Comparison of Temperature and Pain Changes between the Drip and Topical Methods of Administering the Transnasal Sphenopalatine Ganglion Block
Abstract
1. Introduction
2. Materials and Methods
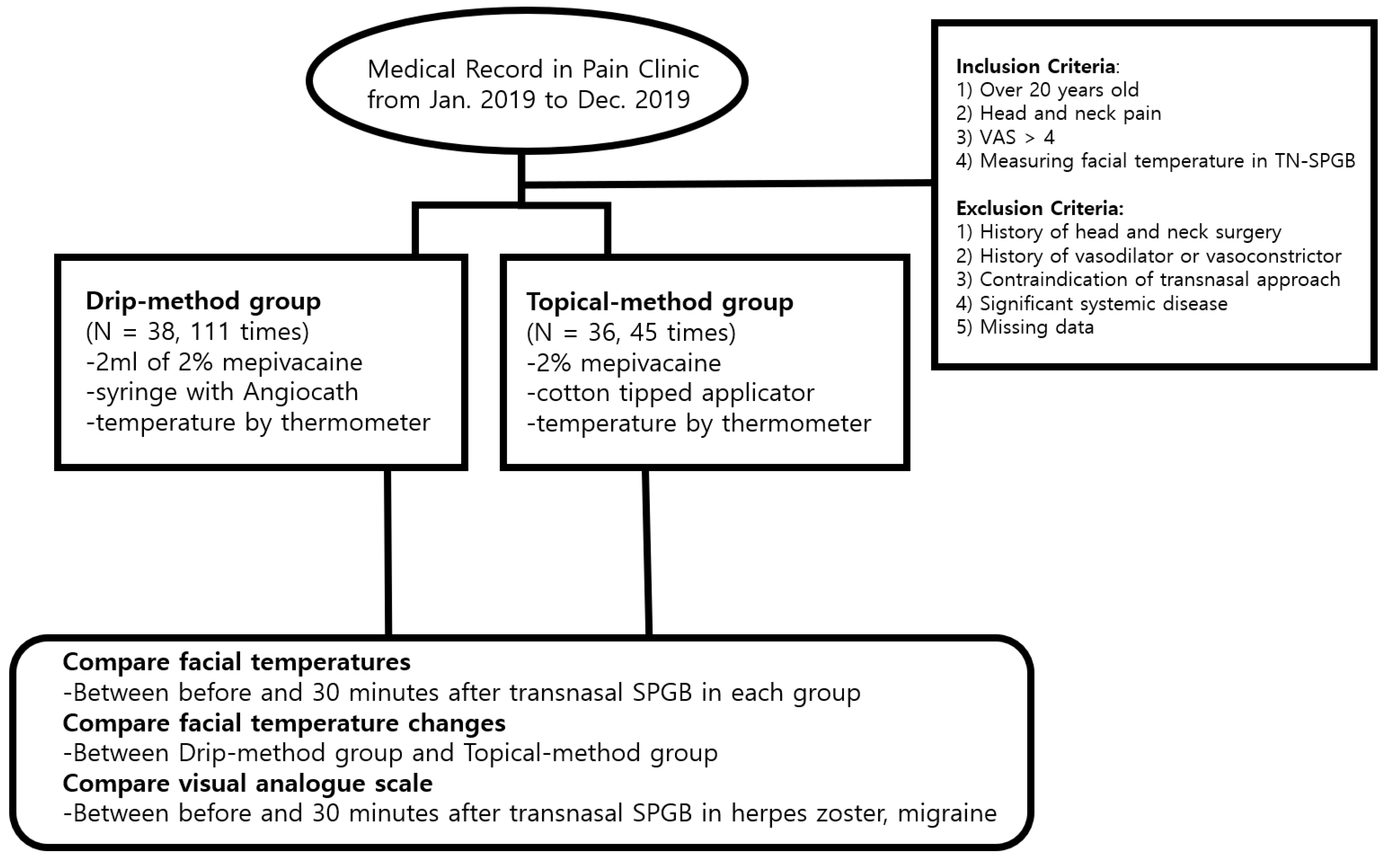
2.1. Participants
2.2. Procedure
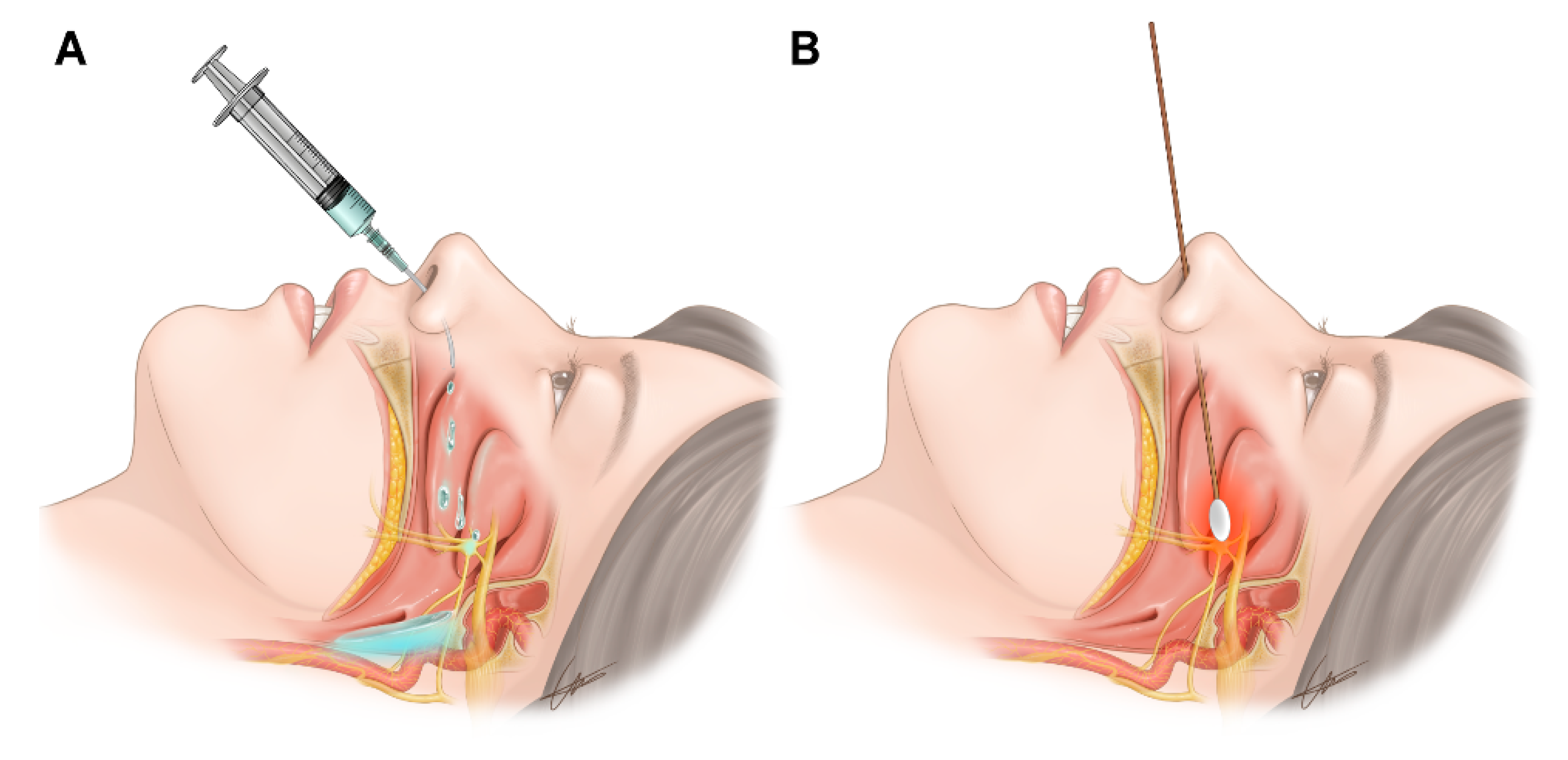
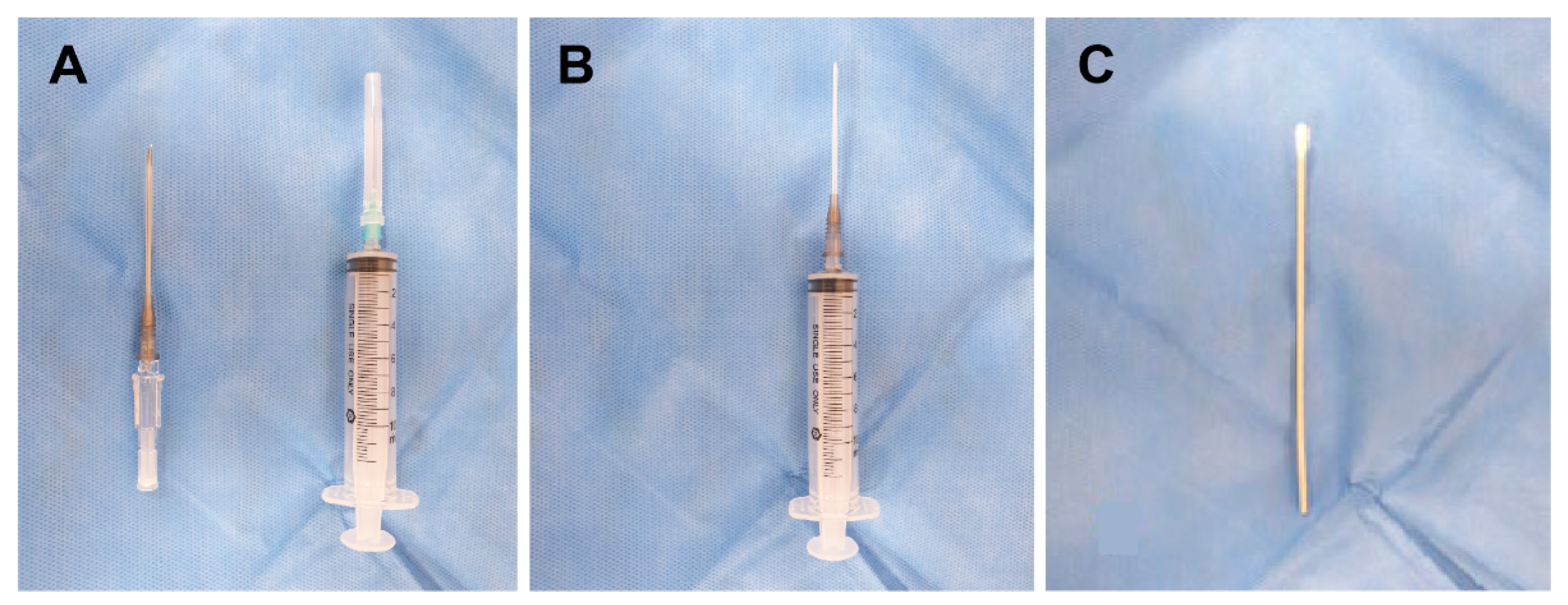
2.2.1. Transnasal SPGB by the Drip Method
2.2.2. Transnasal SPGB by the Topical Method
2.3. Clinical Evaluations
2.3.1. Temperature Measurements
2.3.2. Visual Analogue Scale
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Piagkou, M.; Demesticha, T.; Troupis, T.; Vlasis, K.; Skandalakis, P.; Makri, A.; Mazarakis, A.; Lappas, D.; Piagkos, G.; Johnson, E.O. The pterygopalatine ganglion and its role in various pain syndromes: From anatomy to clinical practice. Pain Pract. 2012, 12, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Robertson, C.E.; Kaplan, E.; Ailani, J.; Charleston, L.T.; Kuruvilla, D.; Blumenfeld, A.; Berliner, R.; Rosen, N.L.; Duarte, R.; et al. The sphenopalatine ganglion: Anatomy, pathophysiology, and therapeutic targeting in headache. Headache 2016, 56, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Additional, I.L.; Candido, K.D.; Masonic, A.I. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician 2013, 16, E769–E778. [Google Scholar]
- Kim, D.Y.; Yu, M.R.; Kang, S.H.; Park, J.M.; Moon, D.E. Pulsed radiofrequency of the sphenopalatine ganglion for treatment of a cluster headache: A case report. Korean J. Pain 2007, 20, 195–198. [Google Scholar] [CrossRef][Green Version]
- Park, S.J.; Moon, D.E.; Kim, W.Y.; Park, J.J.; Cho, E.J.; Yang, S.W. The sphenopalatine ganglion radiofrequency thermocoagulation on a patient of CRPS with facial pain and pruritus. Korean J. Pain 2006, 19, 228–232. [Google Scholar] [CrossRef][Green Version]
- Shin, K.M. Stereotactic sphenopalatine ganglionotomy using radiofrequency thermocoagulation: Case reports. Korean J. Pain Soc. 1999, 12, 227–230. [Google Scholar]
- Raj, P.P.; Lou, L.; Erdine, S.; Staats, P.S.; Waldman, S.D. Radiographic Imaging for Regional Anesthesia and Pain Management; Churchill Livingstone: New York, NY, USA, 2003. [Google Scholar]
- Waldman, S.D. Atlas of Interventional Pain Management; Saunders: Philadelphia, PA, USA, 1998. [Google Scholar]
- Windsor, R.E.; Gore, H.; Merson, M.A. Interventional Sympathetic Blockade. In Pain Procedures in Clinical Practice, 2nd ed.; Hanley & Belfus: Philadelphia, PA, USA, 2000; pp. 321–324. [Google Scholar]
- Wasserman, R.A.; Schack, T.; Moser, S.E.; Brummett, C.M.; Cooper, W. Facial temperature changes following intranasal sphenopalatine ganglion nerve block. J. Nat. Sci. 2017, 3, 354e. [Google Scholar]
- Kim, N.E.; Park, B.; Moon, Y.R.; Lee, S.Y.; Gil, H.Y.; Kim, S.; Lee, S.; Chang, H.S.; Jeong, H.W.; Park, H.; et al. Changes in facial temperature measured by digital infrared thermal imaging in patients after transnasal sphenopalatine ganglion block: Retrospective observational study. Medicine 2019, 98, e15084. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Rayani, B.K. Sphenopalatine ganglion block for relieving postdural puncture headache: Technique and mechanism of action of block with a narrative review of efficacy. Korean J. Pain 2017, 30, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S. Bilateral transnasal sphenopalatine block for treating postdural puncture headache. Korean J. Anesthesiol. 2018, 71, 73–74. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Drip-Method Group (n = 38) | Topical-Method Group (n = 36) | p-Value |
|---|---|---|---|
| Age (years) | 55.8 ± 13.2 | 58.6 ± 11.9 | 0.212 |
| Sex (male/female) | 15/23 | 17/19 | 0.557 |
| Height (cm) | 161.4 ± 7.2 | 162.0 ± 8.6 | 0.738 |
| Weight (kg) | 68.7 ± 10.6 | 67.2 ± 11.85 | 0.566 |
| Diagnosis | Drip-Method Group | Topical-Method Group | Total |
|---|---|---|---|
| Herpes zoster (n/times) | 12/55 | 29/34 | 41/89 |
| Migraine (n/times) | 20/48 | 4/7 | 24/55 |
| Hyperhidrosis (n/times) | 1/1 | -/- | 1/1 |
| Atypical facial pain (n/times) | 4/5 | 1/1 | 5/6 |
| Anosmia (n/times) | -/- | 2/3 | 2/3 |
| Tinnitus (n/times) | 1/2 | -/- | 1/2 |
| Total (n/times) | 38/111 | 36/45 | 74/156 |
| Methods | Regions | Temperatures (°C) (Mean ± SD) | p-Value | |
|---|---|---|---|---|
| Before Transnasal SPGB | 30 min after Completion of the Transnasal SPGB | |||
| Drip-method group | Right forehead area | 33.49 ± 1.09 | 33.73 ± 1.00 | 0.095 |
| Left forehead area | 33.31 ± 1.03 | 33.50 ± 1.06 | 0.174 | |
| Right maxillary area | 32.38 ± 1.76 | 32.98 ± 1.50 | 0.006 * | |
| Left maxillary area | 32.28 ± 1.71 | 32.78 ± 1.53 | 0.025 * | |
| Right mandibular area | 32.52 ± 1.52 | 32.95 ± 1.26 | 0.023 * | |
| Left mandibular area | 32.49 ± 1.59 | 32.93 ± 1.36 | 0.025 * | |
| Total | 32.75 ± 1.54 | 33.15 ± 1.34 | <0.001 * | |
| Right forehead area | 34.25 ± 0.94 | 34.08 ± 0.62 | 0.301 | |
| Topical-method group | Left forehead area | 34.17 ± 1.00 | 34.02 ± 0.68 | 0.403 |
| Right maxillary area | 33.84 ± 1.40 | 34.05 ± 1.01 | 0.415 | |
| Left maxillary area | 33.69 ± 1.40 | 34.01 ± 0.86 | 0.199 | |
| Right mandibular area | 34.09 ± 1.29 | 34.25 ± 0.77 | 0.476 | |
| Left mandibular area | 34.06 ± 1.24 | 34.22 ± 0.70 | 0.446 | |
| Total | 34.02 ± 1.23 | 34.10 ± 0.78 | 0.325 | |
| Methods and Regions | Temperature Difference between before and 30 min after Completion of the Transnasal SPGB (°C) (Mean ± SD) | p-Value | |
|---|---|---|---|
| Drip-Method Group | Topical-Method Group | ||
| Right forehead area | 0.24 ± 0.61 | −0.18 ± 0.73 | 0.001 * |
| Left forehead area | 0.19 ± 0.69 | −0.15 ± 0.81 | 0.015 * |
| Right maxillary area | 0.60 ± 1.21 | 0.21 ± 1.04 | 0.046 * |
| Left maxillary area | 0.49 ± 1.08 | 0.32 ± 1.05 | 0.353 |
| Right mandibular area | 0.43 ± 1.40 | 0.16 ± 0.83 | 0.141 |
| Left mandibular area | 0.45 ± 1.38 | 0.16 ± 0.90 | 0.133 |
| Total | 0.40 ± 1.11 | 0.09 ± 0.91 | <0.001 * |
| Disease | Method | VAS before Transnasal SPGB | VAS 30 min after Completion of the Transnasal SPGB | VAS Change | p-Value |
|---|---|---|---|---|---|
| Herpes zoster | Drip method (55 times) | 5.4 ± 1.6 | 2.9 ± 1.4 | −2.6 ± 1.3 | <0.001 * |
| Topical method (34 times) | 5.4 ± 2.0 | 4.1 ± 1.8 | −1.3 ± 1.1 | 0.008 * | |
| p-value | 0.977 | 0.001 * | <0.001 * | ||
| Migraine | Drip method (4 times) | 6.0 ± 1.9 | 4.1 ± 1.6 | −1.9 ± 1.3 | <0.001 * |
| Topical method (7 times) | 6.4 ± 2.0 | 3.1 ± 1.1 | −3.3 ± 1.1 | 0.004 * | |
| p-value | 0.625 | 0.055 | 0.014 * |
| Transnasal SPGB | Drip Method | Topical Method |
|---|---|---|
| Method |  |  |
| Sympathetic nerve | Block dominantly | Block slightly or exacerbate reactively |
| Parasympathetic nerve | Block slightly or exacerbate reactively | Block dominantly |
| Sensory nerve | Block | Block |
| Dominant effective diseases | Herpes zoster Hyperhidrosis | Migraine Post-dural puncture headache |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.E.; Kim, J.E.; Lee, S.Y.; Gil, H.Y.; Min, S.K.; Park, B.; Kim, S.I.; Cho, R.Y.; Koh, J.C.; Choi, Y.H.; et al. Comparison of Temperature and Pain Changes between the Drip and Topical Methods of Administering the Transnasal Sphenopalatine Ganglion Block. J. Pers. Med. 2022, 12, 830. https://doi.org/10.3390/jpm12050830
Kim NE, Kim JE, Lee SY, Gil HY, Min SK, Park B, Kim SI, Cho RY, Koh JC, Choi YH, et al. Comparison of Temperature and Pain Changes between the Drip and Topical Methods of Administering the Transnasal Sphenopalatine Ganglion Block. Journal of Personalized Medicine. 2022; 12(5):830. https://doi.org/10.3390/jpm12050830
Chicago/Turabian StyleKim, Na Eun, Ji Eun Kim, Sook Young Lee, Ho Young Gil, Sang Kee Min, Bumhee Park, Seung Il Kim, Ra Yoon Cho, Jae Chul Koh, Yi Hwa Choi, and et al. 2022. "Comparison of Temperature and Pain Changes between the Drip and Topical Methods of Administering the Transnasal Sphenopalatine Ganglion Block" Journal of Personalized Medicine 12, no. 5: 830. https://doi.org/10.3390/jpm12050830
APA StyleKim, N. E., Kim, J. E., Lee, S. Y., Gil, H. Y., Min, S. K., Park, B., Kim, S. I., Cho, R. Y., Koh, J. C., Choi, Y. H., Kim, J. H., Park, S. J., & Choi, J. B. (2022). Comparison of Temperature and Pain Changes between the Drip and Topical Methods of Administering the Transnasal Sphenopalatine Ganglion Block. Journal of Personalized Medicine, 12(5), 830. https://doi.org/10.3390/jpm12050830






