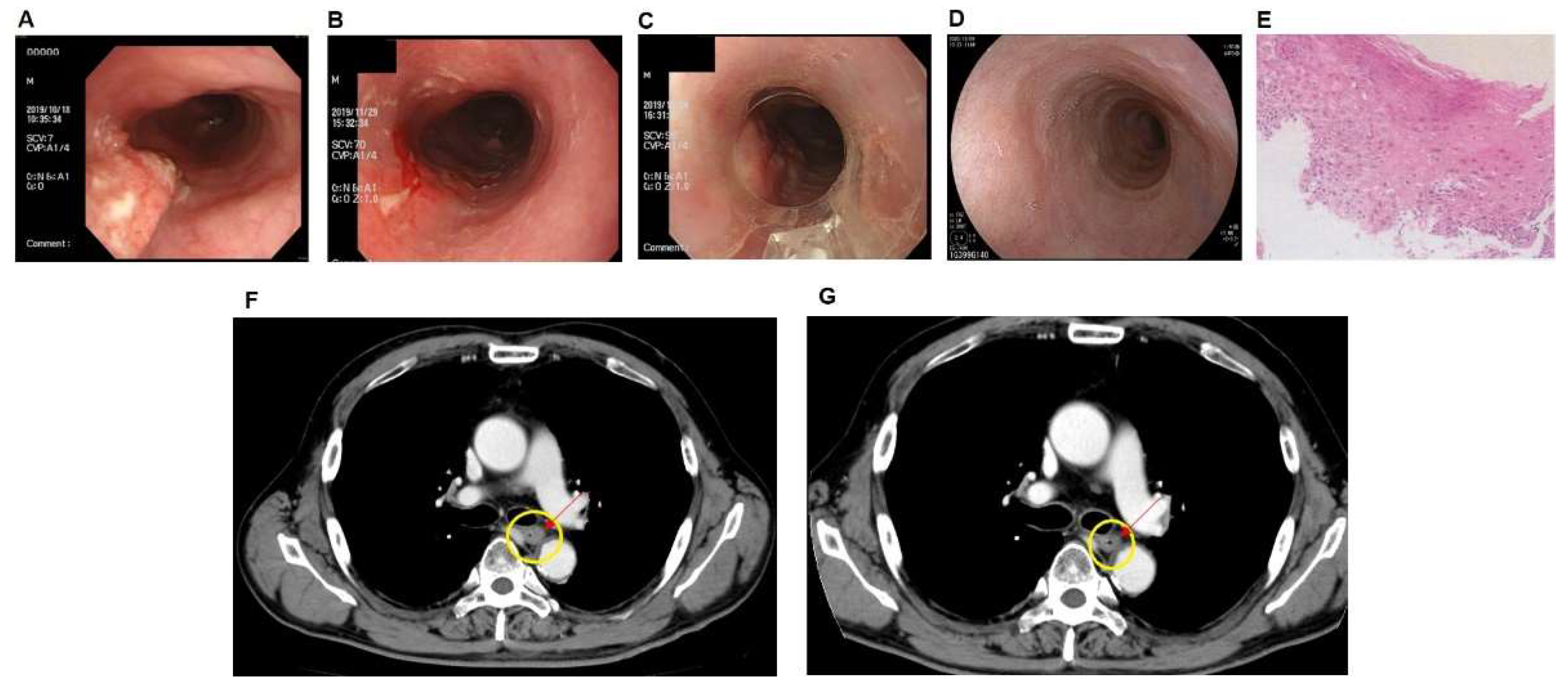
3.1. Case of PDT Combined with Radiotherapy in Middle Intrathoracic Esophagus Carcinoma
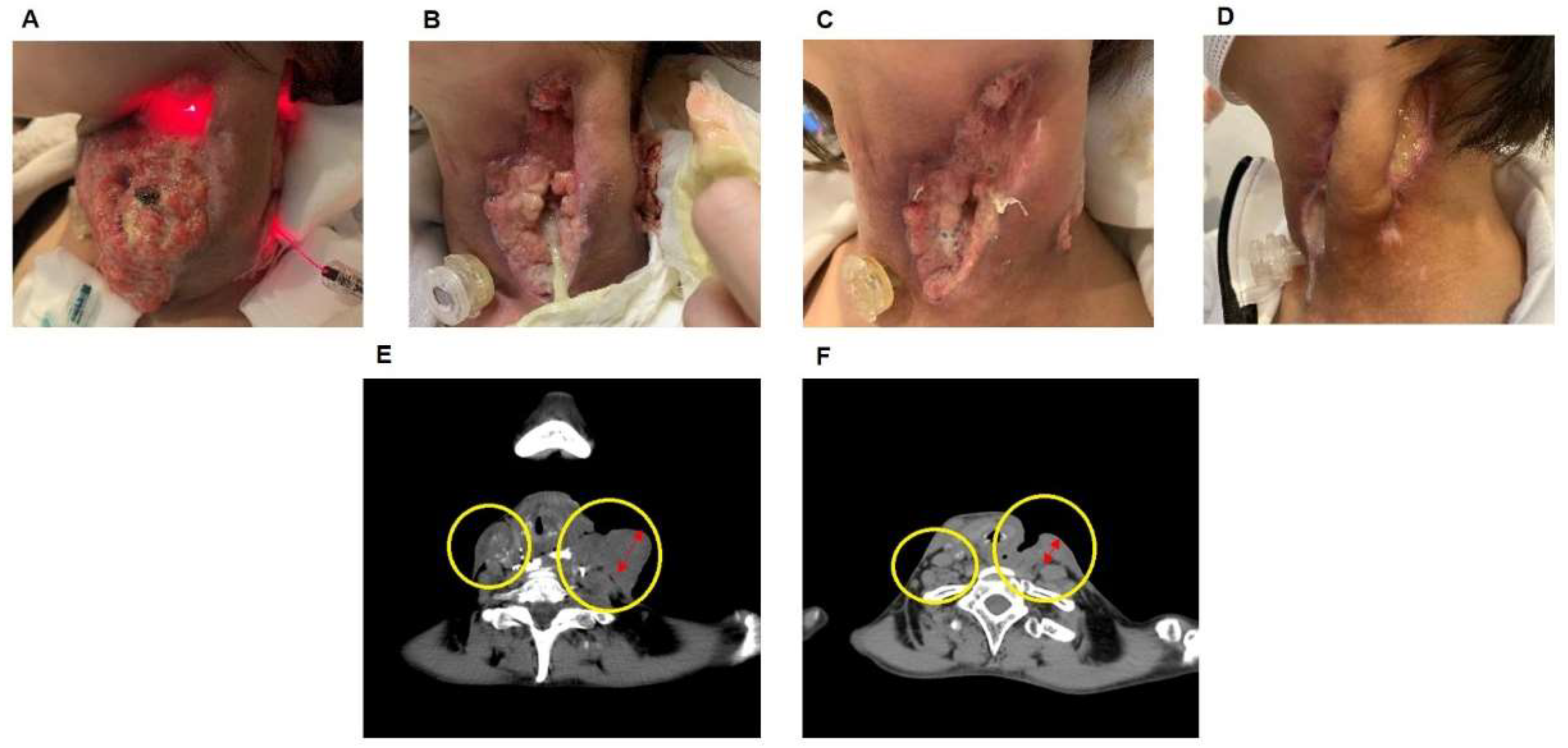
3.2. Case of the Effect of PDT on Hypopharyngeal Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaibori, M.; Kosaka, H.; Matsui, K.; Ishizaki, M.; Matsushima, H.; Tsuda, T.; Hishikawa, H.; Okumura, T.; Sekimoto, M. Near-infrared fluorescence imaging and photodynamic therapy for liver tumors. Front. Oncol. 2021, 11, 638327. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Moretti, A.I.; Abrahão, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for photodynamic therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Polyprodrug nanomedicines: An emerging paradigm for cancer therapy. Adv. Mater. 2022, 34, e2107434. [Google Scholar] [CrossRef]
- Di Mascio, P.D.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Ma, H.; Lu, Y.; Huang, Z.; Long, S.; Cao, J.; Zhang, Z.; Zhou, X.; Shi, C.; Sun, W.; Du, J.; et al. ER-targeting cyanine dye as an NIR photoinducer to efficiently trigger photoimmunogenic cancer cell death. J. Am. Chem. Soc. 2022, 144, 3477–3486. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, Z.; Yang, W.; Lin, L.S.; Wang, S.; Niu, G.; Song, J.; Chen, X. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020, 20, 1928–1933. [Google Scholar] [CrossRef]
- Papayan, G.; Akopov, A. Potential of indocyanine green near-infrared fluorescence imaging in experimental and clinical practice. Photodiagn Photodyn. Ther. 2018, 24, 292–299. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, S.; Wen, L.; Zhu, Y.; Tan, Y.; Qiu, G.; Meng, T.; Yu, F.; Yuan, H.; Hu, F. Enhancing drug delivery for overcoming angiogenesis and improving the phototherapy efficacy of glioblastoma by ICG-loaded glycolipid-like micelles. Int. J. Nanomed. 2020, 15, 2717–2732. [Google Scholar] [CrossRef] [Green Version]
- Hishikawa, H.; Kaibori, M.; Tsuda, T.; Matsui, K.; Okumura, T.; Ozeki, E.; Yoshii, K. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosomes has antineoplastic effects for gallbladder cancer. Oncotarget 2019, 10, 5622–5631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xie, D.; Pan, J.; Xia, C.; Fan, L.; Pu, Y.; Zhang, Q.; Ni, Y.H.; Wang, J.; Hu, Q. A near-infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater. Sci. 2019, 7, 5270–5282. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanigawa, K.; Itoh, Y.; Kobayashi, Y. Improvement of QOL and immunological function With Lentinula edodes mycelia in patients undergoing cancer immunotherapy: An open pilot study. Altern. Ther. Health Med. 2016, 22, 36–42. [Google Scholar] [PubMed]
- Tanaka, K.; Ishikawa, S.; Matsui, Y.; Kawanishi, T.; Tamesada, M.; Harashima, N.; Harada, M. Combining a peptide vaccine with oral ingestion of Lentinula edodes mycelia extract enhances antitumor activity in B16 melanoma-bearing mice. Cancer Immunol. Immunother. 2012, 61, 2143–2152. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kojima, H.; Tamura, A.; Tsuji, K.; Tamesada, M.; Yagi, K.; Murakami, N. Low-molecular-weight lignin-rich fraction in the extract of cultured Lentinula edodes mycelia attenuates carbon tetrachloride-induced toxicity in primary cultures of rat hepatocytes. J. Nat. Med. 2012, 66, 185–191. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Akagi, J.; Baba, H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol. Rep. 2019, 41, 301–311. [Google Scholar] [CrossRef] [Green Version]
- McCall, M.W.; Greenway, H.T.; Mohs, F.E. Mohs’ chemosurgery for skin cancer, microscopically controlled excision. J. Ky. Med. Assoc. 1981, 79, 613–616. [Google Scholar]
- Lamberti, M.J.; Nigro, A.; Mentucci, F.M.; Rumie Vittar, N.B.; Casolaro, V.; Dal Col, J. Dendritic cells and immunogenic cancer cell death: A combination for improving antitumor immunity. Pharmaceutics 2020, 12, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourie, H.R.; Klastersky, J. Immune checkpoint inhibitors side effects and management. Immunotherapy 2016, 8, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mikada, M.; Sukhbaatar, A.; Miura, Y.; Horie, S.; Sakamoto, M.; Mori, S.; Kodama, T. Evaluation of the enhanced permeability and retention effect in the early stages of lymph node metastasis. Cancer Sci. 2017, 108, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, W.; Kimura, S.; Islam, R.; Harada, A.; Ono, K.; Fang, J.; Niidome, T.; Sawa, T.; Maeda, H. EPR-effect enhancers strongly potentiate tumor-targeted delivery of nanomedicines to advanced cancers: Further extension to enhancement of the therapeutic effect. J. Pers. Med. 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, H.; Hou, X.; Wang, L.; Li, Y.; Pang, Y.; Chen, C.; Jiang, G.; Liu, Y. Enhanced antitumor efficacy of temozolomide-loaded carboxylated poly (amido-amine) combined with photothermal/photodynamic therapy for melanoma treatment. Cancer Lett. 2018, 423, 16–26. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yorozu, K.; Kaibori, M.; Kimura, S.; Ichikawa, M.; Matsui, K.; Kaneshige, S.; Kobayashi, M.; Jimbo, D.; Torikai, Y.; Fukuzawa, Y.; et al. Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer. J. Pers. Med. 2022, 12, 1039. https://doi.org/10.3390/jpm12071039
Yorozu K, Kaibori M, Kimura S, Ichikawa M, Matsui K, Kaneshige S, Kobayashi M, Jimbo D, Torikai Y, Fukuzawa Y, et al. Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer. Journal of Personalized Medicine. 2022; 12(7):1039. https://doi.org/10.3390/jpm12071039
Chicago/Turabian StyleYorozu, Kensho, Masaki Kaibori, Shintarou Kimura, Misa Ichikawa, Kosuke Matsui, Soichiro Kaneshige, Masanori Kobayashi, Daiki Jimbo, Yusuke Torikai, Yoshitaka Fukuzawa, and et al. 2022. "Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer" Journal of Personalized Medicine 12, no. 7: 1039. https://doi.org/10.3390/jpm12071039
APA StyleYorozu, K., Kaibori, M., Kimura, S., Ichikawa, M., Matsui, K., Kaneshige, S., Kobayashi, M., Jimbo, D., Torikai, Y., Fukuzawa, Y., & Okamoto, Y. (2022). Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer. Journal of Personalized Medicine, 12(7), 1039. https://doi.org/10.3390/jpm12071039







