Machine Learning Algorithm-Based Prediction Model for the Augmented Use of Clozapine with Electroconvulsive Therapy in Patients with Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview and Participants
2.2. Variable Profiles for the Substantial Prediction Model
2.3. Data Processing and Machine Learning
3. Results
3.1. General Characteristics of the Study Participants
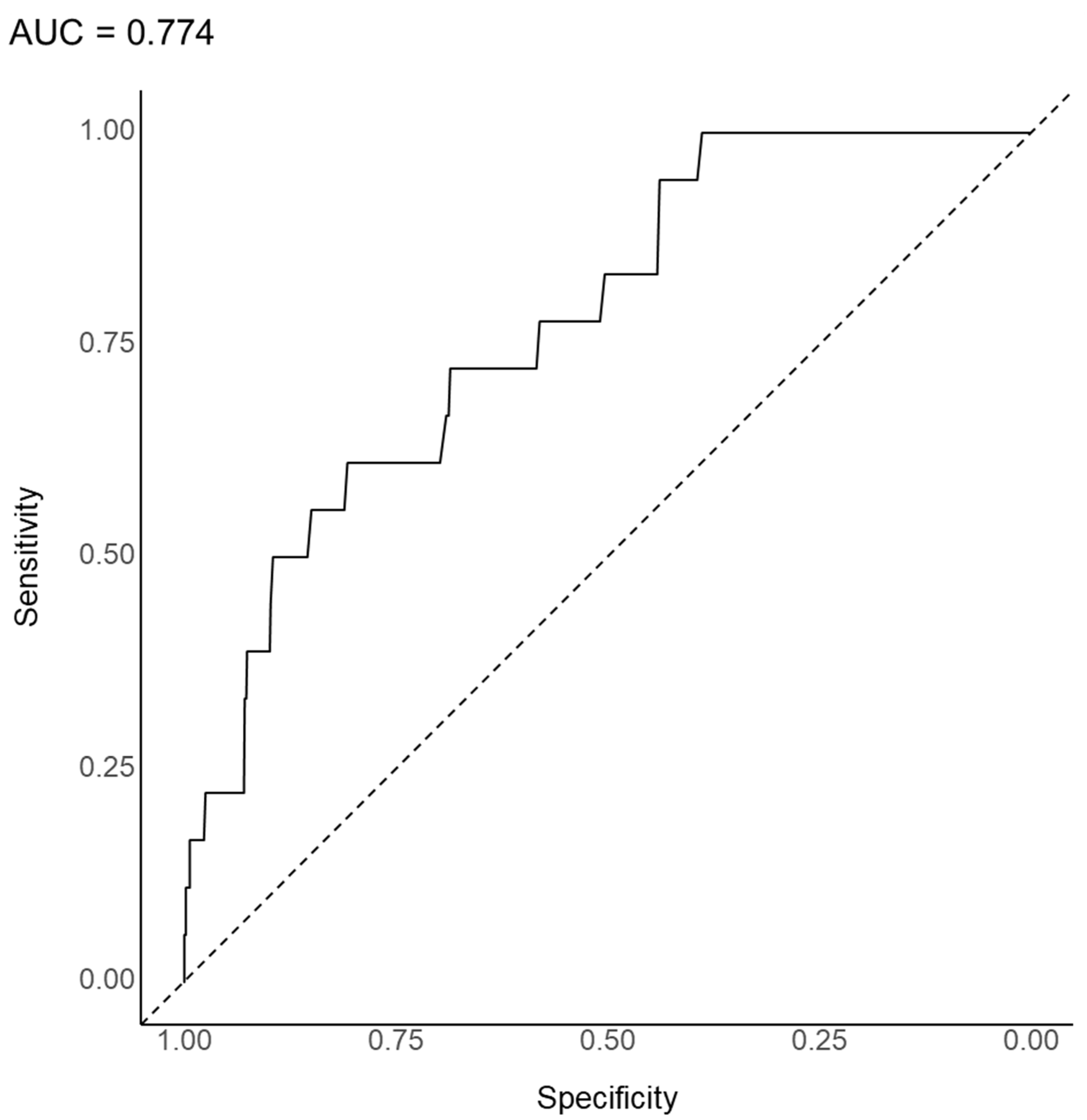
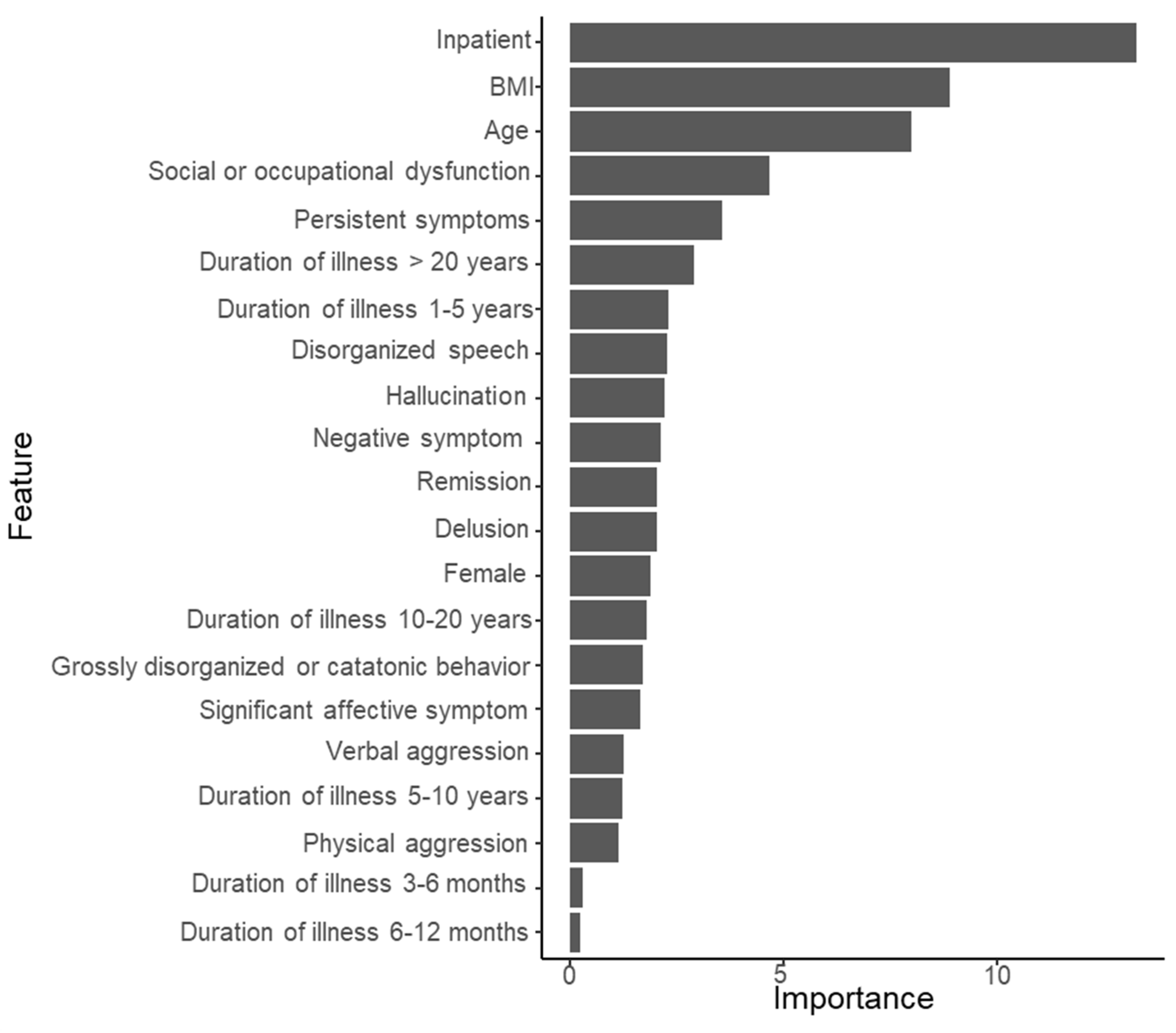
3.2. Substantial Prediction Model Performance and Variable Importance: Random Forest Model
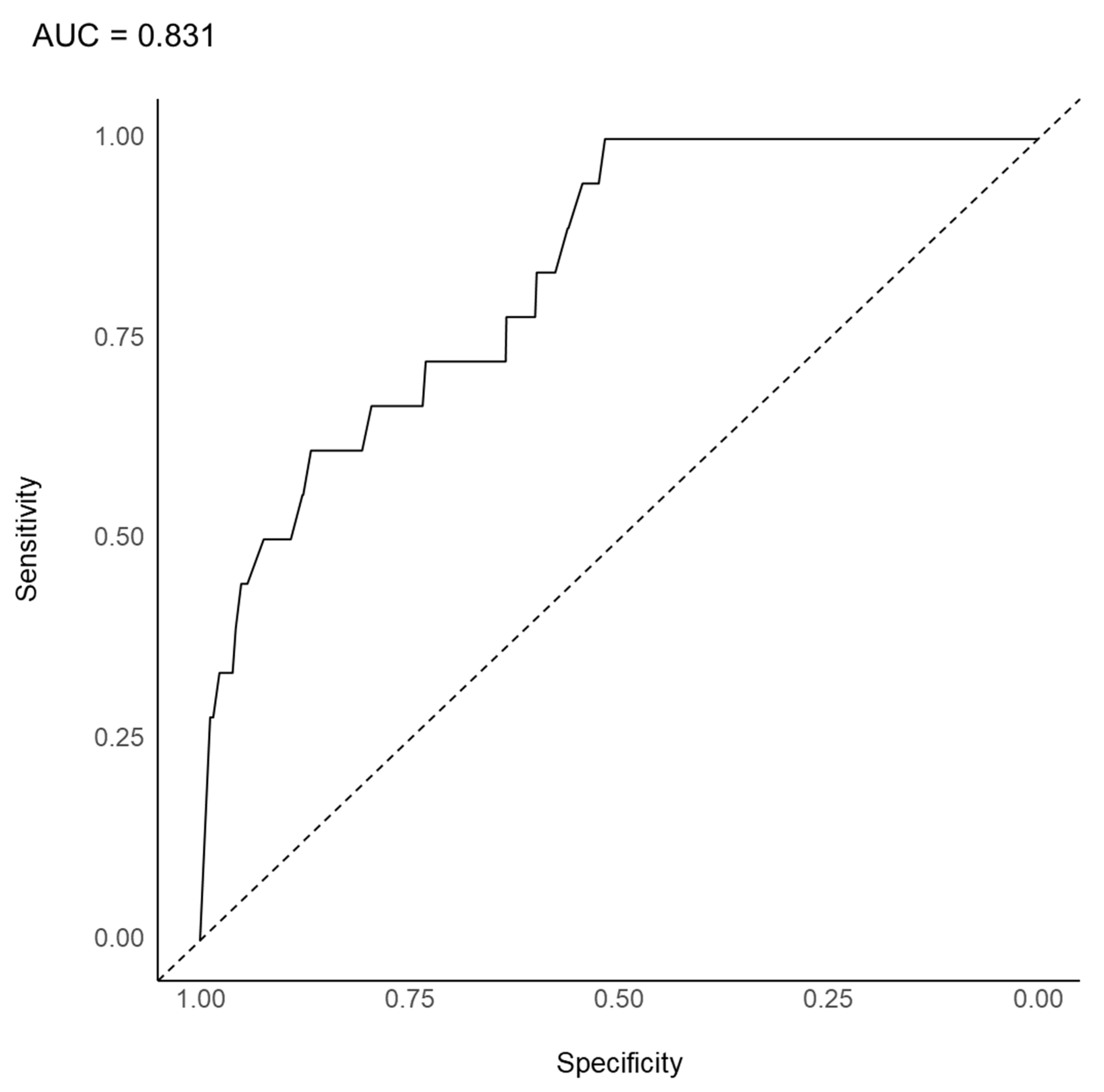
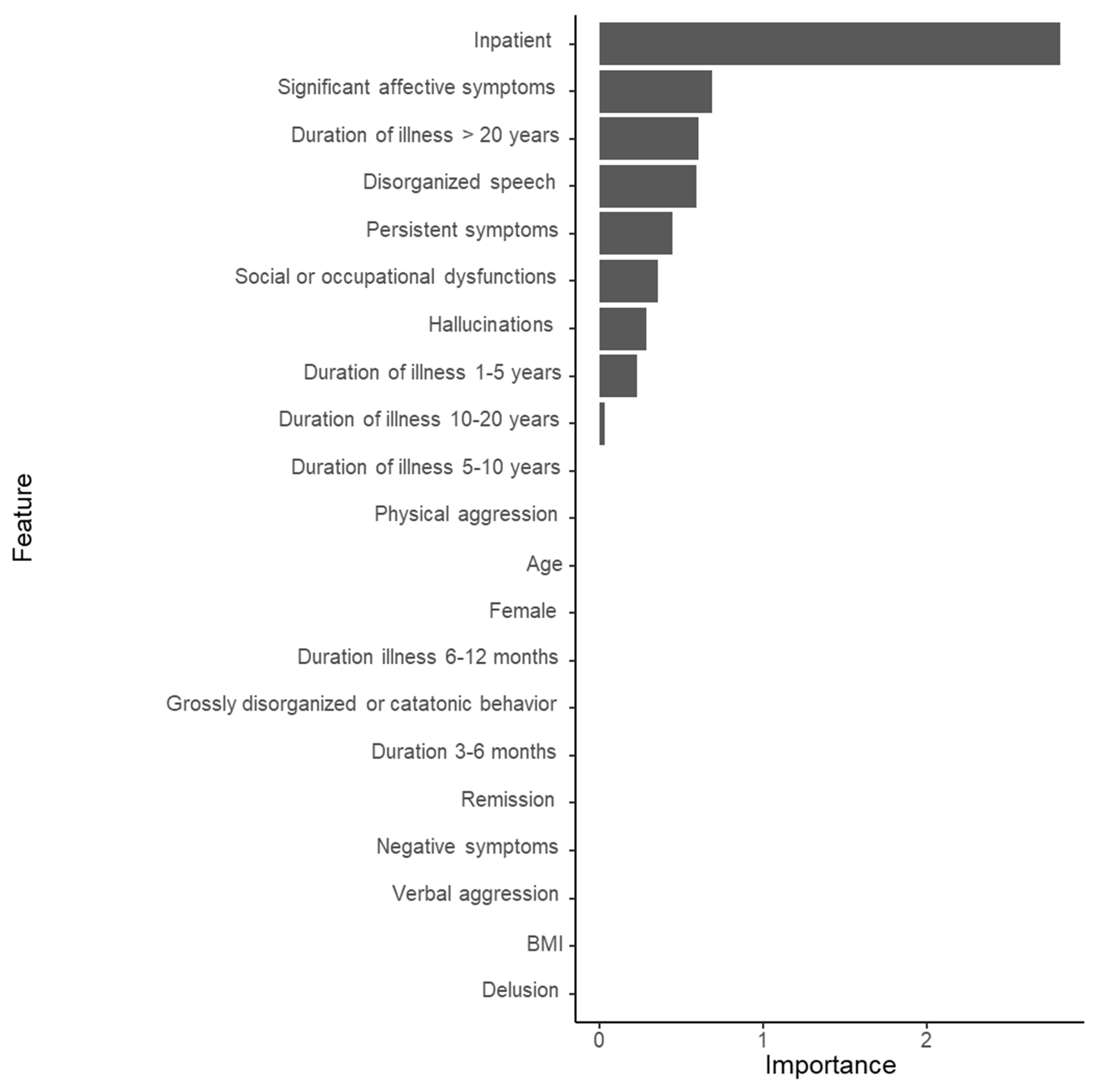
3.3. Substantial Prediction Model Performance and Variable Importance: LASSO Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joo, S.W.; Kim, H.; Jo, Y.T.; Ahn, S.; Choi, Y.J.; Choi, W.; Park, S.; Lee, J. Comparative effectiveness of antipsychotic monotherapy and polypharmacy in schizophrenia patients with clozapine treatment: A nationwide, health insurance data-based study. Eur. Neuropsychopharmacol. 2022, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kanahara, N.; Nakamura, M.; Shiko, Y.; Kawasaki, Y.; Iyo, M. Are serum oxytocin concentrations lower in patients with treatment-resistant schizophrenia?: A 5-year longitudinal study. Asian J. Psychiatry 2022, 6, 103157. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.; Honigfeld, G.; Singer, J.; Melter, H. Clozapine for the treatment resistant schizophrenic: A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Buchanan, R.W.; Kirkpatrick, B.; Davis, O.R.; Irish, D.; Summerfelt, A.; Carpenter, W.T., Jr. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am. J. Psychiatry 1994, 151, 20–26. [Google Scholar] [CrossRef]
- Melzer, H.Y.; Bastani, B.; Kwon, K.Y.; Ramirez, L.F.; Burnett, S.; Sharpe, J. A prospective study of clozapine in treatment-resistant schizophrenic patients, I: Preliminary report. Psychopharmacology 1989, 99, S68–S72. [Google Scholar] [CrossRef]
- Weiner, R.D.; Coffey, C.; Fochtmann, L. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association, 2nd ed.; American Psychiatric Publishing: Washington, DC, USA, 2001. [Google Scholar]
- Petrides, G.; Malur, C.; Braga, R.J.; Bailine, S.H.; Schooler, N.R.; Malhotra, A.K.; Kane, J.M.; Sanghani, S.; Goldberg, T.E.; John, M.; et al. Electroconvulsive Therapy Augmentation in Clozapine-Resistant Schizophrenia: A Prospective, Randomized Study. Am. J. Psychiatry 2015, 172, 52–58. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, I.W.; Kang, U.G.; Ahn, Y.M.; Youn, T. Principle and Practice of Electroconvulsive Therapy; Seoul National University Press: Seoul, Korea, 2019; p. 133. [Google Scholar]
- Manubens, L.K.; Urbina, D.L.; Aceituno, D. Is electroconvulsive therapy effective as augmentation in clozapine-resistant schizophrenia? Medwave 2016, 16 (Suppl. 5), e6577. [Google Scholar] [CrossRef]
- Lally, J.; Tully, J.; Robertson, D.; Stubbs, B.; Gaughran, F.; MacCabe, J.H. Augmentation of clozapine with electroconvulsive therapy in treatment resistant schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2016, 171, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, S.H.; Lee, N.Y.; Youn, T.; Lee, J.H.; Chung, S.; Kim, Y.S.; Chung, I.W. Effectiveness of Electroconvulsive Therapy Augmentation on Clozapine-Resistant Schizophrenia. Psychiatry Investig. 2017, 14, 58–62. [Google Scholar] [CrossRef]
- Kim, J.H.; Youn, T.; Choi, J.G.; Jeong, S.H.; Jung, H.Y.; Kim, Y.S.; Chung, I.W. Combination of Electroconvulsive Therapy and Clozapine in Treatment-Resistant Schizophrenia. Psychiatry Investig. 2018, 15, 829–835. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tognin, S.; Van Hell, H.H.; Merritt, K.; Rossum, I.W.-V.; Bossong, M.G.; Kempton, M.J.; Modinos, G.; Fusar-Poli, P.; Mechelli, A.; Dazzan, P.; et al. Towards Precision Medicine in Psychosis: Benefits and Challenges of Multimodal Multicenter Studies—PSYSCAN: Translating Neuroimaging Findings From Research into Clinical Practice. Schizophr. Bull. 2019, 46, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Grzenda, A.; Kraguljac, N.V.; McDonald, W.M.; Nemeroff, C.; Torous, J.; Alpert, J.E.; Rodriguez, C.I.; Widge, A.S. Evaluating the Machine Learning Literature: A Primer and User’s Guide for Psychiatrists. Am. J. Psychiatry 2021, 178, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yoon, H.-J.; Park, J.H.; Nakagami, Y.; Kubota, C.; Inada, T.; Kato, T.A.; Yang, S.-Y.; Lin, S.-K.; Chong, M.-Y.; et al. Network Analysis-Based Disentanglement of the Symptom Heterogeneity in Asian Patients with Schizophrenia: Findings from the Research on Asian Psychotropic Prescription Patterns for Antipsychotics. J. Pers. Med. 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Kim, G.-M.; Kato, T.A.; Chong, M.-Y.; Lin, S.-K.; Yang, S.-Y.; Avasthi, A.; Grover, S.; Kallivayalil, R.A.; Xiang, Y.-T.; et al. Dyskinesia is most centrally sit-uated in an estimated network of extrapyramidal syndrome in Asian patients with schizophrenia: Findings from Research on Asian Psychotropic Prescription Patterns for Antipsychotics. Nordic. J. Psychiatry 2021, 75, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, B.J.; Park, J.H.; Kawasaki, H.; Avasthi, A.; Grover, S.; Tanra, A.J.; Lin, S.; Javed, A.; Tan, C.H.; et al. QT interval prolongation noted in one percent of 2553 Asian patients with schizophrenia: Findings from the REAP-AP survey. Kaohsiung. J. Med. Sci. 2020, 36, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Anatomical Therapeutic Chemical (ATC) Classification System. Available online: https://www.whocc.no/atc/structure_and_principles/ (accessed on 18 January 2020).
- Sugawara, N.; Yasui-Furukori, N.; Yamazaki, M.; Shimoda, K.; Mori, T.; Sugai, T.; Matsuda, H.; Suzuki, Y.; Ozeki, Y.; Okamoto, K.; et al. Predictive Utility of Body Mass Index for Metabolic Syndrome Among Patients with Schizophrenia in Japan. Neuropsychiatr. Dis. Treat. 2020, 16, 2229–2236. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, K.; Li, H.; Zhou, J.; Xiong, D.; Huang, X.; Li, J.; Liu, Y.; Pan, Z.; Mitchell, D.T.; et al. Homocysteine level, body mass index and clinical correlates in Chinese Han patients with schizophrenia. Sci. Rep. 2020, 10, 16–18. [Google Scholar] [CrossRef]
- Lin, S.H.; Tseng, H.H.; Tsai, H.C.; Chi, M.H.; Lee, I.H.; Chen, P.S.; Chen, K.C.; Yang, Y.K. Positive symptoms in antipsychotic-naïve schizophrenia are associated with increased body mass index after treatment. Clin. Psychopharmacol. Neurosci. 2021, 19, 155–159. [Google Scholar] [CrossRef]
- Luckhoff, H.; du Plessis, S.; Scheffler, F.; Phahladira, L.; Kilian, S.; Buckle, C.; Smit, R.; Chiliza, B.; Asmal, L.; Emsley, R. Fronto-limbic white matter fractional anisotropy and body mass index in first-episode schizophrenia spectrum disorder patients compared to healthy controls. Psychiatry Res. Neuroimag. 2020, 305, 111173. [Google Scholar] [CrossRef]
- Okusaga, O.O.; Kember, R.L.; Peloso, G.M.; Peterson, R.E.; Vujkovic, M.; Mitchell, B.G.; Bernard, J.; Walder, A.; Bigdeli, T.B. History of Suicide Attempts and COVID-19 Infection in Veterans with Schizophrenia or Schizoaffective Disorder: Moderating Effects of Age and Body Mass Index. Complex Psychiatry 2021, 392, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Cho, S.-E.; Geem, Z.W.; Na, K.-S. Development of a Suicide Prediction Model for the Elderly Using Health Screening Data. Int. J. Environ. Res. Public Health 2021, 18, 10150. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, T.J.; Zwinderman, A.H. Machine Learning in Medicine – A Complete Review, 2nd ed.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Breiman, L. Random forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Tibshirani, R.; Freedman, J. The Elements of Statistical Learning, 2nd ed.; Springer: New York, NY, USA, 2017; pp. 593–594. [Google Scholar]
- Sicotte, X.B. Lasso Regression: Implementation of Coordinate Descent. Data Science, Machine Learning and Statistics, Implemented in Python. 2018. Available online: https://xavierbourretsicotte.github.io/lasso_implementation.html (accessed on 3 June 2022).
- Leucht, S.; Kane, J.M.; Kissling, W.; Hamann, J.; Etschel, E.; Engel, R. Clinical implications of Brief Psychiatric Rating Scale scores. Br. J. Psychiatry 2005, 187, 366–371. [Google Scholar] [CrossRef]
- Leucht, S.; Kane, J.M.; Kissling, W.; Hamann, J.; Etschel, E.; Engel, R.R. What does the PANSS mean? Schizophr. Res. 2005, 79, 231–238. [Google Scholar] [CrossRef]
- Correll, C.U.; Howes, O.D. Treatment-resistant schizophrenia: Definition, predictors, and therapy options. J. Clin. Psychiatry 2021, 82, MY20096AH1C. [Google Scholar] [CrossRef]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojitabai, R.; Servis, M.; Walaszek, A.; Buckley, P.; Lenzenweger, M.L.; et al. The American Psychiatric Association Guideline for the Treatment of Patients with Schizophrenia, 3rd ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2021. [Google Scholar]
- Carbon, M.; Correl, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [CrossRef]
- Ortiz, B.B.; Higuchi, C.H.; Noto, C.; Joyce, D.W.; Correll, C.U.; Bressan, R.A.; Gadelha, A. A symptom combination predicting treatment-resistant schizophrenia–A strategy for real-world clinical practice. Schizophr. Res. 2020, 218, 195–200. [Google Scholar] [CrossRef]
- Elkis, H.; Buckley, P.F. Treatment-resistant schizophrenia. Psychiatry Clin. N. Am. 2016, 39, 239–265. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef] [Green Version]
- Tranulis, C.; Mouaffak, F.; Chouchana, L.; Stip, E.; Gourevitch, R.; Poirier, M.F.; Olié, J.P.; Lôo, H.; Gourion, D. Somatic aug-mentation strategies in clozapine resistance—What facts? Clin. Neuropharmacol. 2006, 29, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Mouaffak, F.; Tranulis, C.; Gourevitch, R.; Poirier, M.-F.; Douki, S.; Olié, J.-P.; Lôo, H.; Gourion, D. Augmentation Strategies of Clozapine With Antipsychotics in the Treatment of Ultraresistant Schizophrenia. Clin. Neuropharmacol. 2006, 29, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Takeuchi, H.; Fervaha, G.; Sin, G.L.; Foussias, G.; Agid, O.; Farooq, S.; Remington, G. Subtyping Schizophrenia by Treatment Response: Antipsychotic Development and the Central Role of Positive Symptoms. Can. J. Psychiatry 2015, 60, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, O.D.; McCutcheon, R.; Agid, O.; De Bartolomeis, A.; Van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- Neto, J.H.; Elkis, H. Clinical aspects of super-refractory schizophrenia: A 6-month cohort observational study. Rev. Bras. Psiquiatry 2007, 29, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Buckley, P.; Miller, A.; Olsen, J.; Garver, D.; Miller, D.D.; Csernansky, J. When Symptoms Persist: Clozapine Augmentation Strategies. Schizophr. Bull. 2001, 27, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Pai, N.B.; Laidlaw, M.; Vella, S.-C. Augmentation of clozapine with another pharmacological agent: Treatment for refractory schizophrenia in the ‘real world’. Acta Psychiatr. Scand. 2012, 126, 40–46. [Google Scholar] [CrossRef]
- Tiihonen, J.; Wahlbeck, K.; Kiviniemi, V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2009, 109, 10–14. [Google Scholar] [CrossRef]
- Zheng, W.; Xiang, Y.T.; Xiang, Y.Q.; Li, X.B.; Ungvari, G.S.; Chiu, H.F.; Correll, C.U. Efficacy and safety of adjunctive topiramate for schizophrenia: A meta-analysis of randomized controlled trials. Acta Psychiatry Scand. 2016, 134, 385–398. [Google Scholar] [CrossRef]
- Sommer, I.E.; Begemann, M.J.H.; Temmerman, A.; Leucht, S. Pharmacological Augmentation Strategies for Schizophrenia Patients with Insufficient Response to Clozapine: A Quantitative Literature Review. Schizophr. Bull. 2011, 38, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Veerman, S.R.T.; Schulte, P.F.J.; Begemann, M.J.H.; de Haan, L. Non-Glutamatergic Clozapine Augmentation Strategies: A Review and Meta-Analysis. Pharmacopsychiatry 2014, 47, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; McCutcheon, R.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2019, 7, 64–77. [Google Scholar] [CrossRef]
- Haddad, P. Weight change with atypical antipsychotics in the treatment of schizophrenia. J. Psychopharmacol. 2005, 19, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Youn, T.; Jeong, S.H.; Kim, Y.S.; Chung, I.W. Long-term clinical efficacy of maintenance electroconvulsive therapy in patients with treatment-resistant schizophrenia on clozapine. Psychiatry Res. 2019, 273, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Havaki-Kontaxaki, B.J.; Ferentinos, P.P.; Kontaxakis, V.P.; Paplos, K.G.; Soldatos, C.R. Concurrent Administration of Clozapine and Electroconvulsive Therapy in Clozapine-Resistant Schizophrenia. Clin. Neuropharmacol. 2006, 29, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Chapter 5; John Wiley and Sons: New York, NY, USA, 2000; pp. 160–164. [Google Scholar]
- Rice, M.E.; Harris, G.T. Comparing effect sizes in follow-up studies: ROC Area, Cohen’s d, and r. Law Hum. Behav. 2005, 29, 615–620. [Google Scholar] [CrossRef]
- Sinclair, D.J.; Zhao, S.; Qi, F.; Nyakyoma, K.; Kwong, J.S.; Adams, C.E. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst. Rev. 2019, 3, CD011847. [Google Scholar] [CrossRef]
| Total (n = 3744) | Augmentation of Clozapine with ECT | Statistical Coefficient | p-Value | ||
|---|---|---|---|---|---|
| Yes (n = 47) | No (n = 3697) | ||||
| Country/SAR | χ2 = 19.616 | <0.0001 | |||
| Bangladesh, n (%) | 99 (2.6) | 0 (0.0) | 99 (2.7) | ||
| China, n (%) | 160 (4.3) | 20 (42.6) | 140 (87.5) | ||
| Hong Kong, n (%) | 31 (0.8) | 0 (0.0) | 31 (0.8) | ||
| India, n (%) | 479 (12.8) | 2 (4.3) | 477 (12.9) | ||
| Indonesia, n (%) | 581 (15.5) | 10 (21.3) | 571 (15.4) | ||
| Japan, n (%) | 229 (6.1) | 2 (4.3) | 227 (6.1) | ||
| Korea, n (%) | 131 (3.5) | 0 (0.0) | 131 (3.5) | ||
| Malaysia, n (%) | 305 (8.1) | 4 (8.5) | 301 (8.1) | ||
| Myanmar, n (%) | 164 (4.4) | 0 (0.0) | 164 (4.4) | ||
| Pakistan, n (%) | 298 (8.0) | 0 (0.0) | 298 (8.0) | ||
| Singapore, n (%) | 171 (4.6) | 2 (4.3) | 169 (4.6) | ||
| Sri Lanka, n (%) | 97 (2.6) | 1 (2.1) | 96 (2.6) | ||
| Thailand, n (%) | 322 (8.6) | 2 (4.3) | 320 (8.7) | ||
| Taiwan, n (%) | 403 (10.8) | 2 (4.3) | 401 (10.8) | ||
| Vietnam, n (%) | 274 (7.3) | 2 (4.3) | 272 (7.4) | ||
| Age, mean (SD) years | 39.5 (13.2) | 39.3 (13.6) | 39.5 (13.1) | t = −0.109 | 0.913 |
| Sex | χ2 = 5.142 | 0.023 | |||
| Male, n (%) | 2199 (58.7) | 20 (42.6) | 2179 (58.9) | ||
| Female, n (%) | 1545 (41.3) | 27 (57.4) | 1518 (41.1) | ||
| BMI, mean (SD) kg/m2 | 23.9 (4.7) | 23.2 (3.4) | 23.9 (4.7) | t = −1.376 | 0.319 |
| Hospitalization | χ2 = 39.942 | <0.0001 | |||
| Outpatient, n (%) | 1793 (47.9) | 1 (2.1) | 1792 (48.5) | ||
| Inpatient, n (%) | 1951 (52.1) | 46 (97.9) | 1905 (51.5) | ||
| Duration of illness | χ2 = 19.253 | 0.004 | |||
| <3 months, n (%) | 161 (4.3) | 6 (12.8) | 155 (4.2) | ||
| 3–6 months, n (%) | 125 (3.3) | 0 (0.0) | 125 (3.3) | ||
| 6–12 months, n (%) | 199 (5.3) | 1 (2.1) | 198 (5.4) | ||
| 1–5 years, n (%) | 794 (21.2) | 5 (0.6) | 789 (21.3) | ||
| 5–10 years, n (%) | 729 (19.5) | 8 (17.0) | 721 (19.5) | ||
| 10–20 years, n (%) | 971 (25.9) | 10 (21.3) | 961 (26.0) | ||
| >20 years, n (%) | 765 (20.4) | 17 (36.2) | 748 (20.2) | ||
| Clinical course for the past 1 year | |||||
| Remission, n (%) | 1262 (33.7) | 18 (38.3) | 1244 (33.6) | χ2 = 0.449 | 0.503 |
| Persistent symptoms, n (%) | 1917 (51.2) | 20 (42.6) | 1897 (51.3) | χ2 = 1.423 | 0.233 |
| Current symptoms | |||||
| Delusion, n (%) | 1599 (42.7) | 19 (40.4) | 1580 (42.7) | χ2 = 0.101 | 0.750 |
| Hallucination, n (%) | 1752 (46.8) | 19 (40.4) | 1733 (46.9) | χ2 = 0.776 | 0.378 |
| Disorganized speech, n (%) | 1110 (29.6) | 12 (25.5) | 1098 (29.7) | χ2 = 0.387 | 0.534 |
| Grossly disorganized or catatonic behavior, n (%) | 666 (17.8) | 7 (14.9) | 659 (17.8) | χ2 = 0.273 | 0.601 |
| Negative symptom, n (%) | 1313 (35.1) | 26 (55.3) | 1287 (34.8) | χ2 = 8.571 | 0.003 |
| Social or occupational dysfunction, n (%) | 1693 (45.2) | 28 (59.6) | 1665 (45.0) | χ2 = 3.960 | 0.047 |
| Verbal aggression, n (%) | 942 (25.2) | 9 (19.1) | 933 (25.2) | χ2 = 0.913 | 0.339 |
| Physical aggression, n (%) | 780 (20.8) | 11 (23.4) | 769 (20.8) | χ2 = 0.191 | 0.662 |
| Significant affective symptoms, n (%) | 425 (11.4) | 3 (6.4) | 422 (11.4) | χ2 = 1.168 | 0.280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.S.; Lee, B.J.; Lee, Y.S.; Jang, O.-J.; Nakagami, Y.; Inada, T.; Kato, T.A.; Kanba, S.; Chong, M.-Y.; Lin, S.-K.; et al. Machine Learning Algorithm-Based Prediction Model for the Augmented Use of Clozapine with Electroconvulsive Therapy in Patients with Schizophrenia. J. Pers. Med. 2022, 12, 969. https://doi.org/10.3390/jpm12060969
Oh HS, Lee BJ, Lee YS, Jang O-J, Nakagami Y, Inada T, Kato TA, Kanba S, Chong M-Y, Lin S-K, et al. Machine Learning Algorithm-Based Prediction Model for the Augmented Use of Clozapine with Electroconvulsive Therapy in Patients with Schizophrenia. Journal of Personalized Medicine. 2022; 12(6):969. https://doi.org/10.3390/jpm12060969
Chicago/Turabian StyleOh, Hong Seok, Bong Ju Lee, Yu Sang Lee, Ok-Jin Jang, Yukako Nakagami, Toshiya Inada, Takahiro A. Kato, Shigenobu Kanba, Mian-Yoon Chong, Sih-Ku Lin, and et al. 2022. "Machine Learning Algorithm-Based Prediction Model for the Augmented Use of Clozapine with Electroconvulsive Therapy in Patients with Schizophrenia" Journal of Personalized Medicine 12, no. 6: 969. https://doi.org/10.3390/jpm12060969
APA StyleOh, H. S., Lee, B. J., Lee, Y. S., Jang, O.-J., Nakagami, Y., Inada, T., Kato, T. A., Kanba, S., Chong, M.-Y., Lin, S.-K., Si, T., Xiang, Y.-T., Avasthi, A., Grover, S., Kallivayalil, R. A., Pariwatcharakul, P., Chee, K. Y., Tanra, A. J., Rabbani, G., ... Park, S.-C. (2022). Machine Learning Algorithm-Based Prediction Model for the Augmented Use of Clozapine with Electroconvulsive Therapy in Patients with Schizophrenia. Journal of Personalized Medicine, 12(6), 969. https://doi.org/10.3390/jpm12060969









