Epidemiological Characteristics and Mortality Risk Factors Comparison in Dialysis and Non-Dialysis CKD Patients with COVID-19—A Single Center Experience
Abstract
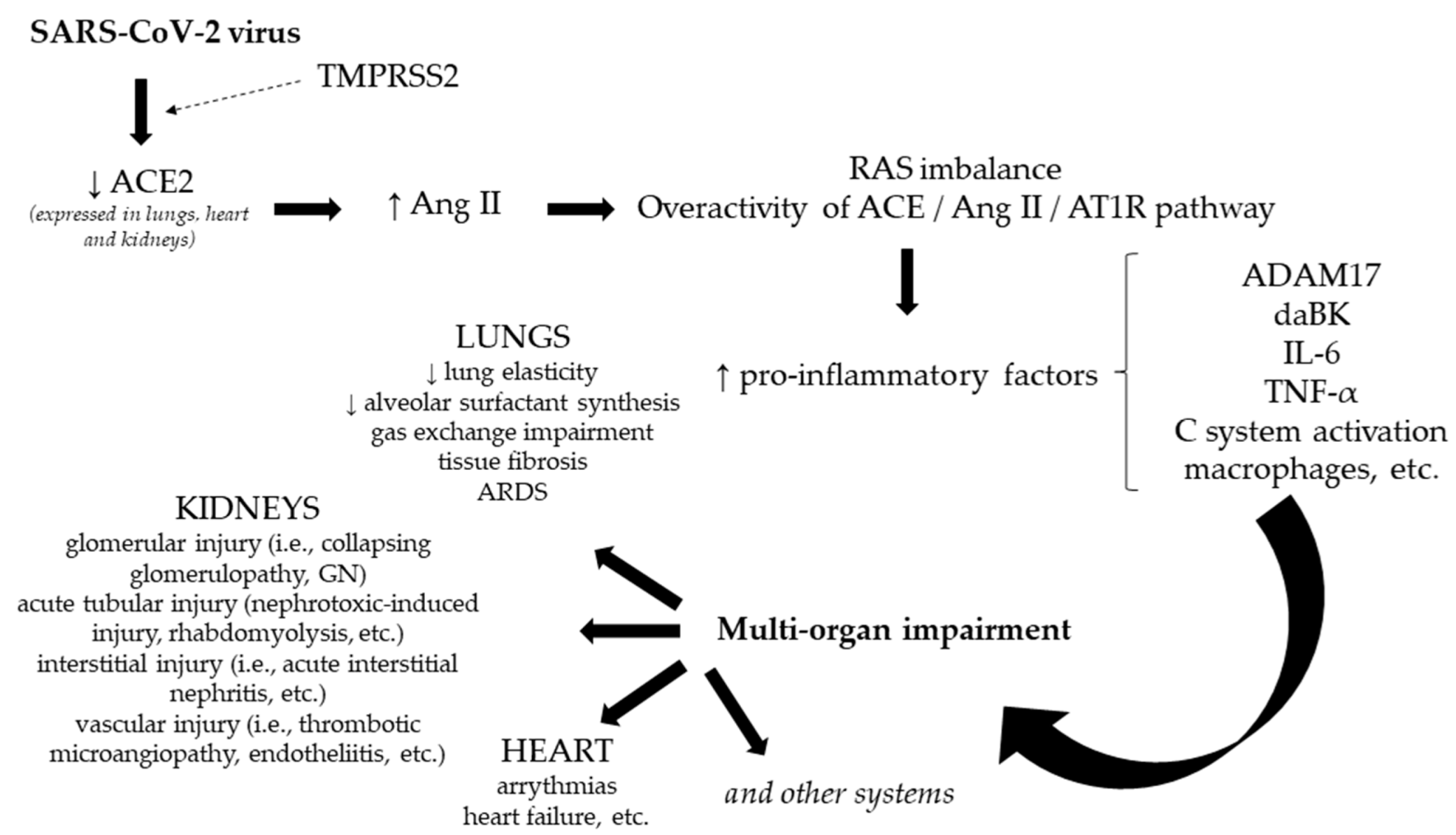
1. Introduction
- The ACE/Ang II/AT1R pathway (angiotensin-converting enzyme/angiotensin II/angiotensin II type 1 receptor)—incriminated in the development of lung injury, cell proliferation, inflammation, etc.
- The ACE2/Ang 1-7/MasR pathway (angiotensin-converting enzyme 2/angiotensin 1-7/Massey receptor)—with a protective role on the respiratory system.
2. Materials and Methods
- Without impairment = grade 1;
- ≤25% = grade 2;
- 26–50% = grade 3;
- 51–75% = grade 4;
- 76–100% = grade 5.
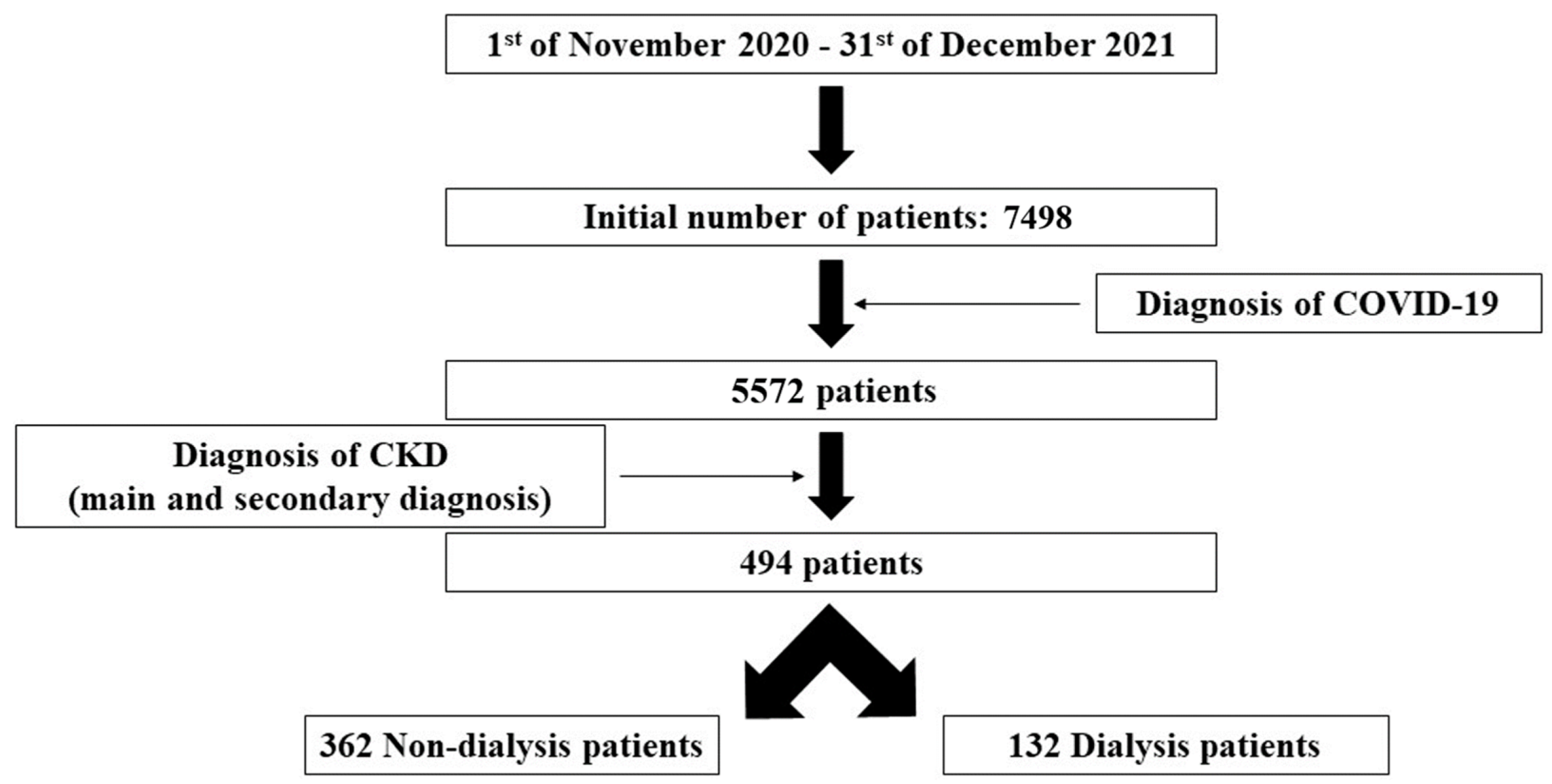
- Time period: between 1 November 2020 and 31 December 2021;
- Diagnosis of COVID-19 and CKD. The diagnosis of CKD was based on the main and secondary diagnosis (according to our national protocol of diagnosis index) related to this pathology, as it was recorded by each physician in the patients’ discharge medical report.
Statistical Analysis
3. Results
- Dialysis group
- Non-dialysis group
4. Discussion
- Hematologic changes—leukocytosis and neutrophilia, lymphopenia, thrombocytopenia, decreased eosinophil count, and anemia;
- Biochemical changes—hypoalbuminemia, increased alanine, and aspartate transaminases, total bilirubin, nitrogenous waste products, LDH, creatinine kinase, creatinine kinase-MB, troponin, and myoglobin;
- Coagulation changes—increased quantitative D-dimer, and prothrombin time;
- Inflammatory syndrome—increased, CRP, ESR, ferritin, IL-6, IL-8, IL-10, and procalcitonin.
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID19, March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 22 February 2022).
- Long, J.D.; Strohbehn, I.; Sawtell, R.; Bhattacharyya, R.; Sise, M.E. COVID-19 Survival and its impact on chronic kidney disease. Transl. Res. 2022, 241, 70–82. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map. Center for Systems Science and Engineering at Johns Hopkins University. 2021. COVID-19 Dashboard Web Site. Available online: https://coronavirus.jhu.edu/map.htmlpublished2021 (accessed on 22 February 2022).
- Barek, M.A.; Aziz, M.A.; Islam, M.S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon 2020, 6, e05684. [Google Scholar] [CrossRef]
- Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Pilling, L.C.; Kuo, C.L.; Kuchel, G.A.; Melzer, D. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2224–2230. [Google Scholar] [CrossRef]
- Jdiaa, S.S.; Mansour, R.; El Alayli, A.; Gautam, A.; Thomas, P.; Mustafa, R.A. COVID-19 and chronic kidney disease: An updated overview of reviews. J. Nephrol. 2022, 35, 69–85. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. COVID-19 and kidney disease: A clinical perspective. Curr. Vasc. Pharmacol. 2022. Epub ahead of . [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Sanders, J.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef]
- Mella, A.; Mingozzi, S.; Gallo, E.; Lavacca, A.; Rossetti, M.; Clari, R.; Randone, O.; Maffei, S.; Salomone, M.; Imperiale, D.; et al. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl. Infect. Dis. 2020, 22, e13348. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Niknam, Z.; Mohammadi Amirabad, L.; Amiri-Dashatan, N.; Koushki, M.; Nemati, M.; Danesh Pouya, F.; Rezaei-Tavirani, M.; Rasmi, Y.; Tayebi, L. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed. Pharmacother. 2022, 145, 112420. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Giaccardi, M. A New Era in Zero X-ray Ablation. Arrhythm. Electrophysiol. Rev. 2020, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Feigin, E.; Levinson, T.; Wasserman, A.; Shenhar-Tsarfaty, S.; Berliner, S.; Ziv-Baran, T. Age-Dependent Biomarkers for Prediction of In-Hospital Mortality in COVID-19 Patients. J. Clin. Med. 2022, 11, 2682. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Mascia, G.; Pescetelli, F.; Baldari, A.; Gatto, P.; Seitun, S.; Sartori, P.; Pieroni, M.; Calò, L.; Della Bona, R.; Porto, I. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int. J. Cardiol. 2021, 326, 248–251. [Google Scholar] [CrossRef]
- Khaloo, P.; Shaqdan, A.; Ledesma, P.A.; Uzomah, U.A.; Galvin, J.; Ptaszek, L.M.; Ruskin, J.N. Distinct etiologies of high-sensitivity troponin T elevation predict different mortality risks for patients hospitalized with COVID-19. Int. J. Cardiol. 2022, 351, 118–125. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Savrun, A.; Aydin, I.E.; Savrun, S.T.; Karaman, U. The predictive role of biomarkers for mortality in COVID-19 patients. Trop. Biomed. 2021, 38, 366–370. [Google Scholar]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Morell-Garcia, D.; Ramos-Chavarino, D.; Bauça, J.M.; Argente Del Castillo, P.; Ballesteros-Vizoso, M.A.; García de Guadiana-Romualdo, L.; Gómez-Cobo, C.; Pou, J.A.; Amezaga-Menéndez, R.; Alonso-Fernández, A.; et al. Urine biomarkers for the prediction of mortality in COVID-19 hospitalized patients. Sci. Rep. 2021, 11, 11134. [Google Scholar] [CrossRef]
- Danwang, C.; Endomba, F.T.; Nkeck, J.R.; Wouna, D.L.A.; Robert, A.; Noubiap, J.J. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19). Biomark. Res. 2020, 8, 37. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.T.; Goncalves, J.; Xiao, Y.; Wang, M.; Guo, Y.; Sun, C.; Tang, X.; Jing, L.; Zhang, M.; et al. An interpretable mortality prediction model for COVID-19 patients. Nat. Mach. Intell. 2020, 2, 283–288. [Google Scholar] [CrossRef]
- Lu, J.; Wei, Z.; Jiang, H.; Cheng, L.; Chen, Q.; Chen, M.; Yan, J.; Sun, Z. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: A retrospective observational study. J. Surg. Res. 2018, 228, 314–321. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar]
- Singh, A.K. Resolved: Targeting a higher hemoglobin is associated with greater risk in patients with CKD anemia: Pro. J. Am. Soc. Nephrol. 2009, 20, 1436–1441. [Google Scholar] [CrossRef][Green Version]
- Braekkan, S.K.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T.; Hansen, J.B. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica 2010, 95, 270–275. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Newsome, B.B.; Warnock, D.G.; McClellan, W.M.; Herzog, C.A.; Kiefe, C.I.; Eggers, P.W.; Allison, J.J. Long-term risk of mortality and end-stage renal disease among the elderly, after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch. Intern. Med. 2008, 168, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.S.; Kim, S.W.; Horwood, C.M.; Hakendorf, P.; Li, J.Y.; Thompson, C.H. Variability in inpatient serum creatinine: Its impact upon short- and long-term mortality. QJM 2015, 108, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Maiese, A.; Passaro, G.; Matteis, A.; Fazio, V.; Raffaele, R.; Paolo, M.D. Thromboinflammatory response in SARS-CoV-2 sepsis. Med. Leg. J. 2020, 88, 78–80. [Google Scholar] [CrossRef]
- Frisoni, P.; Neri, M.; D’Errico, S.; Alfieri, L.; Bonuccelli, D.; Cingolani, M.; Di Paolo, M.; Gaudio, R.M.; Lestani, M.; Marti, M.; et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: From viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci. Med. Pathol. 2022, 18, 4–19. [Google Scholar] [CrossRef]
- Manson, J.J.; Crooks, C.; Naja, M.; Ledlie, A.; Goulden, B.; Liddle, T.; Khan, E.; Mehta, P.; Martin-Gutierrez, L.; Waddington, K.E.; et al. COVID-19-associated hyperinflammation and escalation of patient care: A retrospective longitudinal cohort study. Lancet Rheumatol. 2020, 2, e594–e602. [Google Scholar] [CrossRef]
- Gasparini, M.; Khan, S.; Patel, J.M.; Parekh, D.; Bangash, M.N.; Stvmpfle, R.; Shah, A.; Baharlo, B.; Soni, S.; Collaborators. Renal impairment and its impact on clinical outcomes in patients who are critically ill with COVID-19: A multicentre observational study. Anaesthesia 2021, 73, 320–326. [Google Scholar] [CrossRef]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrol. Dial. Transplant. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, Y.; Chen, J.; Chen, Y.; Luo, P.; Liu, Q.; Yang, C.; Xiong, M.; Zhang, Y.; Liu, X.; et al. COVID-19 and chronic renal disease: Clinical characteristics and prognosis. QJM 2020, 113, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Katwal, P.C.; Jirjees, S.; Htun, Z.M.; Aldawudi, I.; Khan, S. The Effect of Anemia and the Goal of Optimal HbA1c Control in Diabetes and Non-Diabetes. Cureus 2020, 12, e8431. [Google Scholar] [CrossRef]
- Morrow, A.J.; Sykes, R.; McIntosh, A.; Kamdar, A.; Bagot, C.; Bayes, H.K.; Blyth, K.G.; Briscoe, M.; Bulluck, H.; Carrick, D.; et al. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Normal Value Range | Assay | Laboratory Device |
|---|---|---|---|
| Hemoglobin | 12.3–17 g/dL | Photometric method, analyzed using HBG-photometric detection, following SLS hemoglobin chamber | SYSMEX XN-2000-HLG-5diff |
| Serum creatinine | 0.7–1.2 mg/dL | Jaffe method (the colorimetric technique) | COBAS 501 (Roche) |
| Serum urea | 17.4–49 mg/dL | Urease method | COBAS 501 (Roche) |
| Glycemia | 80–115 mg/dL | Hexokinase method | COBAS 501 (Roche) |
| Glycosylated hemoglobin | 4.8–5.6% | Turbidimetric method | COBAS 501 (Roche) |
| Interleukin-6 (IL-6) | <7 pg/mL | Electrochemiluminescence (ECLIA) method | COBAS 601 (Roche) |
| C-reactive protein (CRP) | ≤5 mg/L | Turbidimetric method | COBAS 501 (Roche) |
| Lactate dehydrogenase (LDH) | 135–225 UI/L | Ultraviolet method (with pyruvate) | COBAS 501 (Roche) |
| Serum albumin | 3.4–5.2 g/dL | Colorimetric method | COBAS 501 (Roche) |
| Serum total proteins | 6.4–8.3 g/dL | Colorimetric method | COBAS 501 (Roche) |
| Quantitative D-dimer | 0–0.5 μg/mL | Immunoturbidimetric method | STAR Max 2—STAGO Top Diagnostics |
| Procalcitonin | ≤0.05 ng/mL | Electrochemiluminescence (ECLIA) method | COBAS 601 (Roche) |
| Ferritin | 30–400 ng/mL | Electrochemiluminescence (ECLIA) method | COBAS 601 (Roche) |
| Erythrocyte sedimentation rate (ESR) | 2–20 mm/h | Capillary microphotometry (automatic method) | ALIFAX |
| Fibrinogen | 200–400 mg/dL | Mechanical method to determine fibrinogen concentration (measurement of the conversion of fibrinogen to fibrin, in the presence of excess thrombin) | STAR Max 2—STAGO Top Diagnostics |
| Data | Non-Dialysis Group (n = 362) | Dialysis Group (n = 132) | p Value | |
|---|---|---|---|---|
| Age (years; mean ± SD values) | 72.56 ± 13.10 | 64.89 ± 12.07 | <0.001 | |
| Gender | male | 213 (58.8%) | 81 (61.4%) | 0.613 |
| female | 149 (41.2%) | 51 (38.6%) | ||
| Length of hospital stay (days; mean ± SD values) | 15.24 ± 9.70 | 15.82 ± 9.78 | 0.557 | |
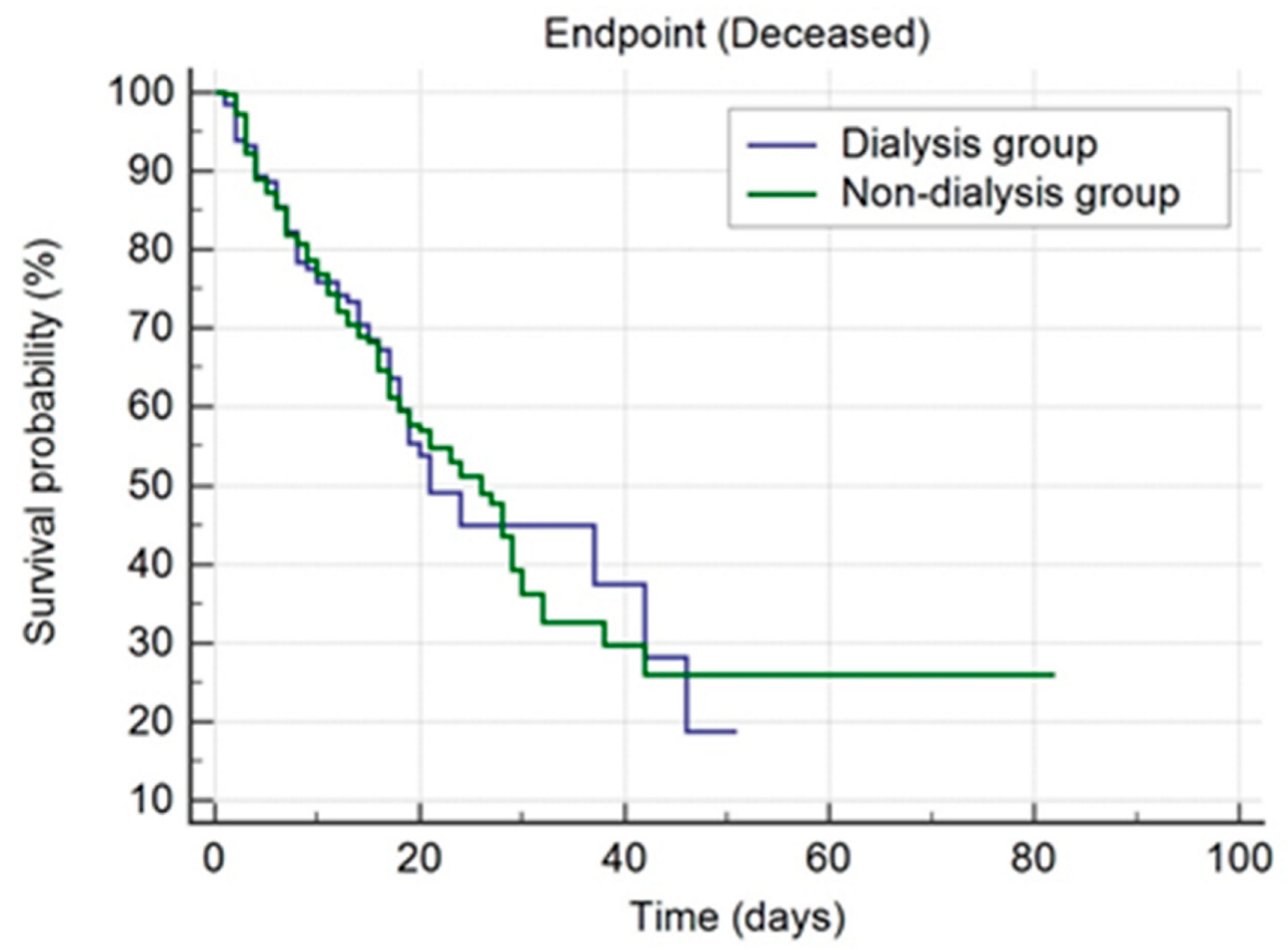
| Discharge status | Discharge | 183 (50.6%) | 57 (43.2%) | 0.207 |
| Deceased | 152 (42%) | 58 (43.9%) | ||
| Transferred to another hospital | 11 (3%) | 8 (6.1%) | ||
| Discharge by request | 16 (4.4%) | 9 (6.8%) | ||
| Patient’s environment | Urban | 280 (77.3%) | 100 (75.8%) | 0.710 |
| Rural | 82 (22.7%) | 32 (24.2%) | ||
| Medical departments (patients’ admission) | Nephrology | 155 (42.8%) | 123 (93.2%) | <0.001 |
| Urology | 34 (9.4%) | 3 (2.3%) | 0.0183 | |
| Cardiology | 83 (22.9%) | 2 (1.5%) | <0.001 | |
| Internal medicine | 31 (8.6%) | 0 (0%) | <0.001 | |
| Vascular surgery | 8 (2.2%) | 0 (0%) | 0.086 | |
| Plastic surgery | 10 (2.8%) | 0 (0%) | 0.052 | |
| Gastroenterology | 23 (6.4%) | 1 (0.8%) | 0.01 | |
| Orthopedy | 5 (1.4%) | 0 (0%) | 0.172 | |
| General surgery | 11 (3%) | 3 (2.3%) | 0.677 | |
| Thoracic surgery | 1 (0.3%) | 0 (0%) | 0.529 | |
| Gynecology | 1 (0.3%) | 0 (0%) | 0.529 | |
| Diabetes mellitus | 178 (49.2%) | 52 (39.4%) | 0.054 | |
| Obesity | 141 (39%) | 49 (37.1%) | 0.712 | |
| Grade of pulmonary impairment | without | 27 (7.5%) | 14 (10.6%) | 0.270 |
| ≤25% | 122 (33.7%) | 44 (33.3%) | 0.933 | |
| 26–50% | 85 (23.5%) | 26 (19.7%) | 0.371 | |
| 51–75% | 74 (20.4%) | 26 (19.7%) | 0.864 | |
| 76–100% | 54 (14.9%) | 22 (16.7%) | 0.624 | |
| Oxygen therapy | High flow oxygen therapy (AIRVO) | 7 (1.9%) | 1 (0.8%) | 0.359 |
| Invasive mechanical ventilation | 143 (39.5%) | 53 (40.2%) | 0.896 | |
| Non-invasive mechanical ventilation | 52 (14.4%) | 29 (22%) | 0.043 | |
| Admission hemoglobin (g/dL; mean ± SD values) | 11.64 ± 2.64 | 9.91 ± 1.96 | <0.001 | |
| Admission serum creatinine (mg/dL; mean ± SD values) | 3.64 ± 3.56 | 8.23 ± 3.23 | <0.001 | |
| Admission serum urea (mg/dL; mean ± SD values) | 141.49 ± 89.93 | 155.82 ± 81.89 | 0.109 | |
| Admission glycemia (mg/dL; mean ± SD values) | 157.12 ± 88.49 | 145.31 ± 88.37 | 0.190 | |
| Admission glycosylated hemoglobin (%; mean ± SD values) | 6.77 ± 1.68 | 6.63 ± 1.79 | 0.552 | |
| Admission IL-6 (pg/mL; mean ± SD values) | 216 ± 661.08 | 231.99 ± 472.38 | 0.001 | |
| Admission CRP (mg/L; mean ± SD values) | 112.21 ± 98.13 | 123.91 ± 105.23 | 0.254 | |
| Admission LDH (UI/L; mean ± SD values) | 428.50 ± 258.58 | 422.89 ± 276.29 | 0.839 | |
| Admission serum albumin (g/dL; mean ± SD values) | 3.36 ± 0.59 | 3.51 ± 0.53 | 0.016 | |
| Admission serum total proteins (g/dL; mean ± SD values) | 6.49 ± 0.86 | 6.62 ± 0.79 | 0.168 | |
| Admission quantitative D-dimer (μg/mL; mean ± SD values) | 2.71 ± 2.69 | 2.93 ± 2.77 | 0.117 | |
| Admission procalcitonin (ng/mL; mean ± SD values) | 3.29 ± 12.85 | 7.32 ± 19.52 | 0.002 | |
| Admission ferritin (ng/mL; mean ± SD values) | 1352.26 ± 1499.85 | 2213.20 ± 2327.83 | <0.001 | |
| Admission ESR (mm/h; mean ± SD values) | 66.74 ± 31.22 | 72.31 ± 29.30 | 0.083 | |
| Admission fibrinogen (mg/dL; mean ± SD values) | 602.68 ± 184.38 | 585 ± 182.10 | 0.349 | |
| Variables | b | S.E. | Wald | df | p | Exp(b) | 95% CI of Exp(b) |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.049 | 0.010 | 25.365 | 1 | <0.001 | 1.051 | 1.031 to 1.071 |
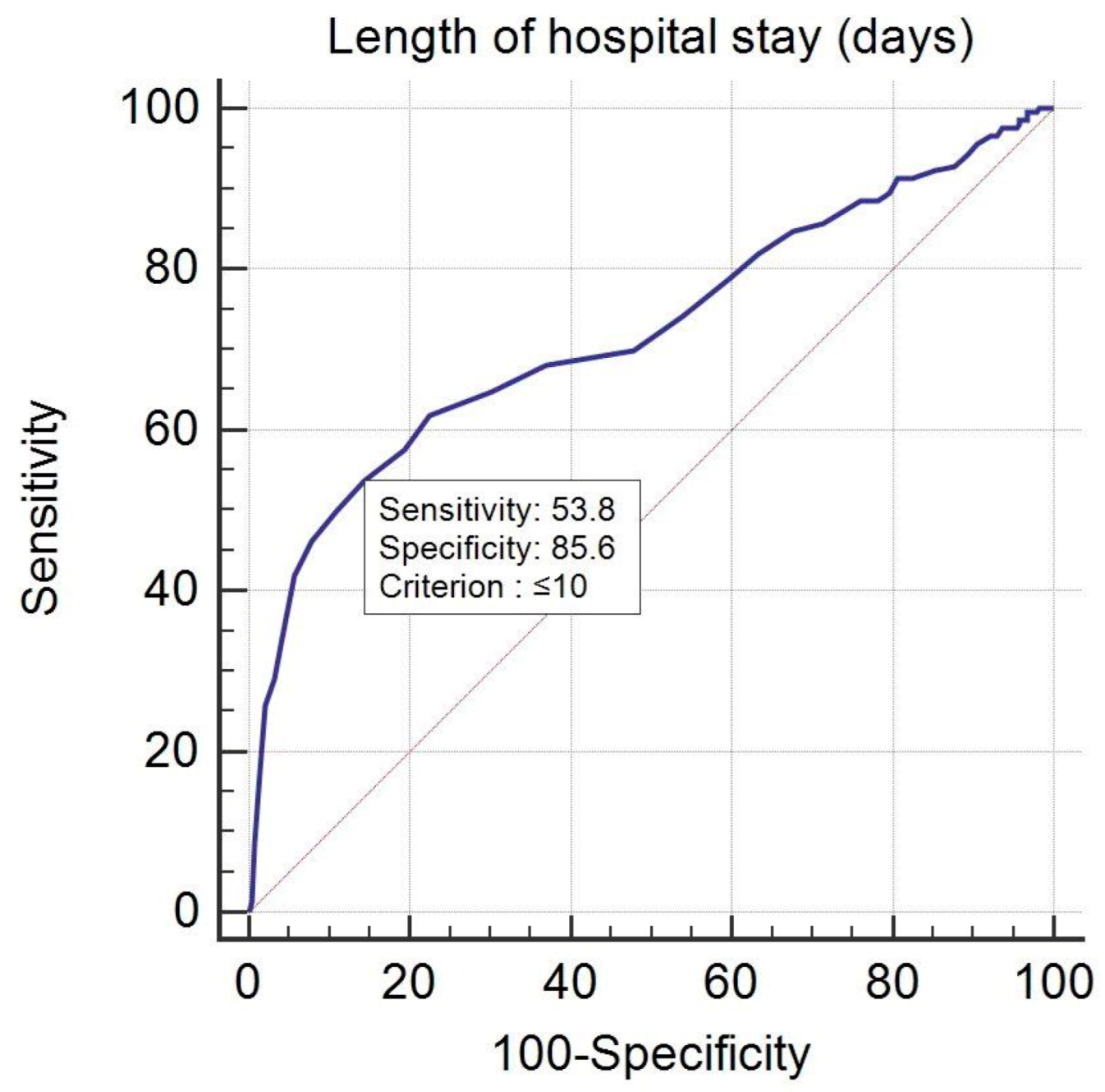
| LOS (≤10 days) | 2.027 | 0.256 | 62.566 | 1 | <0.001 | 7.590 | 4.593 to 12.541 |
| Gender (male) | 0.496 | 0.234 | 4.506 | 1 | 0.034 | 1.642 | 1.039 to 2.595 |
| Grade of pulmonary impairment (>25%) | 2.038 | 0.250 | 66.607 | 1 | <0.001 | 7.675 | 4.704 to 12.520 |
| Dialysis group | 0.609 | 0.269 | 5.131 | 1 | 0.023 | 1.839 | 1.086 to 3.116 |
| Variables | b | S.E. | Wald | df | p | Exp(b) | 95% CI of Exp(b) |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.049 | 0.010 | 25.365 | 1 | <0.001 | 1.051 | 1.031 to 1.071 |
| LOS (≤10 days) | 2.027 | 0.256 | 62.566 | 1 | <0.001 | 7.590 | 4.593 to 12.541 |
| Gender (male) | 0.496 | 0.234 | 4.506 | 1 | 0.034 | 1.642 | 1.039 to 2.595 |
| Grade of pulmonary impairment (>25%) | 2.038 | 0.250 | 66.607 | 1 | <0.001 | 7.675 | 4.704 to 12.520 |
| Non-dialysis group | −0.609 | 0.269 | 5.131 | 1 | 0.023 | 0.544 | 0.321 to 0.921 |
| Age (Years) | Logit (p) | p |
|---|---|---|
| 30 | 0.435 | 0.6071 |
| 35 | 0.680 | 0.6637 |
| 40 | 0.925 | 0.7161 |
| 45 | 1.170 | 0.7631 |
| 50 | 1.415 | 0.8046 |
| 55 | 1.660 | 0.8402 |
| 60 | 1.905 | 0.8705 |
| 65 | 2.150 | 0.8957 |
| 70 | 2.395 | 0.9164 |
| 75 | 2.640 | 0.9334 |
| 80 | 2.885 | 0.9471 |
| Age (Years) | Logit (p) | p |
|---|---|---|
| 30 | −0.159 | 0.4603 |
| 35 | 0.088 | 0.5220 |
| 40 | 0.336 | 0.5831 |
| 45 | 0.583 | 0.6418 |
| 50 | 0.831 | 0.6965 |
| 55 | 1.078 | 0.7461 |
| 60 | 1.325 | 0.7901 |
| 65 | 1.573 | 0.8282 |
| 70 | 1.820 | 0.8606 |
| 75 | 2.068 | 0.8877 |
| 80 | 2.315 | 0.9101 |
| Variables | b | S.E. | Wald | df | p | Exp(b) | 95% CI of Exp(b) |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.047 | 0.015 | 10.367 | 1 | 0.001 | 1.048 | 1.019 to 1.079 |
| LOS (≤10 days) | 2.365 | 0.392 | 36.387 | 1 | <0.001 | 10.643 | 4.936 to 22.950 |
| Hb | 0.231 | 0.084 | 7.459 | 1 | 0.006 | 1.260 | 1.067 to 1.486 |
| Serum creatinine | 0.153 | 0.054 | 8.041 | 1 | 0.005 | 1.165 | 1.048 to 1.295 |
| LDH | 0.003 | 0.001 | 10.829 | 1 | 0.001 | 1.003 | 1.001 to 1.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, A.; Peride, I.; Nechita, A.-M.; Petcu, L.C.; Tiglis, M.; Checherita, I.A. Epidemiological Characteristics and Mortality Risk Factors Comparison in Dialysis and Non-Dialysis CKD Patients with COVID-19—A Single Center Experience. J. Pers. Med. 2022, 12, 966. https://doi.org/10.3390/jpm12060966
Niculae A, Peride I, Nechita A-M, Petcu LC, Tiglis M, Checherita IA. Epidemiological Characteristics and Mortality Risk Factors Comparison in Dialysis and Non-Dialysis CKD Patients with COVID-19—A Single Center Experience. Journal of Personalized Medicine. 2022; 12(6):966. https://doi.org/10.3390/jpm12060966
Chicago/Turabian StyleNiculae, Andrei, Ileana Peride, Ana-Maria Nechita, Lucian Cristian Petcu, Mirela Tiglis, and Ionel Alexandru Checherita. 2022. "Epidemiological Characteristics and Mortality Risk Factors Comparison in Dialysis and Non-Dialysis CKD Patients with COVID-19—A Single Center Experience" Journal of Personalized Medicine 12, no. 6: 966. https://doi.org/10.3390/jpm12060966
APA StyleNiculae, A., Peride, I., Nechita, A.-M., Petcu, L. C., Tiglis, M., & Checherita, I. A. (2022). Epidemiological Characteristics and Mortality Risk Factors Comparison in Dialysis and Non-Dialysis CKD Patients with COVID-19—A Single Center Experience. Journal of Personalized Medicine, 12(6), 966. https://doi.org/10.3390/jpm12060966







