Personalized Care in Late-Stage Parkinson’s Disease: Challenges and Opportunities
Abstract
1. Introduction
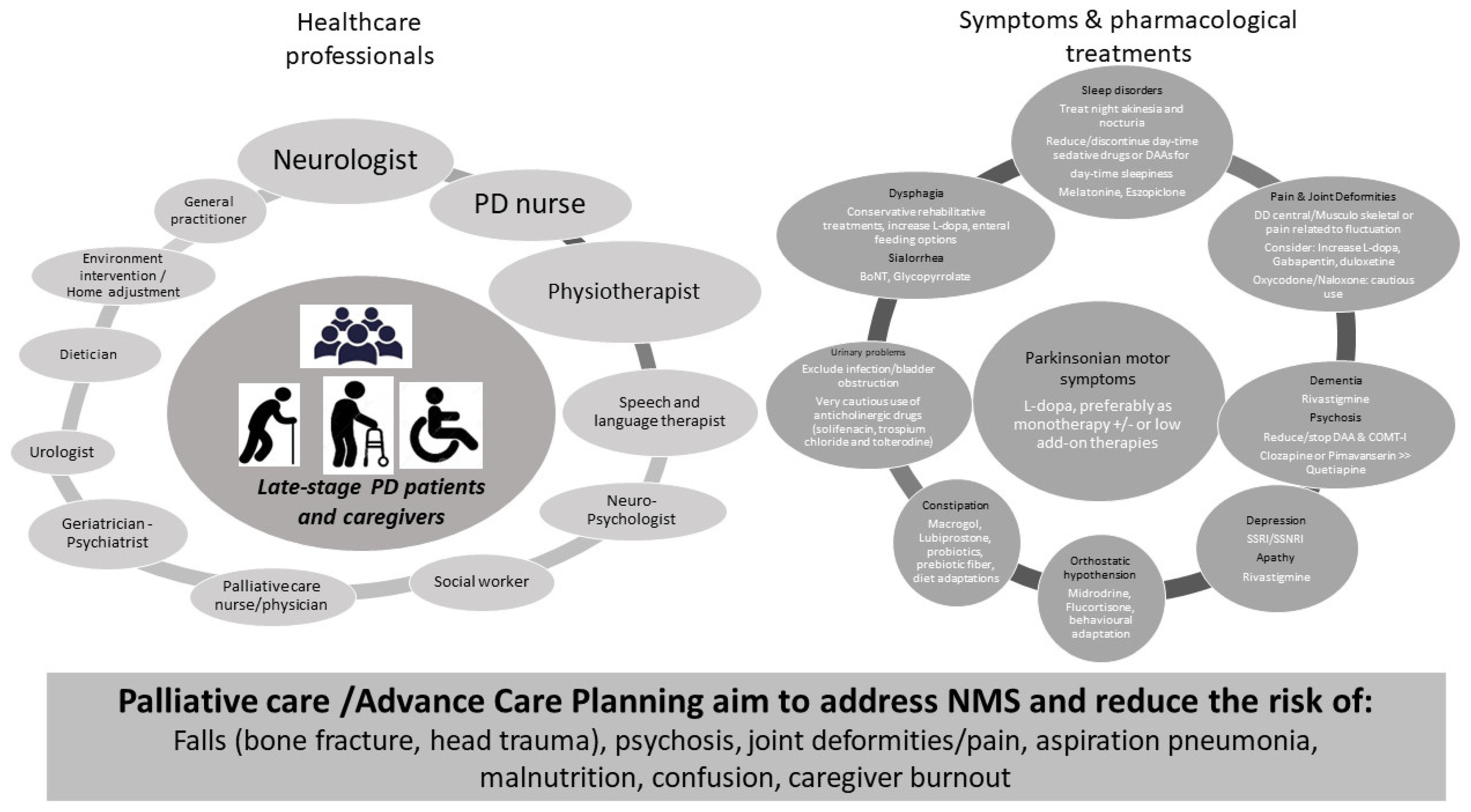
2. An Atypical Clinical Phenotype and Unique Clinical Needs
3. Therapeutic Challenges: Oral and Non-Pharmacological Approaches
4. Management of Device-Aided Therapies in LSPD
5. Caregiver Burden in LSPD
6. Management of LSPD Living in Nursing Homes
7. Risks and Management of LSPD during Hospitalizations Due to Systemic Illness
8. Home Care for Late-Stage PD
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, M.; Ferreira, J.J. Late-stage Parkinson disease. Nat. Rev. Neurol. 2012, 8, 435–442. [Google Scholar] [CrossRef]
- Schrag, A.; Hommel, A.; Lorenzl, S.; Meissner, W.G.; Odin, P.; Coelho, M.; Bloem, B.R.; Dodel, R.; Clasp, C. The late stage of Parkinson’s-results of a large multina-tional study on motor and non-motor complications. Parkinsonism Relat. Disord. 2020, 75, 91–96. [Google Scholar] [CrossRef]
- Kruse, C.; Kretschmer, S.; Lipinski, A.; Verheyen, M.; Mengel, D.; Balzer-Geldsetzer, M.; Lorenzl, S.; Richinger, C.; Schmotz, C.; Tönges, L.; et al. Resource Utilization of Patients with Parkinson’s Disease in the Late Stages of the Disease in Germany: Data from the CLaSP Study. PharmacoEconomics 2021, 39, 601–615. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic—A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- De Pablo-Fernández, E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Faculty Opinions recommendation of Prognosis and neuropathologic correlation of clinical subtypes of parkinson disease. JAMA Neurol. 2020, 76, 470–479. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Postuma, R.B. Subtypes of Parkinson’s Disease: What Do They Tell Us About Disease Progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. [Google Scholar] [CrossRef]
- Fabbri, M.; Coelho, M.; Abreu, D.; Guedes, L.C.; Rosa, M.M.; Godinho, C.; Cardoso, R.; Guimaraes, I.; Antonini, A.; Zibetti, M.; et al. Dysphagia predicts poor outcome in late-stage Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 64, 73–81. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Antonini, A. Cognitive decline in Parkinson’s disease: The complex picture. Npj Park. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Fabbri, M.; Kauppila, L.A.; Ferreira, J.J.; Rascol, O. Challenges and Perspectives in the Management of Late-Stage Parkinson’s Disease. J. Park. Dis. 2020, 10, S75–S83. [Google Scholar] [CrossRef]
- Fabbri, M.; Coelho, M.; Abreu, D.; Ferreira, J.J. Levodopa response in later stages of Parkinson’s disease: A case-control study. Parkinsonism Relat. Disord. 2019, 77, 160–162. [Google Scholar] [CrossRef]
- Fabbri, M.; Coelho, M.; Abreu, D.; Guedes, L.C.; Rosa, M.M.; Costa, N.; Antonini, A.; Ferreira, J.J. Do patients with late-stage Parkinson’s disease still respond to levodopa? Parkinsonism Relat. Disord. 2016, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, K.; Horne, M.; Hagell, P.; Iwarsson, S.; Nilsson, M.H.; Odin, P. Levodopa Effect and Motor Function in Late Stage Parkinson’s Disease. J. Park. Dis. 2018, 8, 59–70. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov. Disord. Off. J. Mov. Dis-Order Soc. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Rukavina, K.; Batzu, L.; Boogers, A.; Abundes-Corona, A.; Bruno, V.; Chaudhuri, K.R. Non-motor complications in late stage Parkinson’s disease: Recognition, management and unmet needs. Expert Rev. Neurother. 2021, 21, 335–352. [Google Scholar] [CrossRef]
- Hommel, A.L.; Meinders, M.J.; Weerkamp, N.J.; Richinger, C.; Schmotz, C.; Lorenzl, S.; Dodel, R.; Coelho, M.; Ferreira, J.J.; Tison, F.; et al. Optimizing Treatment in Undertreated Late-Stage Parkinsonism: A Pragmatic Randomized Trial. J. Park. Dis. 2020, 10, 1171–1184. [Google Scholar] [CrossRef]
- Modugno, N.; Antonini, A.; Tessitore, A.; Marano, P.; Pontieri, F.E.; Tambasco, N.; Canesi, M.; Fabbrini, G.; Sensi, M.; Quatrale, R.; et al. Impact of Supporting People with Advanced Parkinson’s Disease on Carer’s Quality of Life and Burden. Neuropsychiatr. Dis. Treat. 2020, 16, 2899–2912. [Google Scholar] [CrossRef]
- Leta, V.; Dafsari, H.; Sauerbier, A.; Metta, V.; Titova, N.; Timmermann, L.; Ashkan, K.; Samuel, M.; Pekkonen, E.; Odin, P.; et al. Personalised Advanced Therapies in Parkinson’s Disease: The Role of Non-Motor Symptoms Profile. J. Pers. Med. 2021, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Zibetti, M.; Rizzone, M.G.; Giannini, G.; Borellini, L.; Stefani, A.; Bove, F.; Bruno, A.; Calandra-Buonaura, G.; Modugno, N.; et al. Should We Consider Deep Brain Stimulation Discontin-uation in Late-Stage Parkinson’s Disease? Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Morgante, F.; Oppo, V.; Fabbri, M.; Olivola, E.; Sorbera, C.; De Micco, R.; Ielo, G.C.; Colucci, F.; Bonvegna, S.; Novelli, A.; et al. Levodopa–carbidopa intrajejunal infusion in Parkinson’s disease: Untangling the role of age. J. Neurol. 2020, 268, 1728–1737. [Google Scholar] [CrossRef]
- Bove, F.; Bentivoglio, A.R.; Naranian, T.; Fasano, A. Enteral feeding in Parkinson’s patients receiving levodopa/carbidopa intestinal gel. Parkinsonism Relat. Disord. 2017, 42, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, K.; Gray, W.K.; van Wersch, A.; van Schaik, P.; Walker, R. Predictors of the psychosocial impact of being a carer of people living with Parkinson’s disease: A systematic review. Parkinsonism Relat. Disord. 2015, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mosley, P.E.; Moodie, R.; Dissanayaka, N. Caregiver Burden in Parkinson Disease: A Critical Review of Recent Literature. J. Geriatr. Psychiatry Neurol. 2017, 30, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Kalampokini, S.; Hommel, A.L.A.J.; Lorenzl, S.; Ferreira, J.J.; Meissner, W.G.; Odin, P.; Bloem, B.R.; Dodel, R.; Schrag, A.-E. Caregiver Burden in Late-Stage Parkinsonism and Its Associations. J. Geriatr. Psychiatry Neurol. 2020, 35, 110–120. [Google Scholar] [CrossRef]
- Rosqvist, K.; Kylberg, M.; Löfqvist, C.; Schrag, A.; Odin, P.; Iwarsson, S. Perspectives on Care for Late-Stage Parkinson’s Disease. Park. Dis. 2021, 2021, 9475026. [Google Scholar] [CrossRef]
- Rosqvist, K.; Schrag, A.; Odin, P. The CLaSP Consortium Caregiver Burden and Quality of Life in Late Stage Parkinson’s Disease. Brain Sci. 2022, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Kiely, D.K.; Kiel, D.P.; Lipsitz, L.A. The epidemiology, clinical characteristics, and natural history of older nursing home residents with a diagnosis of Parkinson’s disease. J. Am. Geriatr. Soc. 1996, 44, 394–399. [Google Scholar] [CrossRef]
- Hosking, A.; Hommel, A.A.; Lorenzl, S.; Coelho, M.; Ferreira, J.J.; Meissner, W.G.; Odin, P.; Bloem, B.R.; Dodel, R.; Schrag, A. Characteristics of Patients with Late-Stage Parkin-sonism Who are Nursing Home Residents Compared with those Living at Home. J. Am. Med. Dir. Assoc. 2021, 22, 440–445.e2. [Google Scholar] [CrossRef]
- Weerkamp, N.J.; Zuidema, S.U.; Tissingh, G.; Poels, P.J.; Munneke, M.; Koopmans, R.T.; Bloem, B.R. Motor profile and drug treatment of nursing home residents with Parkinson’s disease. J. Am. Geriatr. Soc. 2012, 60, 2277–2282. [Google Scholar] [CrossRef]
- Safarpour, D.; Thibault, D.P.; DeSanto, C.L.; Boyd, C.M.; Dorsey, E.R.; Racette, B.A.; Willis, A.W. Nursing home and end-of-life care in Parkinson disease. Neurology 2015, 85, 413–419. [Google Scholar] [CrossRef]
- Makoutonina, M.; Iansek, R.; Simpson, P. Optimizing care of residents with Parkinsonism in supervised facilities. Park. Relat. Disord. 2010, 16, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Okunoye, O.; Horsfall, L.; Marston, L.; Walters, K.; Schrag, A. Rate of Hospitalizations and Underlying Reasons Among People with Parkinson’s Disease: Population-Based Cohort Study in UK Primary Care. J. Park. Dis. 2022, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Pederzoli, M.; Antonini, A.; Beretta, F.; Crespi, V. Reasons for hospitalization in Parkinson’s disease: A case-control study. Parkinsonism Relat. Disord. 2014, 20, 488–492. [Google Scholar] [CrossRef]
- Low, V.; Ben-Shlomo, Y.; Coward, E.; Fletcher, S.; Walker, R.; Clarke, C.E. Measuring the burden and mortality of hospitalisation in Parkinson’s disease: A cross-sectional analysis of the English Hospital Episodes Statistics database 2009–2013. Parkinsonism Relat. Disord. 2015, 21, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, E.; Godi, L.; Citterio, A.; Zangaglia, R.; Riboldazzi, G.; Calandrella, D.; Pacchetti, C.; Nappi, G. Comorbid disorders and hospitalisation in Parkinson’s disease: A prospective study. Neurol. Sci. 2004, 25, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.; Walker, R. Emergency hospital admissions in idiopathic Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2005, 20, 1104–1108. [Google Scholar] [CrossRef]
- Mahajan, A.; Balakrishnan, P.; Patel, A.; Konstantinidis, I.; Nistal, D.; Annapureddy, N.; Poojary, P.; Nadkarni, G.N.; Sidiropoulos, C. Epidemiology of inpatient stay in Parkinson’s disease in the United States: Insights from the Nationwide Inpatient Sample. J. Clin. Neurosci. 2016, 31, 162–165. [Google Scholar] [CrossRef]
- Gerlach, O.H.; Winogrodzka, A.; Weber, W.E. Clinical problems in the hospitalized Parkinson’s disease patient: Systematic review. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 197–208. [Google Scholar] [CrossRef]
- Fletcher, P.; Leake, A.; Marion, M.H. Patients with Parkinson’s disease dementia stay in the hospital twice as long as those without dementia. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 919. [Google Scholar] [CrossRef]
- Martins, J.; Rua, A.; Vila Chã, N. Hospital Mortality in Parkinson’s Disease: Retrospective Analysis in a Portuguese Tertiary Centre. Acta Med. Port. 2016, 29, 315–318. [Google Scholar] [CrossRef]
- Magdalinou, K.N.; Martin, A.; Kessel, B. Prescribing medications in Parkinson’s disease (PD) patients during acute admissions to a District General Hospital. Parkinsonism Relat. Disord. 2007, 13, 539–540. [Google Scholar] [CrossRef]
- Chou, K.L.; Zamudio, J.; Schmidt, P.; Price, C.C.; Parashos, S.A.; Bloem, B.R.; Lyons, K.E.; Christine, C.W.; Pahwa, R.; Bodis-Wollner, I.; et al. Hospitalization in Parkinson disease: A survey of National Parkinson Foundation Centers. Parkinsonism Relat. Disord. 2011, 17, 440–445. [Google Scholar] [CrossRef]
- Miyasaki, J.M.; Lim, S.; Chaudhuri, K.R.; Antonini, A.; Pt, M.P.; Richfield, E.; Gonzalez, D.A.; Lorenzl, S.; Walker, R.; Bhidayasiri, R.; et al. Access and Attitudes Toward Palliative Care Among Movement Disorders Clinicians. Mov. Disord. 2021, 37, 182–189. [Google Scholar] [CrossRef]
- Fleisher, J.E.; Sweeney, M.M.; Oyler, S.; Meisel, T.; Friede, N.; Di Rocco, A.; Chodosh, J. Disease severity and quality of life in homebound people with advanced Parkinson disease: A pilot study. Neurol. Clin. Pract. 2020, 10, 277–286. [Google Scholar] [CrossRef]
- Rektorova, I.; Biundo, R. Non-invasive brain stimulation to treat cognitive symptoms of Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 66, 1–2. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Fiorenzato, E.; Antonini, A. Cognitive Rehabilitation in Parkinson’s Disease: Is it Feasible? Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2017, 32, 840–860. [Google Scholar] [CrossRef]
- World Health Organization. Strengthening of palliative care as a component of integrated treatment throughout the life course. J. Pain Palliat. Care Pharmacother. 2014, 28, 130–134. [Google Scholar] [CrossRef]
- Sethi, S.; Hohler, A.D. The Application of Palliative Care Principles in Advanced Parkinson’s Disease. Adv. Parkinson’s Dis. 2016, 5, 73–86. [Google Scholar] [CrossRef][Green Version]
- Meinders, M.J.; Gentile, G.; Schrag, A.E.; Konitsiotis, S.; Eggers, C.; Taba, P.; Lorenzl, S.; Odin, P.; Rosqvist, K.; Chaudhuri, K.R.; et al. Advance Care Planning and Care Coordination for People With Parkinson’s Disease and Their Family Caregivers-Study Protocol for a Multicentre, Randomized Controlled Trial. Front. Neurol. 2021, 12, 673893. [Google Scholar] [CrossRef]
- Spilsbury, K.; Rosenwax, L. Community-based specialist palliative care is associated with reduced hospital costs for people with non-cancer conditions during the last year of life. BMC Palliat. Care 2017, 16, 68. [Google Scholar] [CrossRef]
- Luis-Martínez, R.; Monje, M.H.G.; Antonini, A.; Sánchez-Ferro, Á.; Mestre, T.A. Technology-Enabled Care: Integrating Multidisciplinary Care in Parkinson’s Disease Through Digital Technology. Front. Neurol. 2020, 11, 575975. [Google Scholar] [CrossRef]
- Antonini, A.; Gentile, G.; Giglio, M.; Marcante, A.; Gage, H.; Touray, M.M.; Fotiadis, D.I.; Gatsios, D.; Konitsiotis, S.; Timotijevic, L.; et al. Acceptability to patients, carers and clinicians of an mHealth platform for the management of Parkinson’s disease (PD_Manager): Study protocol for a pilot randomised controlled trial. Trials 2018, 19, 492. [Google Scholar] [CrossRef]
- Gatsios, D.; Antonini, A.; Gentile, G.; Konitsiotis, S.; Fotiadis, D.; Nixina, I.; Taba, P.; Weck, C.; Lorenzl, S.; Lex, K.M.; et al. Education on palliative care for Parkinson patients: Development of the “Best care for people with late-stage Parkinson’s disease” curriculum toolkit. BMC Med. Educ. 2021, 21, 538. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, M.; Coelho, M.; Garon, M.; Biundo, R.; Mestre, T.A.; Antonini, A.; on behalf of iCARE-PD Consortium. Personalized Care in Late-Stage Parkinson’s Disease: Challenges and Opportunities. J. Pers. Med. 2022, 12, 813. https://doi.org/10.3390/jpm12050813
Fabbri M, Coelho M, Garon M, Biundo R, Mestre TA, Antonini A, on behalf of iCARE-PD Consortium. Personalized Care in Late-Stage Parkinson’s Disease: Challenges and Opportunities. Journal of Personalized Medicine. 2022; 12(5):813. https://doi.org/10.3390/jpm12050813
Chicago/Turabian StyleFabbri, Margherita, Miguel Coelho, Michela Garon, Roberta Biundo, Tiago A. Mestre, Angelo Antonini, and on behalf of iCARE-PD Consortium. 2022. "Personalized Care in Late-Stage Parkinson’s Disease: Challenges and Opportunities" Journal of Personalized Medicine 12, no. 5: 813. https://doi.org/10.3390/jpm12050813
APA StyleFabbri, M., Coelho, M., Garon, M., Biundo, R., Mestre, T. A., Antonini, A., & on behalf of iCARE-PD Consortium. (2022). Personalized Care in Late-Stage Parkinson’s Disease: Challenges and Opportunities. Journal of Personalized Medicine, 12(5), 813. https://doi.org/10.3390/jpm12050813








