A Randomized Controlled Trial Evaluating Outcome Impact of Cilostazol in Patients with Coronary Artery Disease or at a High Risk of Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
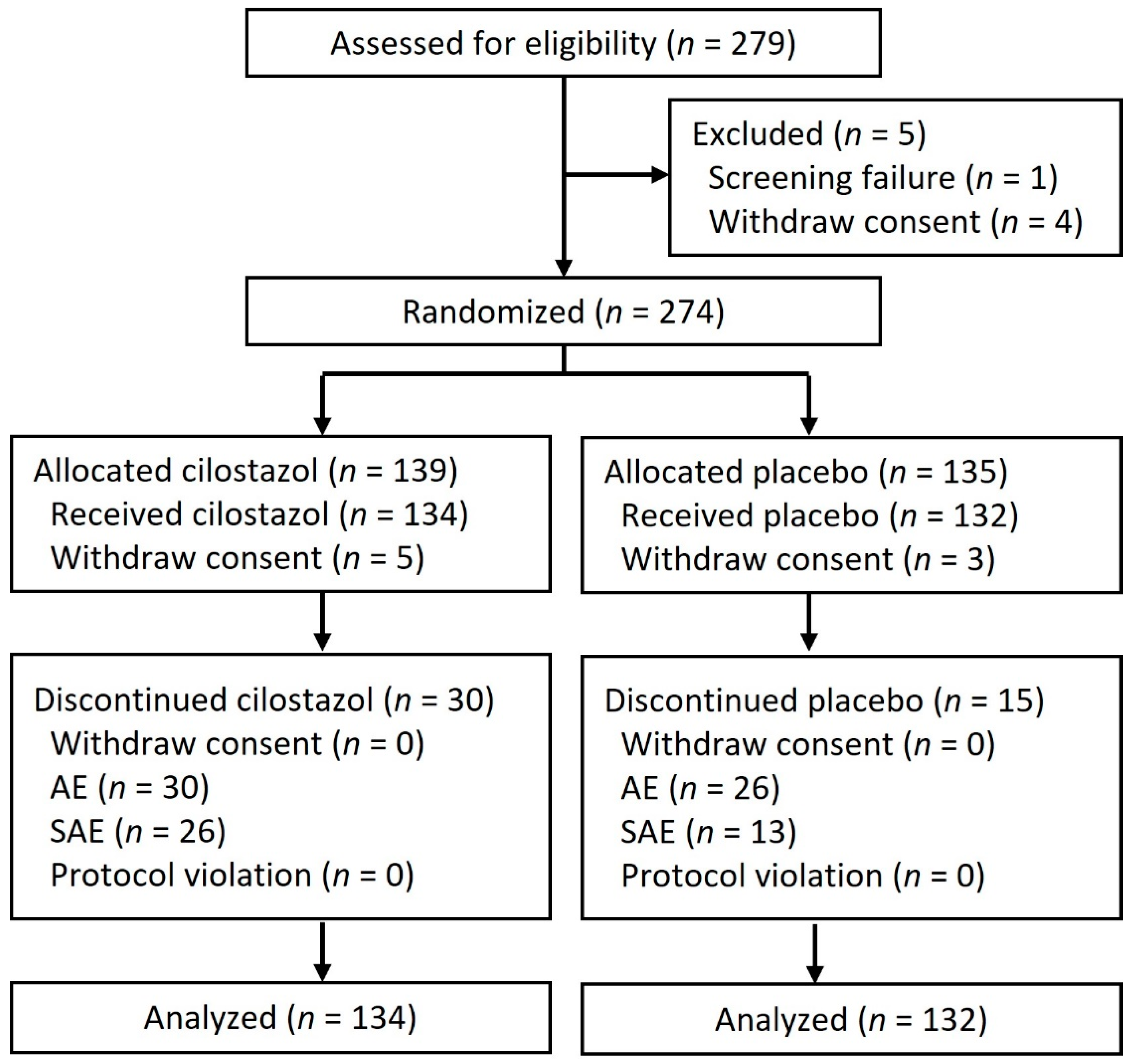
2.2. Patient Population
2.3. Outcomes and Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, K.C.; Oliphant, C.S.; Finks, S.W. Clinical efficacy and safety of cilostazol: A critical review of the literature. Drugs 2015, 75, 377–395. [Google Scholar] [CrossRef]
- Tseng, S.Y.; Chao, T.H.; Li, Y.H.; Liu, P.Y.; Lee, C.H.; Cho, C.L.; Wu, H.L.; Chen, J.H. Cilostazol improves high glucose-induced impaired angiogenesis in human endothelial progenitor cells and vascular endothelial cells as well as enhances vasculoangiogenesis in hyperglycemic mice mediated by the adenosine monophosphate-activated protein kinase pathway. J. Vasc. Surg. 2016, 63, 1051–1062. [Google Scholar] [CrossRef][Green Version]
- Chao, T.H.; Chen, I.C.; Lee, C.H.; Chen, J.Y.; Tsai, W.C.; Li, Y.H.; Tseng, S.Y.; Tsai, L.M.; Tseng, W.K. Cilostazol enhances mobilization of circulating endothelial progenitor cells and improves endothelium-dependent function in patients at high risk of cardiovascular disease. Angiology 2016, 67, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.H.; Tseng, S.Y.; Chen, I.C.; Tsai, Y.S.; Huang, Y.Y.; Liu, P.Y.; Ou, H.Y.; Li, Y.H.; Wu, H.L.; Cho, C.L.; et al. Cilostazol enhances mobilization and proliferation of endothelial progenitor cells and collateral formation by modifying vasculo-angiogenic biomarkers in peripheral arterial disease. Int. J. Cardiol. 2014, 172, e371–e374. [Google Scholar] [CrossRef]
- Chao, T.H.; Tseng, S.Y.; Li, Y.H.; Liu, P.Y.; Cho, C.L.; Shi, G.Y.; Wu, H.L.; Chen, J.H. A novel vasculo-angiogenic effect of cilostazol mediated by cross-talk between multiple signalling pathways including the ERK/p38 MAPK signalling transduction cascade. Clin. Sci. 2012, 123, 147–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tseng, S.Y.; Chao, T.H.; Li, Y.H.; Cho, C.L. Cilostazol improves proangiogenesis functions in human early endothelial progenitor cells through the stromal cell-derived factor system and hybrid therapy provides a synergistic effect in vivo. Biomed. Res. Int. 2016, 2016, 3639868. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Fraccarollo, D.; Schultheiss, M.; Froese, S.; Galuppo, P.; Widder, J.D.; Tsikas, D.; Ertl, G.; Bauersachs, J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 2007, 56, 666–674. [Google Scholar] [CrossRef]
- Delva, P.; Degan, M.; Vallerio, P.; Arosio, E.; Minuz, P.; Amen, G.; Di Chio, M.; Lechi, A. Endothelial progenitor cells in patients with essential hypertension. J. Hypertens. 2007, 25, 127–132. [Google Scholar] [CrossRef]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Böhm, M.; Nickenig, G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Desai, K.; Han, B.; Kuziez, L.; Yan, Y.; Zayed, M.A. Literature review and meta-analysis of the efficacy of cilostazol on limb salvage rates after infrainguinal endovascular and open revascularization. J. Vasc. Surg. 2021, 73, 711–721. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Saha, P.; Diamantopoulos, A.; Karunanithy, N.; Krokidis, M.; Modarai, B.; Karnabatidis, D. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: A systematic review and network meta-analysis. PLoS ONE 2015, 10, e0135692. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Kim, Y.S.; Kawamori, R.; Yamasaki, Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus: Principal results of the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) study: A randomized trial. Circulation 2010, 121, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.U.; Cho, Y.J.; Koo, J.S.; Bae, H.J.; Lee, Y.S.; Hong, K.S.; Lee, J.H.; Kim, J.S. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: The multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005, 36, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Katayama, Y.; Uchiyama, S.; Yamaguchi, T.; Handa, S.; Matsuoka, K.; Ohashi, Y.; Tanahashi, N.; Yamamoto, H.; Genka, C.; et al. Cilostazol for prevention of secondary stroke (CSPS 2): An aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010, 9, 959–968. [Google Scholar] [CrossRef]
- Douglas, J.S., Jr.; Holmes, D.R., Jr.; Kereiakes, D.J.; Grines, C.L.; Block, E.; Ghazzal, Z.M.; Morris, D.C.; Liberman, H.; Parker, K.; Jurkovitz, C.; et al. Coronary stent restenosis in patients treated with cilostazol. Circulation 2005, 112, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, S.W.; Kim, Y.H.; Yun, S.C.; Park, D.W.; Lee, C.W.; Kang, S.J.; Park, S.J.; Lee, J.H.; Choi, S.W.; et al. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: Results from the DECLARE-LONG II (Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions) trial. J. Am. Coll. Cardiol. 2011, 57, 1264–1270. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, J.Y.; Park, G.M.; Lee, S.W.; Kim, H.S.; Choi, Y.J.; Nam, C.W.; Cho, J.H.; Shin, W.Y.; Seo, J.B.; et al. Comparison of 1-year outcomes of triple (aspirin + clopidogrel + cilostazol) versus dual antiplatelet therapy (aspirin + clopidogrel + placebo) after implantation of second-generation drug-eluting stents into one or more coronary arteries: From the DECREASE-PCI Trial. Am. J. Cardiol. 2018, 121, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.J.; Lee, J.W.; Ahn, S.G.; Lee, S.H.; Choi, H.; Yu, C.W.; Hong, Y.J.; Kwon, H.M.; Hong, M.K.; Jang, Y.; et al. Multicenter randomized trial of 3-month cilostazol use in addition to dual antiplatelet therapy after biolimus-eluting stent implantation for long or multivessel coronary artery disease. Am. Heart J. 2014, 167, 241–248. [Google Scholar] [CrossRef]
- Suh, J.W.; Lee, S.P.; Park, K.W.; Lee, H.Y.; Kang, H.J.; Koo, B.K.; Cho, Y.S.; Youn, T.J.; Chae, I.H.; Choi, D.J.; et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: Results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J. Am. Coll. Cardiol. 2011, 57, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kido, A.; Matsuhisa, S.; Asawa, K.; Yoshida, N.; Tsujimoto, M.; Sasaki, Y.; Kuga, Y.; Yamasaki, M.; Ueda, K.; et al. Addition of cilostazol to aspirin therapy for secondary prevention of cardiovascular and cerebrovascular disease in patients undergoing percutaneous coronary intervention: A randomized, open-label trial. Am. Heart J. 2016, 173, 134–142. [Google Scholar] [CrossRef]
- Shin, E.S.; Lee, J.H.; Yoo, S.Y.; Park, Y.; Hong, Y.J.; Kim, M.H.; Lee, J.Y.; Nam, C.W.; Tahk, S.J.; Kim, J.S.; et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart 2014, 100, 1531–1536. [Google Scholar] [CrossRef]
- Lee, D.H.; Chun, E.J.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Effect of cilostazol, a phosphodiesterase-3 inhibitor, on coronary artery stenosis and plaque characteristics in patients with type 2 diabetes: ESCAPE study. Diabetes Obes. Metab. 2019, 21, 1409–1418. [Google Scholar] [CrossRef]
- Muzurović, E.M.; Mikhailidis, D.P. Diabetes mellitus and noncardiac atherosclerotic vascular disease-pathogenesis and pharmacological treatment options. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Asal, N.J.; Wojciak, K.A. Effect of cilostazol in treating diabetes-associated microvascular complication. Endocrine 2017, 56, 240–244. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet 2008, 372, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012, 126, e354–e471. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Feng, Z.W.; Li, X.Y.; Hu, Z.H.; Xu, Q.; Wang, Z.; Cheng, J.H.; Shi, H.T.; Wang, Q.B.; Wu, H.Y.; et al. The efficacy and safety of cilostazol as an alternative to aspirin in Chinese patients with aspirin intolerance after coronary stent implantation: A combined clinical study and computational system pharmacology analysis. Acta Pharmacol. Sin. 2018, 39, 205–212. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Z.; Fu, J.; Qian, J.; Ge, J. Cilostazol for Chinese patients with aspirin intolerance after coronary drug-eluting stent implantation. Thromb. Haemost. 2020, 120, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, S.; Zhou, R.; Liu, G.; Tang, P.; He, J.; Ma, C.; He, Y.; Yang, J. The effectiveness and safety of triple-antiplatelet treatment based on cilostazol for patients receiving percutaneous coronary intervention: A meta-analysis. Clin. Cardiol. 2012, 35, 598–604. [Google Scholar] [CrossRef]
- Bangalore, S.; Singh, A.; Toklu, B.; DiNicolantonio, J.J.; Croce, K.; Feit, F.; Bhatt, D.L. Efficacy of cilostazol on platelet reactivity and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: Insights from a meta-analysis of randomised trials. Open Heart 2014, 1, e000068. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Capranzano, P.; Goto, S.; Aslam, M.; Desai, B.; Charlton, R.K.; Suzuki, Y.; Box, L.C.; Shoemaker, S.B.; Zenni, M.M.; et al. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: Results of the OPTIMUS-2 study. Eur. Heart J. 2008, 29, 2202–2211. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, M.H.; Jeong, J.W.; Kim, K.H.; Ahn, T.H.; Kang, W.C.; Park, C.G.; Kim, J.H.; Chae, I.H.; Nam, C.W.; et al. Randomized comparison of cilostazol vs clopidogrel after drug-eluting stenting in diabetic patients—Clilostazol for diabetic patients in drug-eluting stent (CIDES) trial. Circ. J. 2008, 72, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, S.W.; Kim, Y.H.; Yun, S.C.; Park, D.W.; Lee, C.W.; Hong, M.K.; Kim, H.S.; Ko, J.K.; Park, J.H.; et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients). J. Am. Coll. Cardiol. 2008, 51, 1181–1187. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Qin, T.; Chen, M.H. Comparing the effectiveness and safety between triple antiplatelet therapy and dual antiplatelet therapy in type 2 diabetes mellitus patients after coronary stents implantation: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2015, 15, 118. [Google Scholar] [CrossRef]
- Chen, P.W.; Tseng, S.Y.; Chang, H.Y.; Lee, C.H.; Chao, T.H. Diverse Effects of Cilostazol on Proprotein Convertase Subtilisin/Kexin Type 9: Obesity or Not; National Chen Kung University Hospital: Tainan, Taiwan, 2022. [Google Scholar]
- Park, K.W.; Kang, S.H.; Park, J.J.; Yang, H.M.; Kang, H.J.; Koo, B.K.; Park, B.E.; Cha, K.S.; Rhew, J.Y.; Jeon, H.K.; et al. Adjunctive cilostazol versus double-dose clopidogrel after drug-eluting stent implantation: The HOST-ASSURE randomized trial (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-Safety & Effectiveness of Drug-Eluting Stents & Anti-platelet Regimen). JACC Cardiovasc. Interv. 2013, 6, 932–942. [Google Scholar] [CrossRef]
- Lee, D.H.; Chun, E.J.; Moon, J.H.; Yun, H.M.; Lim, S. Effect of cilostazol on carotid plaque volume measured by three-dimensional ultrasonography in patients with type 2 diabetes: The FANCY study. Diabetes Obes. Metab. 2020, 22, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- De Donato, G.; Setacci, F.; Mele, M.; Giannace, G.; Galzerano, G.; Setacci, C. Restenosis after coronary and peripheral intervention: Efficacy and clinical impact of cilostazol. Ann. Vasc. Surg. 2017, 41, 300–307. [Google Scholar] [CrossRef] [PubMed]
| Cilostazol (n = 134) | Placebo (n = 132) | p-Value | |
|---|---|---|---|
| Age, year | 64.5 (9.6) | 67.2 (9.9) | 0.03 |
| Male gender | 104 (77.6) | 91 (68.9) | 0.11 |
| Indications | |||
| Stable CAD | 103 (76.9) | 97 (73.5) | 0.52 |
| Old MI | 38 (28.4) | 29 (22.0) | 0.23 |
| High risk of CV disease | 31 (23.1) | 35 (26.5) | 0.52 |
| Risk factors only | 6 (4.5) | 13 (9.8) | 0.09 |
| Underlying disease | |||
| Diabetes mellitus | 54 (40.3) | 51 (38.6) | 0.78 |
| Hypertension | 101 (75.4) | 106 (80.3) | 0.33 |
| Hyperlipidemia | 108 (80.6) | 102 (77.3) | 0.51 |
| Metabolic syndrome | 77 (57.5) | 79 (59.8) | 0.69 |
| Tobacco smoking | 30 (22.4) | 27 (20.5) | 0.70 |
| Chronic kidney disease | 13 (9.7) | 14 (10.6) | 0.81 |
| Anemia | 24 (18.2) | 41 (31.1) | 0.02 |
| CAD | 103 (76.9) | 97 (73.5) | 0.52 |
| MI | 38 (28.4) | 29 (22.0) | 0.23 |
| Peripheral artery disease | 27 (20.1) | 14 (10.6) | 0.03 |
| Cerebrovascular disease | 8 (6.0) | 5 (3.8) | 0.40 |
| PCI | 67 (50.0) | 65 (49.2) | 0.90 |
| CABG | 6 (4.5) | 0 | 0.03 |
| PTA | 0 | 0 | |
| Medication | |||
| Aspirin | 94 (70.1) | 77 (58.3) | 0.04 |
| Clopidogrel | 12 (9.0) | 19 (14.4) | 0.17 |
| Ticagrelor | 1 (0.7) | 2 (1.5) | 0.62 |
| ACEi | 37 (27.6) | 31 (23.5) | 0.44 |
| ARB | 47 (35.1) | 51 (38.6) | 0.55 |
| Statin | 76 (56.7) | 66 (50.0) | 0.27 |
| Objective data | |||
| Blood pressure, mmHg | |||
| Systolic | 132.1 (13.0) | 132.2 (14.2) | 0.95 |
| Diastolic | 76 (70–84) | 74 (70–83.75) | 0.24 |
| Creatinine, mg/dL | 0.87 (0.75–1.11) | 0.87 (0.71–1.07) | 0.30 |
| eGFR, ml/min/1.73 m2 | 84 (64–90) | 82.69 (66.25–90) | 0.86 |
| Hemoglobin A1C, % | 6.15 (5.7–7.2) | 6.1 (5.8–7.0) | 0.77 |
| LDL cholesterol, mg/dL | 108.0 (30.2) | 109.7 (30.3) | 0.63 |
| Cilostazol (n = 134) | Placebo (n = 132) | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Composite endpoints | ||||
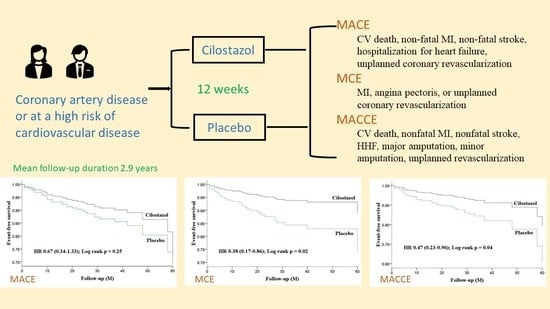
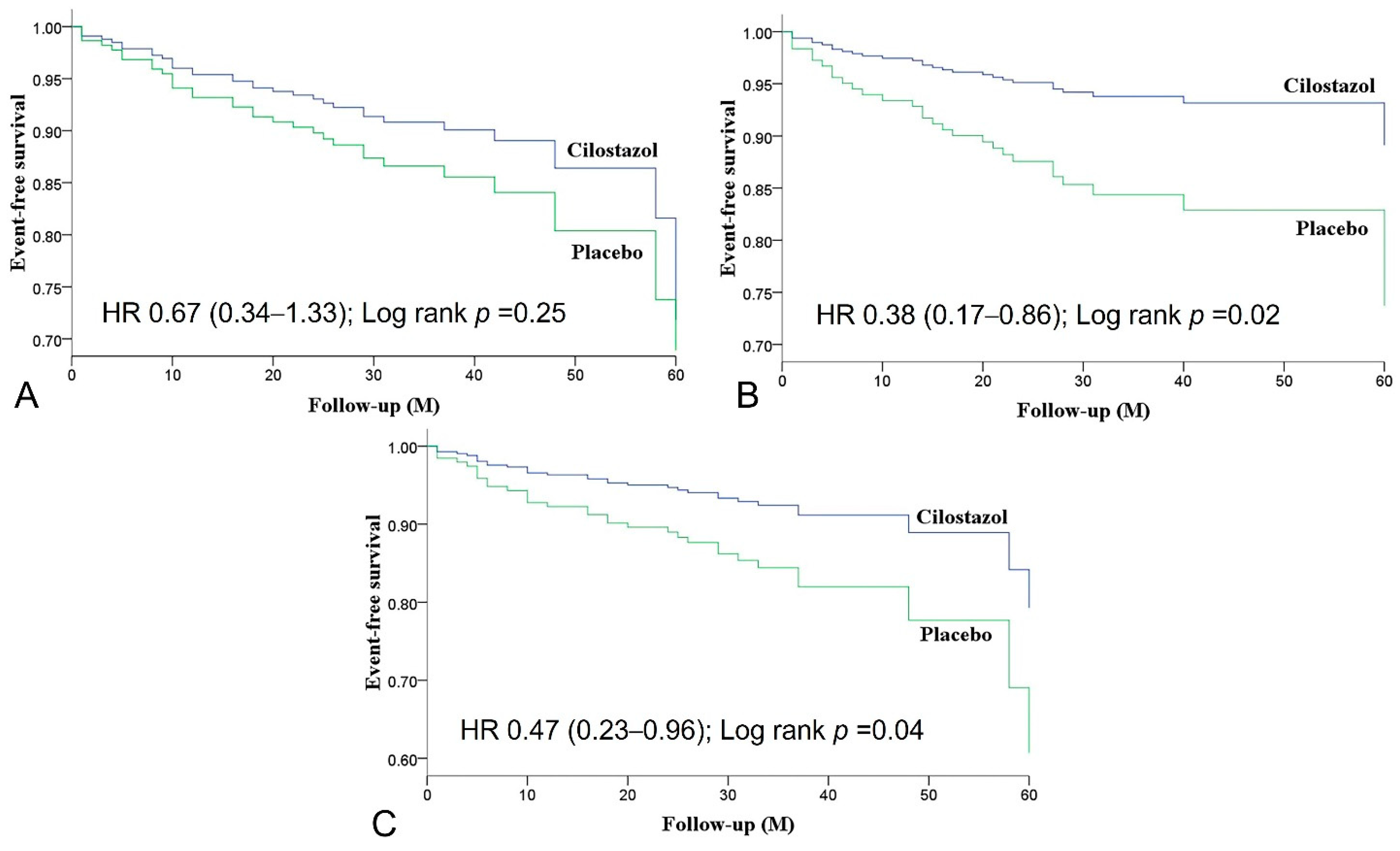
| MACE | 14 (10.4) | 20 (15.2) | 0.67 (0.34–1.33) | 0.25 |
| MCE | 8 (6.0) | 20 (15.2) | 0.38 (0.17–0.86) | 0.02 |
| MACCE | 11 (8.2) | 22 (16.7) | 0.47 (0.23–0.96) | 0.04 |
| Individual endpoints | ||||
| CV death | 2 (1.5) | 2 (1.5) | 0.99 (0.14–7.00) | 0.99 |
| Nonfatal MI | 1 (0.7) | 4 (3.0) | 0.23 (0.03–2.09) | 0.19 |
| Nonfatal stroke | 5 (3.7) | 5 (3.8) | 0.93 (0.27–3.23) | 0.91 |
| HHF | 3 (2.2) | 7 (5.3) | 0.42 (0.11–1.62) | 0.21 |
| Angina pectoris | 8 (6.0) | 20 (15.2) | 0.38 (0.17–0.86) | 0.02 |
| Unplanned coronary revascularization | 5 (3.7) | 8 (6.1) | 0.60 (0.20–1.83) | 0.37 |
| Unplanned revascularization | 1 (0.7) | 7 (5.3) | 0.13 (0.02–1.09) | 0.06 |
| Major amputation | 0 | 0 | ||
| Minor amputation | 0 | 0 |
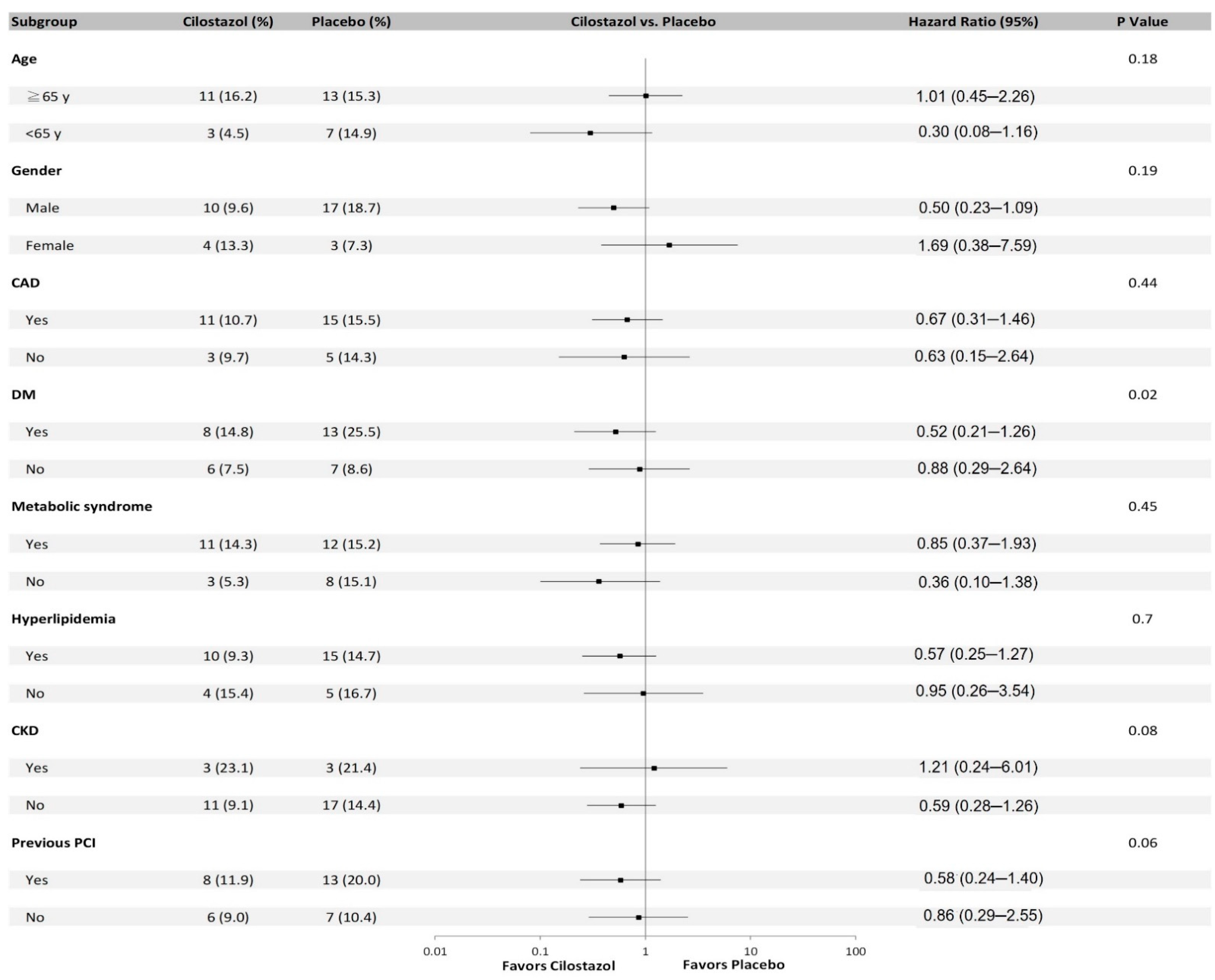
| Variables | Uni-Variables HR (95% CI) | p-Value | Multi-Variables HR (95% CI) | p-Value |
|---|---|---|---|---|
| Cilostazol | 0.38 (0.17–0.86) | 0.02 | 0.34 (0.15–0.78) | 0.01 |
| Age | 1.01 (0.97–1.05) | 0.56 | ||
| Peripheral artery disease | 0.89 (0.32–2.48) | 0.82 | ||
| Coronary bypass surgery | 0.05 (0–10296.19) | 0.63 | ||
| Anemia | 0.93 (0.37–2.31) | 0.88 | ||
| Aspirin | 2.52 (0.96–6.63) | 0.06 | 2.88 (1.09–7.61) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-L.; Tseng, W.-K.; Lee, P.-T.; Lee, C.-H.; Tseng, S.-Y.; Chen, P.-W.; Chang, H.-Y.; Chao, T.-H. A Randomized Controlled Trial Evaluating Outcome Impact of Cilostazol in Patients with Coronary Artery Disease or at a High Risk of Cardiovascular Disease. J. Pers. Med. 2022, 12, 938. https://doi.org/10.3390/jpm12060938
Lin J-L, Tseng W-K, Lee P-T, Lee C-H, Tseng S-Y, Chen P-W, Chang H-Y, Chao T-H. A Randomized Controlled Trial Evaluating Outcome Impact of Cilostazol in Patients with Coronary Artery Disease or at a High Risk of Cardiovascular Disease. Journal of Personalized Medicine. 2022; 12(6):938. https://doi.org/10.3390/jpm12060938
Chicago/Turabian StyleLin, Jia-Ling, Wei-Kung Tseng, Po-Tseng Lee, Cheng-Han Lee, Shih-Ya Tseng, Po-Wei Chen, Hsien-Yuan Chang, and Ting-Hsing Chao. 2022. "A Randomized Controlled Trial Evaluating Outcome Impact of Cilostazol in Patients with Coronary Artery Disease or at a High Risk of Cardiovascular Disease" Journal of Personalized Medicine 12, no. 6: 938. https://doi.org/10.3390/jpm12060938
APA StyleLin, J.-L., Tseng, W.-K., Lee, P.-T., Lee, C.-H., Tseng, S.-Y., Chen, P.-W., Chang, H.-Y., & Chao, T.-H. (2022). A Randomized Controlled Trial Evaluating Outcome Impact of Cilostazol in Patients with Coronary Artery Disease or at a High Risk of Cardiovascular Disease. Journal of Personalized Medicine, 12(6), 938. https://doi.org/10.3390/jpm12060938