Prognostic Significance of Lymphocyte Infiltrate Localization in Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histopathology and Immunohistochemistry
2.3. Statistical Analysis
3. Results
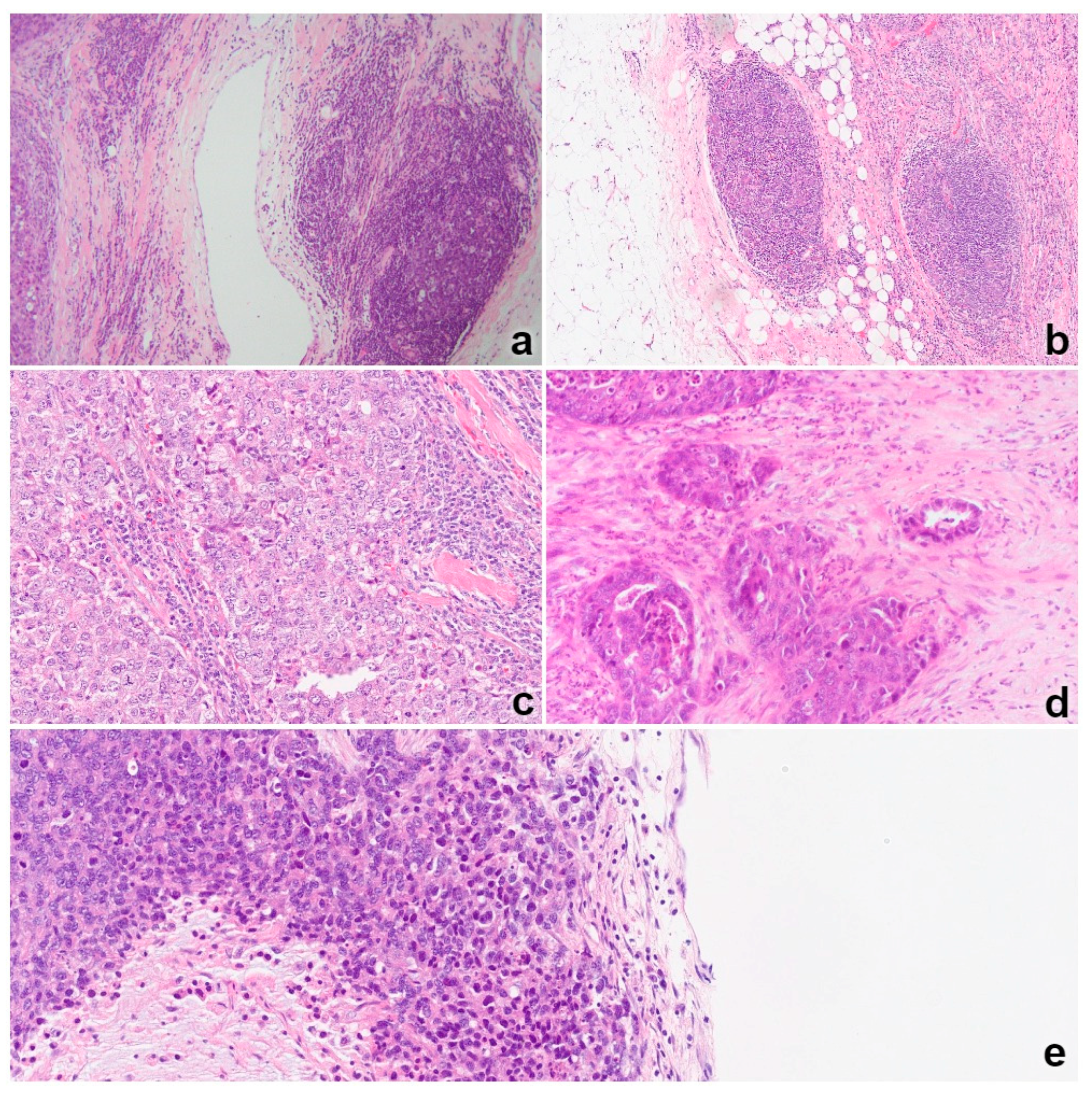
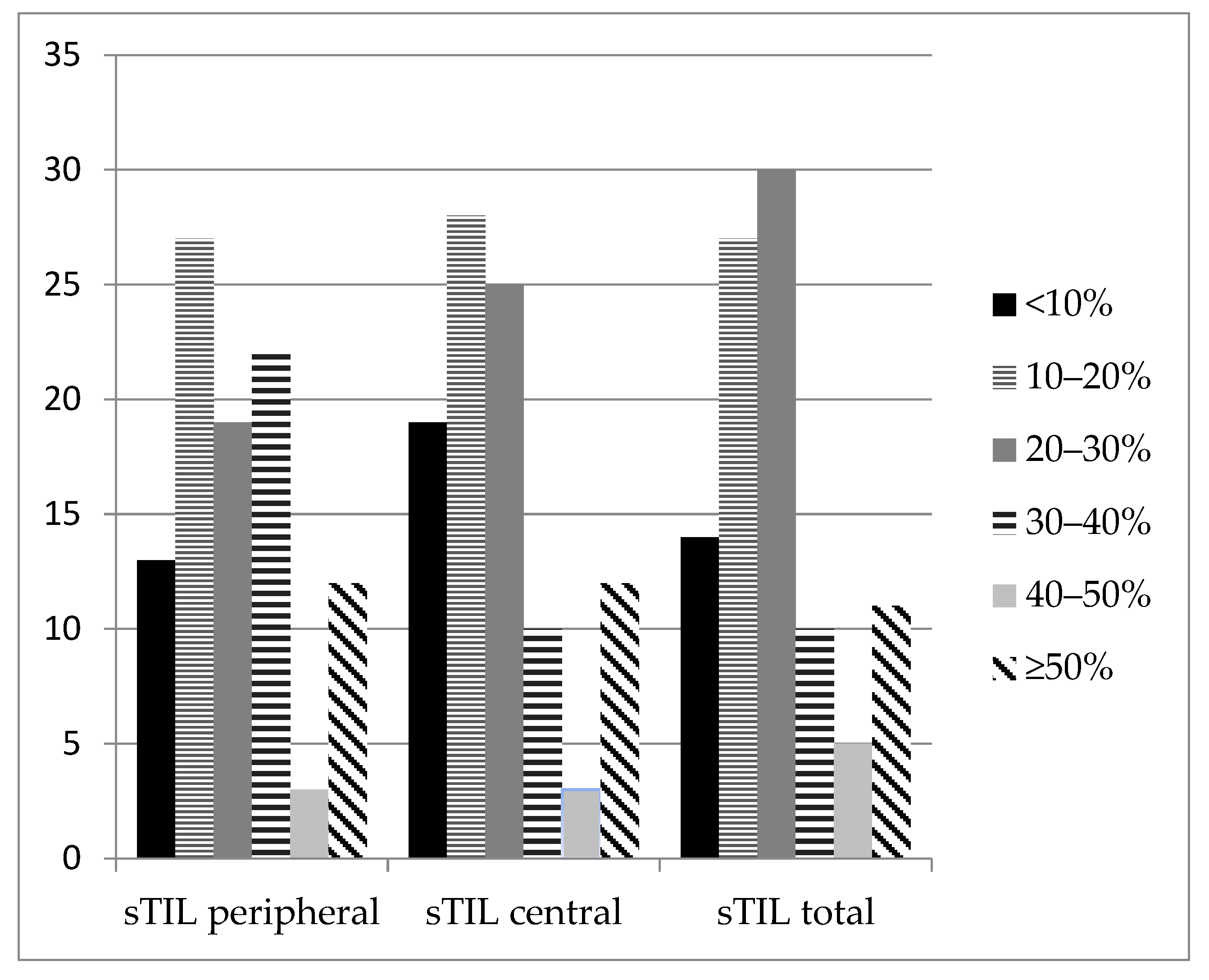
3.1. TNBC Infiltration by Lymphocytes
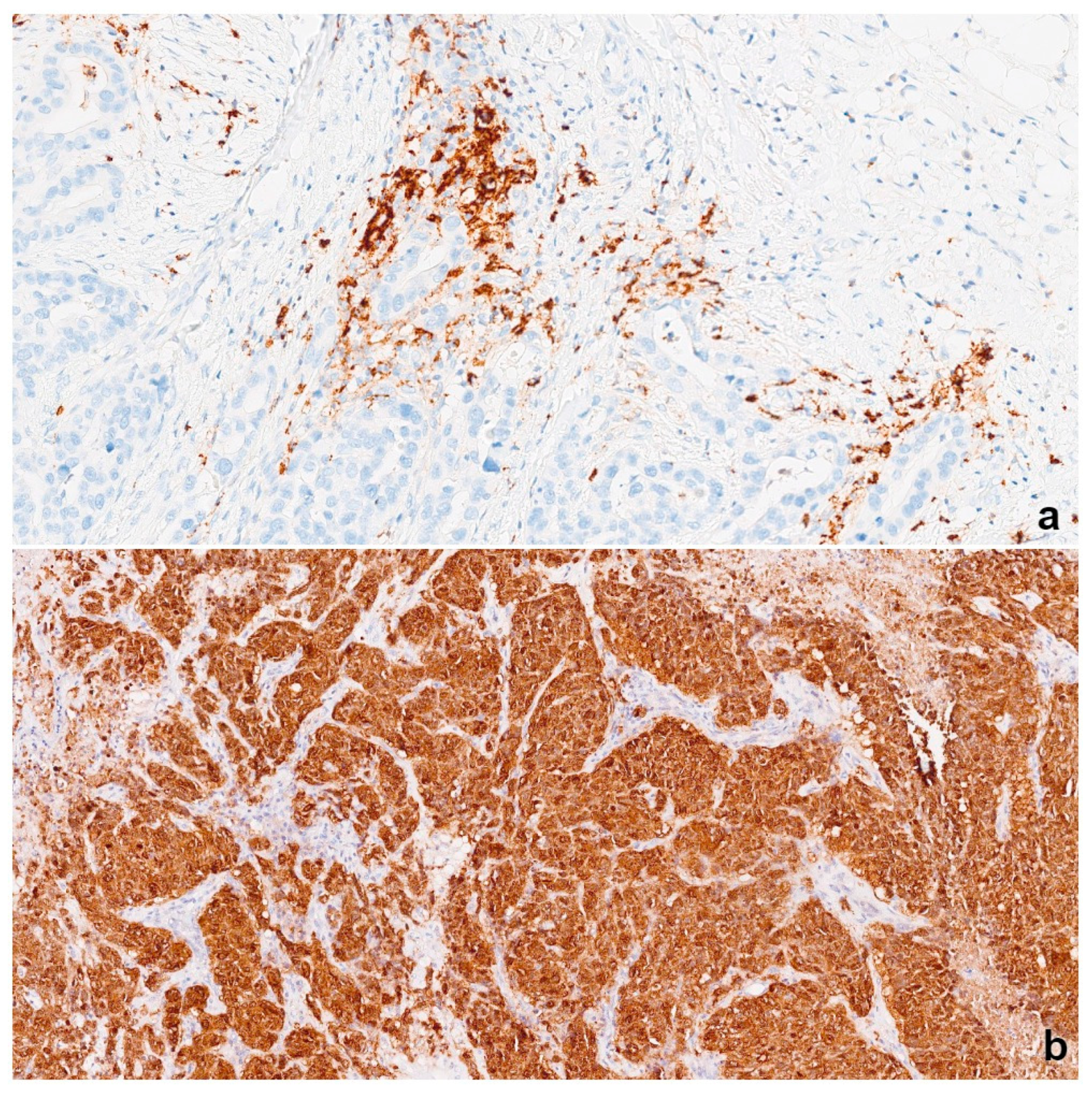
3.2. NY-ESO-1 Expression
3.3. PD-L1 Expression
3.4. Prognostic Significance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowam, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, M.; Toillon, R.A.; Leclercq, G. Stable portrait of breast tumors during progression: Data from biology, pathology and genetics. Endocr.-Relatated Cancer 2004, 11, 497–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulford, L.G.; Reis-Filho, J.S.; Ryder, K.; Jones, C.; Gillett, C.E.; Hanby, A.; Easton, D.; Lakhani, S.R. Basal-like grade III invasive ductal carcinoma of the breast: Patterns of metastasis and long-term survival. Breast Cancer Res. 2007, 9, R4. [Google Scholar] [CrossRef] [Green Version]
- Hicks, D.G.; Short, S.M.; Prescott, N.L.; Tarr, S.; Coleman, K.A.; Yoder, B.J.; Crowe, J.P.; Choueiri, T.K.; Dawson, A.E.; Budd, G.T.; et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am. J. Surg. Pathol. 2006, 30, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [Green Version]
- Balatoni, T.; Mohos, A.; Papp, E.; Sebestyén, T.; Liszkay, G.; Oláh, J.; Varga, J.; Lengyel, Z.; Emri, G.; Gaudi, I.; et al. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol. Immunother. 2018, 67, 141–151. [Google Scholar] [CrossRef]
- Zheng, X.; Song, X.; Shao, Y.; Xu, B.; Chen, L.; Zhou, Q.; Wenwei, H.; Dachuan, Z.; Wu, C.; Tao, M.; et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 57386. [Google Scholar] [CrossRef] [Green Version]
- Galon, J.; Pagès, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef]
- Liu, S.; Lachapelle, J.; Leung, S.; Gao, D.; Foulkes, W.D.; Nielsen, T.O. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Gao, D.; Leung, S.; Chia, S.K.; Nielsen, T.O. LAG-3+ tumor infiltrating lymphocytes in breast cancer: Clinical correlates and association with PD-1/PD-L1+ tumors. Ann. Oncol. 2017, 28, 2977–2984. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Muller, B.M.; Komor, M.; Dudczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Ahn, S.; Cho, J.; Sung, J.; Lee, J.E.; Nam, J.S.; Kim, K.M.; Cho, E.Y. The prognostic significance of tumor-associated stroma in invasive breast carcinoma. Tumor Biol. 2012, 33, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Takada, K.; Takahashi, K.; Shibutani, M.; Amano, R.; Takashima, T.; Tomita, S.; Hirakawa, K.; et al. Predicting therapeutic efficacy of endocrine therapy for stage IV breast cancer by tumorinfiltrating lymphocytes. Mol. Clin. Oncol. 2020, 13, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Salgado, R.; Fox, S.; Savas, P.; Eng-Wong, J.; Clark, E.; Kiermaier, A.; Swain, S.M.; Baselga, J.; Michiels, S.; et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017, 18, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Simpson, A.J.; Old, L.J. The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 2004, 4, 1. [Google Scholar]
- Grigoriadis, A.; Caballero, O.L.; Hoek, K.S.; da Silva, L.; Chen, Y.T.; Shin, S.J.; Jungbluth, A.A.; Miller, L.D.; Clouston, D.; Cebon, J.; et al. CT-X antigen expression in human breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 13493–13498. [Google Scholar] [CrossRef] [Green Version]
- Curigliano, G.; Viale, G.; Ghioni, M.; Jungbluth, A.A.; Bagnardi, V.; Spagnoli, G.C.; Neville, A.M.; Nole, F.; Rotmensz, N.; Goldhirsch, A. Cancer–testis antigen expression in triple-negative breast cancer. Ann. Oncol. 2011, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, F.O.; Bshara, W.; Attwood, K.; Morrison, C.; Edge, S.B.; Ambrosone, C.B.; O’Connor, T.L.; Levine, E.G.; Miliotto, A.; Ritter, E.; et al. NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS ONE 2012, 7, e38783. [Google Scholar] [CrossRef]
- Mrklić, I.; Spagnoli, G.C.; Juretić, A.; Pogorelić, Z.; Tomić, S. Co-expression of cancer testis antigens and topoisomerase 2-alpha in triple negative breast carcinomas. Acta Histochem. 2014, 116, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Karn, T.; Pusztai, L.; Ruckhäberle, E.; Liedtke, C.; Muller, V.; Schmidt, M.; Metzler, D.; Wang, J.; Coombes, K.R.; Gätje, R.; et al. Melanoma antigen family A identified by the bimodality index defines a subset of triple negative breast cancers as candidates for immune response augmentation. Eur. J. Cancer 2012, 48, 12–23. [Google Scholar] [CrossRef]
- Tessari, A.; Pilla, L.; Silvia, D.; Duca, M.; Paolini, B.; Carcangiu, M.L.; Mariani, L.; de Braud, F.G.; Cresta, S. Expression of NY-ESO-1, MAGE-A3, PRAME and WT1 in different subgroups of breast cancer: An indication to immunotherapy? Breast 2018, 42, 68–73. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–825. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Brierly, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Clasiffication of Malignant Tumors, 8th ed.; UICC. John Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lokuhetty, D.; White, V.A.; Watanabe, R.; Cree, I.A. (Eds.) WHO Classification of Tumors, Breast Tumours, 5th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.R. The International Conference on Harmonization Good Clinical Practice Guideline. Qual. Assur. 1998, 6, 65–74. [Google Scholar] [CrossRef]
- Juretic, A.; Spagnoli, G.C.; Schultz-Thater, E.; Sarcevic, B. Cancer/testis tumour-associated antigens: Immunohistochemical detection with monoclonal antibodies. Lancet Oncol. 2003, 4, 104–109. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 2018, 142, 2105–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, K.H.; Hammond, M.E.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists guideline update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Wright, G.S.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehnerm, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef] [Green Version]
- Ho-Yen, C.; Bowen, R.L.; Jones, J.L. Characterization of basal-like breast cancer: An update. Diagn. Histopathol. 2012, 18, 104–111. [Google Scholar] [CrossRef]
- Mrklić, I.; Pogorelić, Z.; Ćapkun, V.; Tomić, S. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 2013, 115, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Milne, K.; Nelson, B.H. Location, location, location: CD103 demarcates intraepithelial, prognostically favorable CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Oncoimmunology 2014, 3, e27668. [Google Scholar] [CrossRef] [Green Version]
- Solinas, C.; Gombos, A.; Latifyan, S.; Piccart-Gebhart, M.; Kok, M.; Buisseret, L. Targeting immune checkpoints in breast cancer: An update of early results. ESMO Open 2017, 2, e000255. [Google Scholar] [CrossRef] [Green Version]
- Monnot, G.C.; Romero, P. Rationale for immunological approaches to breast cancer therapy. Breast 2018, 37, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Zgura, A.; Galesa, L.; Bratila, E.; Anghel, R. Relationship between tumor infiltrating lymphocytes and progression in breast cancer. Maedica 2018, 13, 317–320. [Google Scholar] [PubMed]
- Chin, Y.; Janseens, J.; Vandepitte, J.; Vandenbrande, J.; Opdebeek, L.; Raus, J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992, 12, 1463–1466. [Google Scholar] [PubMed]
- Zou, Y.; Zou, X.; Zheng, S.; Tang, H.; Zhang, L.; Liu, P.; Xie, X. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and metaanalysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940928. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitrem, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicinbased chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959. [Google Scholar] [CrossRef] [PubMed]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; van de Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. International Immuno-Oncology Biomarker Working Group. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 2020, 6, 17. [Google Scholar]
- Amgad, M.; Stovgaard, E.S.; Balslev, E.; Thagaard, J.; Chen, W.; Dudgeon, S.; Sharma, A.; Kerner, J.K.; Denkert, C.; Yuan, Y.; et al. Report on computational assessment of tumor infiltrating lymphocytes from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2020, 6, 16. [Google Scholar] [CrossRef]
- Vihervuori, H.; Autere, T.A.; Repo, H.; Kurki, S.; Kallio, L.; Lintunen, M.M.; Talvinen, K.; Kronqvist, P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3105–3114. [Google Scholar] [CrossRef] [Green Version]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Rosario, A.; Wang, R.; Solomon, S.; Srinivasan, G.; Nelson, M.S.; Huang, Y.; Lim, M.H.; et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight 2019, 4, e130000. [Google Scholar] [CrossRef] [Green Version]
- Remark, R.; Alifano, M.; Cremer, I.; Lupo, A.; Dieu-Nosjean, M.C.; Riquet, M.; Crozet, L.; Ouakrim, H.; Goc, J.; Cazes, A.; et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: Influence of tumor origin. Clin. Cancer Res. 2013, 19, 4079–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Caro, G.; Bergomas, F.; Grizzi, F.; Doni, A.; Bianchi, P.; Malesci, A.; Laghi, L.; Allavena, P.; Mantovani, A.; Marchesi, F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014, 20, 2147–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieu-Nosjean, M.C.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Sautès-Fridman, C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 2016, 271, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, S.L.; Fismen, S.; Fenton, K.A.; Fenton, C.; Mortensen, E.S. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015, 15, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronella, J.A.; Spier, C.; Welch, M.; Trevor, K.T.; Stopeck, A.T.; Villar, H.; Hersh, E.M. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J. Immunol. 2002, 169, 1829–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goenka, R.; Barnett, L.G.; Silver, J.S.; O’Neill, P.J.; Hunter, C.A.; Cancro, M.P.; Laufer, T.M. Cutting edge: Dendritic cellrestricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 2011, 187, 1091–1095. [Google Scholar] [CrossRef] [Green Version]
- Roncati, L.; Barbolini, G.; Piacentini, F.; Piscioli, F.; Pusiol, T.; Maiorana, A. Prognostic factors for breast cancer: An immunomorphological update. Pathol. Oncol. Res. 2016, 22, 449–452. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, I.A.; Song, I.H.; Shin, S.J.; Kim, J.Y.; Yu, J.H.; Gong, G. Tertiary lymphoid structures: Prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J. Clin. Pathol. 2016, 69, 422–430. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol.-Res. Pract. 2020, 216, 152802. [Google Scholar] [CrossRef]
- Cerbelli, B.; Pernazza, A.; Botticelli, A.; Fortunato, L.; Monti, M.; Sciattella, P.; Campagna, D.; Mazzuca, F.; Mauri, M.; Naso, G.; et al. PD-L1 expression in TNBC: A predictive biomarker of response to neoadjuvant chemotherapy? BioMed Res. Int. 2017, 2017, 1750925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vennapusa, B.; Chang, C.W.; Tran, D.; Nakamura, R.; Sumiyoshi, T.; Hegde, P.; Molinero, L. Prevalence study of PD-L1 SP142 assay in metastatic triple-negative breast cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 258. [Google Scholar] [CrossRef] [PubMed]
- Polónia, A.; Pinto, R.; Cameselle-Teijeiro, J.F.; Schmitt, F.C.; Paredes, J. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J. Clin. Pathol. 2017, 70, 860–867. [Google Scholar] [CrossRef]
- Evangelou, Z.; Papoudou-Bai, A.; Karpathiou, G.; Kourea, H.; Kamina, S.; Goussia, A.; Harissis, H.; Peschos, D.; Batistatou, A. PD-L1 expression and tumor-infiltrating lymphocytes in breast cancer: Clinicopathological analysis in women younger than 40 years old. In Vivo 2020, 34, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catacchio, I.; Silvestris, N.; Scarpi, E.; Schirosi, L.; Scattone, A.; Mangia, A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019, 12, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Ross, D.S.; Chiu, R.; Zhou, X.K.; Chen, Y.Y.; Lee, P.; Hoda, S.A.; Simpson, A.J.; Old, L.J.; Caballero, O.; et al. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS ONE 2011, 6, e17876. [Google Scholar]
- Badovinac Crnjevic, T.; Spagnoli, G.; Juretic, A.; Jakic-Razumovic, J.; Podolski, P.; Saric, N. High expression of MAGE-A10 cancer-testis antigen in triple-negative breast cancer. Med. Oncol. 2012, 29, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Raghavendra, A.; Kalita-de Croft, P.; Vargas, A.C.; Smart, C.E.; Simpson, P.T.; Saunus, J.M.; Lakhani, S.R. Expression of MAGE-A and NY-ESO-1 cancer/testis antigens is enriched in triple-negative invasive breast cancers. Histopathology 2018, 73, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Bandic, D.; Juretic, A.; Sarcevic, B.; Separovic, V.; Kujundzic, M.; Hudolin, T.; Giulio, C.; Covic, D.; Samija, M. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: Retrospective immunohistochemical study. Croat. Med. J. 2006, 47, 32–41. [Google Scholar]
- Lee, H.J.; Kim, J.Y.; Song, I.H.; Park, I.A.; Yu, J.H.; Gong, G. Expression of NY-ESO-1 in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and a good prognosis. Oncology 2015, 89, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Bagnardi, V.; Ghioni, M.; Louahed, J.; Brichard, V.; Lehmann, F.F.; Marra, A.; Trapani, D.; Criscitiello, C.; Viale, G. Expression of tumor-associated antigens in breast cancer subtypes. Breast 2020, 49, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | itTIL < 10% (85) | itTIL ≥ 10% (12) | p | OR | p | |
|---|---|---|---|---|---|---|
| Age (years) | Median value (q1–q3; minimum–maximum) | 65 (54–74; 29–91) | 70 (60–80; 51–83) | 0.153 | ||
| Ki67 | Median value (q1–q3; minimum–maximum) | 55% (35–75; 5–90) | 62% (38–80; 27–98) | 0.413 | ||
| Tumor size median value | Median value (q1–q3; minimum–maximum) | 2.2 (1.5–3; 0.9–10) | 1.9 (1.7–2.9; 1.1–4.5) | 0.709 | ||
| Histologic grade * | 2 | 18 (21) | 0 | 0.166 | ||
| 3 | 66 (79) | 12 (100) | ||||
| Histologic type | NOS | 68 (80) | 11 (92) | 0.564 | ||
| Other subtypes | 17 (20) | 1 (8) | ||||
| sTIL peripheral | Median value (q1–q3; minimum–maximum) | 20 (10–30; 0–80) | 40 (25–55; 10–70) | 0.005 | ||
| sTIL central | Median value (q1–q3; minimum–maximum) | 15 (10–25; 1–85) | 45 (27–80; 10–80) | <0.001 | ||
| sTIL total | Median value (q1–q3; minimum–maximum) | 20 (10–25; 1–85) | 40 (26–64; 10–70) | <0.001 | ||
| Primary lymphoid aggregates | No | 30 (35) | 1 (8) | 0.123 | ||
| Yes | 55 (65) | 11 (92) | ||||
| Secondary lymphoid aggregates | No | 75 (88) | 8 (67) | 0.121 | ||
| Yes | 10 (12) | 4 (33) | ||||
| PD-L1 | Negative | 50 (59) | 0 | <0.001 | ||
| Positive | 35 (41) | 12 (100) | ||||
| NY-ESO-1 | 0% | 56 (66) | 4 (33) | 0.064 | 3.9 (1.1–13.9) | p = 0.039 |
| ≥1% | 29 (34) | 8 (67) |
| Variables | PD-L1 Negative (50; 51%) | PD-L1 Positive (47; 49%) | p | |
|---|---|---|---|---|
| Age (years) | Median value (q1–q3; minimum–maximum) | 66 (54–78; 39–91) | 65(55–72; 34–88) | 0.509 |
| Ki67 | Median value (q1–q3; minimum–maximum) | 42 (30–70; 5–90) | 65 (50–80; 24–98) | 0.005 |
| Tumor size median value | Median value (q1–q3; minimum–maximum) | 2.1 (1.6–4.5; 0.9–10) | 2 (1.5–3; 0.9–5) | 0.411 |
| Histologic grade * | 2 | 15 (31) | 3 (6) | 0.005 |
| 3 | 34 (69) | 44 (94) | ||
| Histologic type | NOS | 37 (74) | 42 (89) | 0.092 |
| Other subtypes | 13 (26) | 5 (11) | ||
| Clinical stage ** | I | 20 (40) | 20 (43.5) | 0.795 |
| II | 21 (42) | 20 (43.5) | ||
| III | 9 (18) | 6 (13) | ||
| sTIL peripheral | Median value (q1–q3; minimum–maximum) | 15 (6.5–25; 1–35) | 30 (25–50; 0–80) | <0.001 |
| sTIL peripheral | ≤25% | 41 (82) | 19 (40) | <0.001 |
| >25% | 9 (18) | 28 (60) | ||
| sTIL central | Median value (q1–q3; minimum–maximum) | 10 (5–30; 1–35) | 25 (20–50; 2–85) | <0.001 |
| sTIL central | ≤20% | 43 (86) | 17 (36) | <0.001 |
| >20% | 7 (14) | 30 (64) | ||
| sTIL total | Median value (q1–q3; minimum–maximum) | 15 (9–20; 1–30) | 30 (20–45; 5–85) | <0.001 |
| sTIL total | ≤20% | 45 (90) | 17 (36) | <0.001 |
| >20% | 5 (10) | 30 (64) | ||
| itTIL | Median value (q1–q3; min–max) | 1 (1–2; 0–5) | 5 (1–10; 1–15) | <0.001 |
| itTIL | ≤2% | 42 (84) | 20 (43) | <0.001 |
| >2% | 8 (16) | 27 (57) | ||
| Primary lymphoid aggregates | No | 23 (46) | 8 (17) | 0.003 |
| Yes | 27 (54) | 39 (83) | ||
| Secondary lymphoid aggregates | No | 46 (92) | 37 (79) | 0.116 |
| Yes | 4 (8) | 10 (21) | ||
| NY-ESO-1 | 0 | 33 (66) | 27 (57) | 0.511 |
| ≥1 | 17 (34) | 20 (43) |
| Variables | Log-Rank Test | Cox Regression Univariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Average DFS (Months) (SE) | 95% CI | LR | p | RR | 95% CI | p | ||
| Ki67 (ROC analysis) | ≤55.5% | 45.1 (1.4) | 42–48 | 1.39 | 0.238 | 2.11 | 0.64–7 | 0.221 |
| >55.5% | 40 (2) | 36–44 | ||||||
| Histological grade * | 2 | 41.9 (3.2) | 35.7–48 | 0.404 | 0.525 | 0.698 | 0.189–2.6 | 0.590 |
| 3 | 41.8 (1.3) | 39–44 | ||||||
| Histological type | NOS | 41.7 (1.3) | 39–44 | 0.570 | 0.450 | 1.45 | 0.39–5.4 | 0.577 |
| Other subtypes | 41.6 (3.6) | 35–48 | ||||||
| Positive lymph nodes | No | 45 (1.1) | 43–48 | 6.4 | 0.011 | 4.1 | 1.3–12.8 | 0.017 |
| Yes | 36.4 (3.2) | 30–43 | ||||||
| sTIL peripheral by median value | ≤25% | 42.5 (1.7) | 39–46 | 0.957 | 0.328 | 0.547 | 0.15–2 | 0.366 |
| >25% | 42.7 (1.8) | 39–46.3 | ||||||
| sTIL central by median value | ≤20% | 42.5 (1.7) | 39–46 | 0.945 | 0.331 | 0.549 | 0.15–2 | 0.369 |
| >20% | 42.6 (1.9) | 39–46 | ||||||
| sTIL total by median value | ≤20% | 42.7 (1.6) | 39–46 | 0.677 | 0.411 | 0.603 | 0.163–2.23 | 0.448 |
| >20% | 42.4 (2) | 38–46 | ||||||
| Primary lymphoid aggregates | No | 37.7 (2.7) | 32–43 | 4.9 | 0.027 | 0.319 | 1–9.9 | 0.051 |
| Yes | 45.1 (1.3) | 42.6–46 | ||||||
| PD-L1 | Negative | 41.4 (2) | 37–45 | 2.97 | 0.085 | 0.351 | 0.095–1.3 | 0.117 |
| Positive | 43.3 (1.5) | 40–46 | ||||||
| NY-ESO-1 | 0% | 42.4 (1.7) | 39–46 | 0.841 | 0.359 | 0.518 | 0.14–1.9 | 0.324 |
| ≥1% | 43 (1.6) | 40–46 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čeprnja, T.; Mrklić, I.; Perić Balja, M.; Marušić, Z.; Blažićević, V.; Spagnoli, G.C.; Juretić, A.; Čapkun, V.; Tečić Vuger, A.; Vrdoljak, E.; et al. Prognostic Significance of Lymphocyte Infiltrate Localization in Triple-Negative Breast Cancer. J. Pers. Med. 2022, 12, 941. https://doi.org/10.3390/jpm12060941
Čeprnja T, Mrklić I, Perić Balja M, Marušić Z, Blažićević V, Spagnoli GC, Juretić A, Čapkun V, Tečić Vuger A, Vrdoljak E, et al. Prognostic Significance of Lymphocyte Infiltrate Localization in Triple-Negative Breast Cancer. Journal of Personalized Medicine. 2022; 12(6):941. https://doi.org/10.3390/jpm12060941
Chicago/Turabian StyleČeprnja, Toni, Ivana Mrklić, Melita Perić Balja, Zlatko Marušić, Valerija Blažićević, Giulio Cesare Spagnoli, Antonio Juretić, Vesna Čapkun, Ana Tečić Vuger, Eduard Vrdoljak, and et al. 2022. "Prognostic Significance of Lymphocyte Infiltrate Localization in Triple-Negative Breast Cancer" Journal of Personalized Medicine 12, no. 6: 941. https://doi.org/10.3390/jpm12060941
APA StyleČeprnja, T., Mrklić, I., Perić Balja, M., Marušić, Z., Blažićević, V., Spagnoli, G. C., Juretić, A., Čapkun, V., Tečić Vuger, A., Vrdoljak, E., & Tomić, S. (2022). Prognostic Significance of Lymphocyte Infiltrate Localization in Triple-Negative Breast Cancer. Journal of Personalized Medicine, 12(6), 941. https://doi.org/10.3390/jpm12060941







