Patterns of Recurrent Disease in Cervical Cancer
Abstract
:1. Introduction
- 1.
- Local or central-pelvic (including recurrence in the vaginal vault alone, cervix, uterus, parametria);
- 2.
- Regional (with or without vaginal involvement) defined as anterior, invading bladder, ureters, urethra, or as posterior, invading rectum, anal sphincter, or as lateral, invading pelvic side wall, vessels and nerves, or involving pelvic lymph nodes stations;
- 3.
- Distant, including infra-diaphragmatic (para-aortic lymph nodes) or supra-diaphragmatic nodal recurrence, or distant organ metastasis (lungs, liver) [4].
2. Imaging Surveillance
3. Normal Imaging Findings
4. Pelvic Recurrence
4.1. Local
4.2. Regional
4.2.1. Anterior
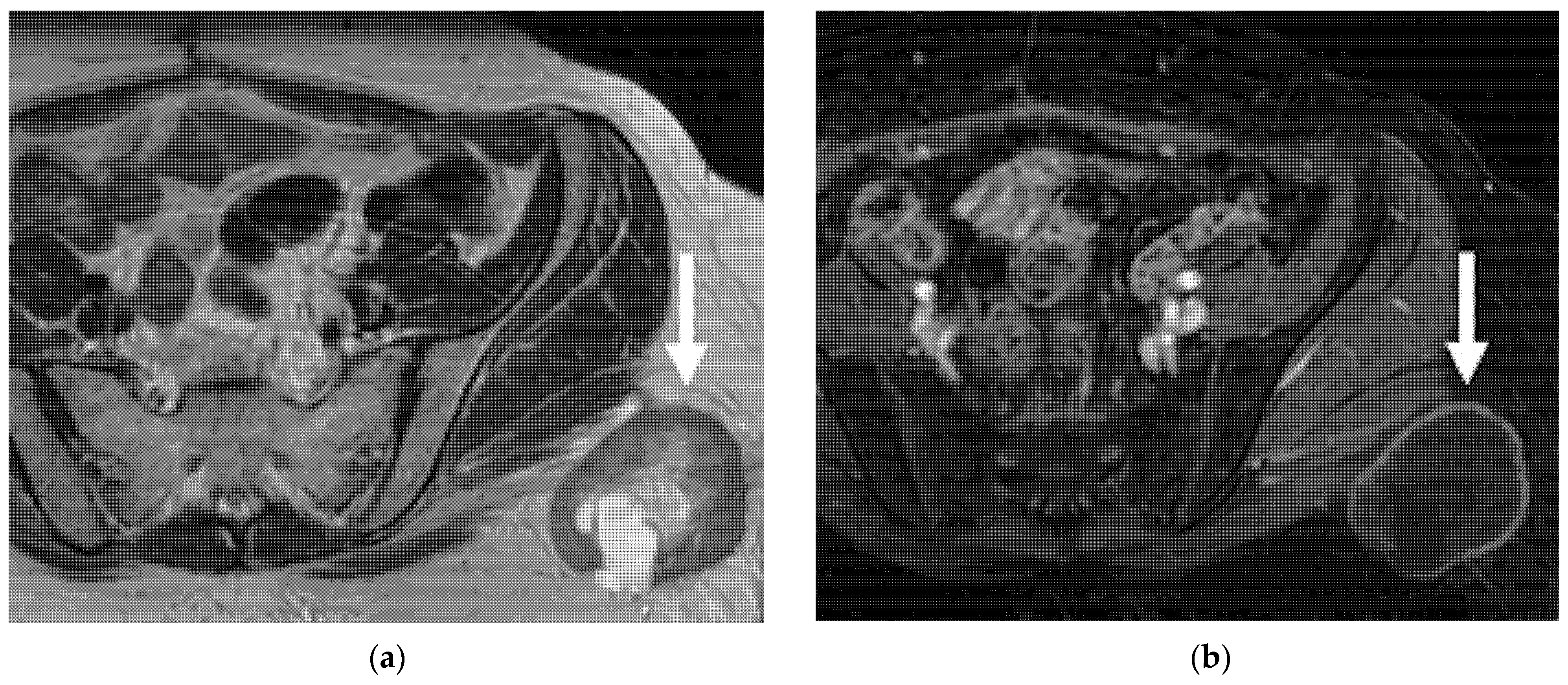
4.2.2. Posterior
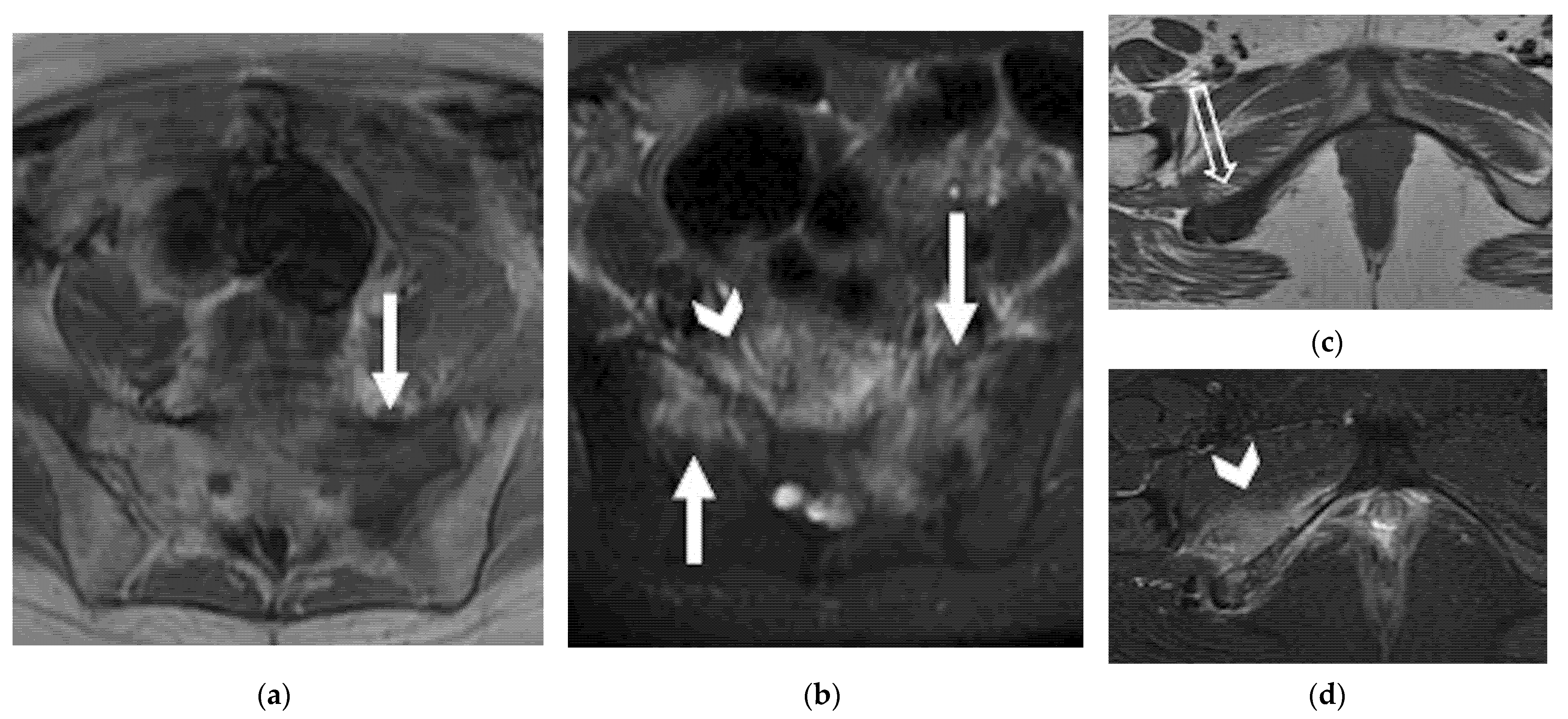
4.2.3. Lateral
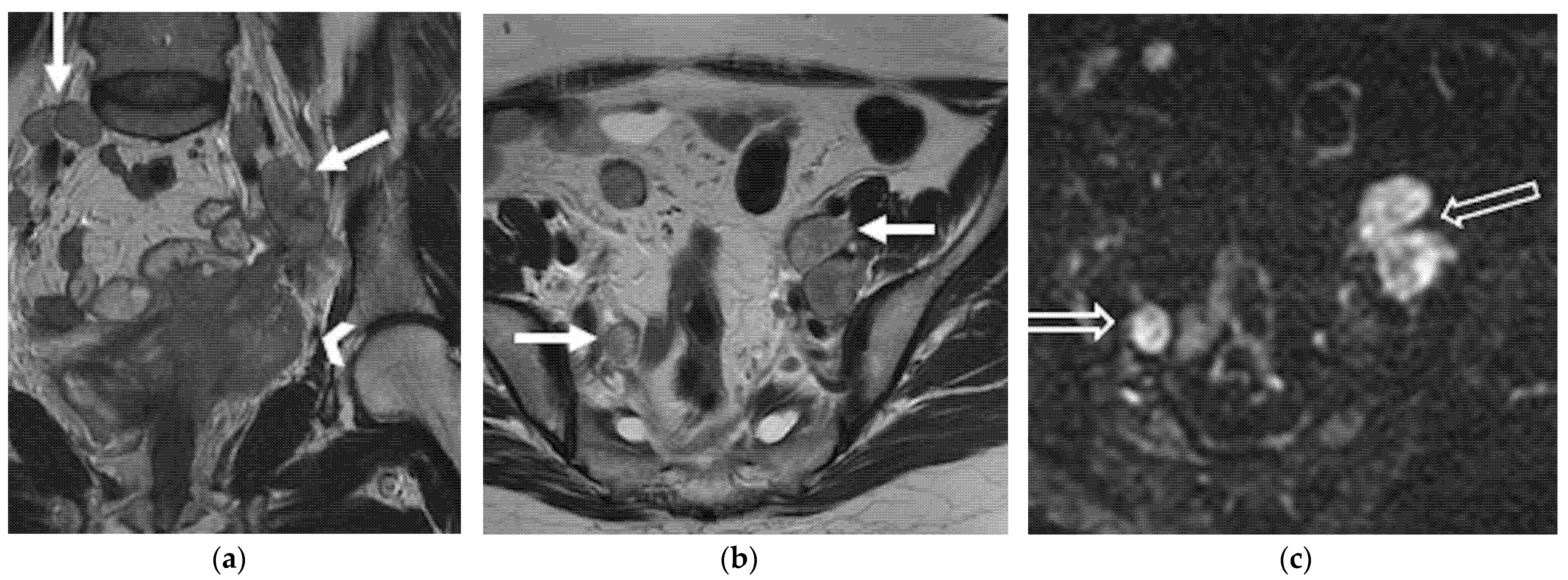
5. Lymphadenopathies
6. Distant Recurrence
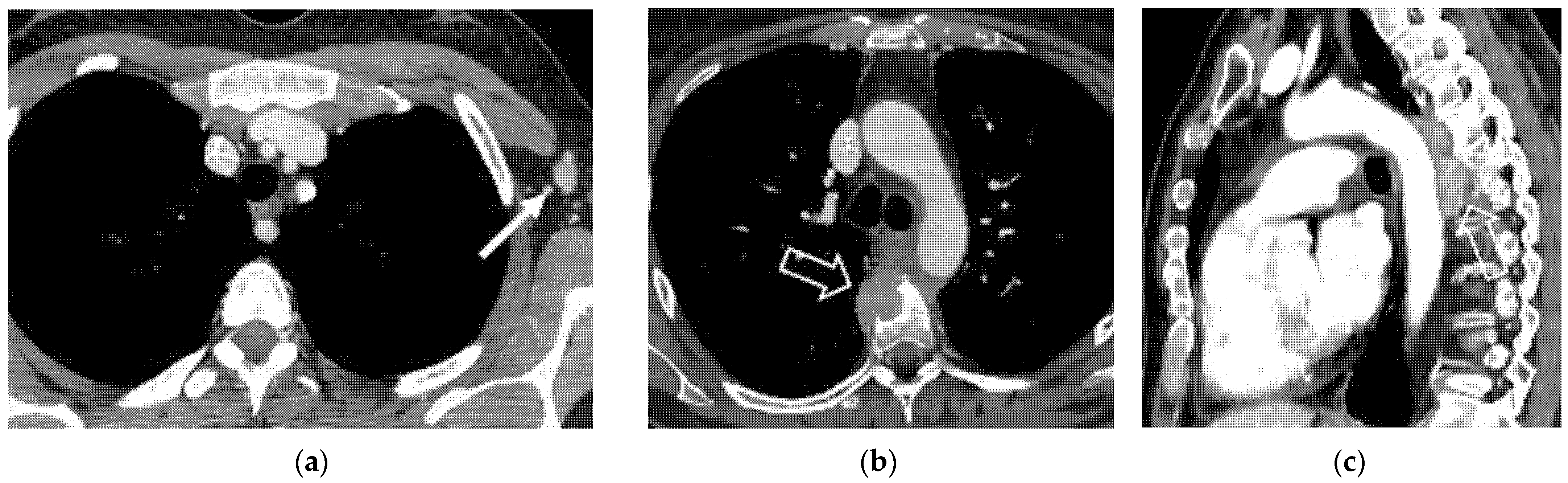
6.1. Supra-Diaphragmatic Lymph Nodes
6.2. Abdominal and Extra-Abdominal Recurrence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quinn, M.; Benedet, J.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.; Heintz, A.; Nan, H.; Pecorelli, S. Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2006, 95, S43–S103. [Google Scholar] [CrossRef]
- Antunes, D. Recurrent Cervical Cancer: How Can Radiology Be Helpfull. Omi. J. Radiol. 2013, 2, 138. [Google Scholar] [CrossRef] [Green Version]
- Bendifallah, S.; de Foucher, T.; Bricou, A.; Ouldamer, L.; Lavoue, V.; Varinot, J.; Canlorbe, G.; Carcopino, X.; Raimond, E.; Huguet, F.; et al. Cervical Cancer Recurrence: Proposal for a Classification Based on Anatomical Dissemination Pathways and Prognosis. Surg. Oncol. 2019, 30, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, M.; Zapardiel, I.; Zanagnolo, V.; Landoni, F.; Morrow, C.P.; Maggioni, A. Management of Recurrent Cervical Cancer: A Review of the Literature. Surg. Oncol. 2012, 21, e59–e66. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.H.; Roberts, C.A.; Rockall, A.G. MRI and PET Scans for Primary Staging and Detection of Cervical Cancer Recurrence. Women’s Health 2010, 6, 251–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beriwal, S.; Gan, G.N.; Heron, D.E.; Selvaraj, R.N.; Kim, H.; Lalonde, R.; Kelley, J.L.; Edwards, R.P. Early Clinical Outcome With Concurrent Chemotherapy and Extended-Field, Intensity-Modulated Radiotherapy for Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 166–171. [Google Scholar] [CrossRef]
- Zola, P.; Fuso, L.; Mazzola, S.; Piovano, E.; Perotto, S.; Gadducci, A.; Galletto, L.; Landoni, F.; Maggino, T.; Raspagliesi, F.; et al. Could Follow-up Different Modalities Play a Role in Asymptomatic Cervical Cancer Relapses Diagnosis?. An Italian Multicenter Retrospective Analysis. Gynecol. Oncol. 2007, 107, 28. [Google Scholar] [CrossRef]
- Webb, M.J.; Symmonds, R.E. Site of Recurrence of Cervical Cancer after Radical Hysterectomy. Am. J. Obstet. Gynecol. 1980, 138, 813–817. [Google Scholar] [CrossRef]
- Kobayashi, R.; Yamashita, H.; Okuma, K.; Ohtomo, K.; Nakagawa, K. Details of Recurrence Sites after Definitive Radiation Therapy for Cervical Cancer. J. Gynecol. Oncol. 2016, 27, e16. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, A.; Mahantshetty, U.M.; Gurram, L.; Chopra, S.; Engineer, R.; Maheshwari, A.; Gupta, S.; Deodhar, K.; Rangarajan, V.; Thakur, M.; et al. Patterns of First Relapse and Outcome in Patients with Locally Advanced Cervical Cancer After Radiochemotherapy: A Single Institutional Experience. Indian J. Gynecol. Oncol. 2020, 18, 3–8. [Google Scholar] [CrossRef]
- Tan, L.T.; Pötter, R.; Sturdza, A.; Fokdal, L.; Haie-Meder, C.; Schmid, M.; Gregory, D.; Petric, P.; Jürgenliemk-Schulz, I.; Gillham, C.; et al. Change in Patterns of Failure After Image-Guided Brachytherapy for Cervical Cancer: Analysis From the RetroEMBRACE Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Taarnhøj, G.A.; Christensen, I.J.; Lajer, H.; Fuglsang, K.; Jeppesen, M.M.; Kahr, H.S.; Høgdall, C. Risk of Recurrence, Prognosis, and Follow-up for Danish Women with Cervical Cancer in 2005-2013: A National Cohort Study. Cancer 2018, 124, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salani, R.; Khanna, N.; Frimer, M.; Bristow, R.E.; Chen, L. An Update on Post-Treatment Surveillance and Diagnosis of Recurrence in Women with Gynecologic Malignancies: Society of Gynecologic Oncology (SGO) Recommendations. Gynecol. Oncol. 2017, 146, 3–10. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Elit, L.; Fyles, A.W.; Devries, M.C.; Oliver, T.K.; Fung-Kee-Fung, M. Follow-up for Women after Treatment for Cervical Cancer: A Systematic Review. Gynecol. Oncol. 2009, 114, 528–535. [Google Scholar] [CrossRef]
- Sartori, E.; Pasinetti, B.; Carrara, L.; Gambino, A.; Odicino, F.; Pecorelli, S. Pattern of Failure and Value of Follow-up Procedures in Endometrial and Cervical Cancer Patients. Gynecol. Oncol. 2007, 107, 25. [Google Scholar] [CrossRef]
- Burger, I.A.; Vargas, H.A.; Donati, O.F.; Andikyan, V.; Sala, E.; Gonen, M.; Goldman, D.A.; Chi, D.S.; Schöder, H.; Hricak, H. The Value of 18F-FDG PET/CT in Recurrent Gynecologic Malignancies Prior to Pelvic Exenteration. Gynecol. Oncol. 2013, 129, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Meads, C.; Davenport, C.; Małysiak, S.; Kowalska, M.; Zapalska, A.; Guest, P.; Martin-Hirsch, P.; Borowiack, E.; Auguste, P.; Barton, P.; et al. Evaluating PET-CT in the Detection and Management of Recurrent Cervical Cancer: Systematic Reviews of Diagnostic Accuracy and Subjective Elicitation. BJOG An. Int. J. Obstet. Gynaecol. 2014, 121, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, Recurrence and Follow-up of Uterine Cervical Cancer Using MRI: Updated Guidelines of the European Society of Urogenital Radiology after Revised FIGO Staging 2018. Eur. Radiol. 2021, 31, 7802–7816. [Google Scholar] [CrossRef]
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of Uterine Cervical Cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2011, 21, 1102–1110. [Google Scholar] [CrossRef]
- Reznek, F.R.H. Appearances of the Female Pelvis After. Cancer 2005, 11, 41–52. [Google Scholar]
- Otero-García, M.M.; Mesa-Álvarez, A.; Nikolic, O.; Blanco-Lobato, P.; Basta-Nikolic, M.; de Llano-Ortega, R.M.; Paredes-Velázquez, L.; Nikolic, N.; Szewczyk-Bieda, M. Role of MRI in Staging and Follow-up of Endometrial and Cervical Cancer: Pitfalls and Mimickers. Insights Imaging 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, I.; Stewart, V.; Barwick, T.D.; Park, W.H.E.; Soneji, N.; Rockall, A.G.; Bharwani, N. Post–Radiation Therapy Imaging Appearances in Cervical Carcinoma. Radiographics 2016, 36, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Addley, H.C.; Vargas, H.A.; Moyle, P.L.; Crawford, R.; Sala, E. Pelvic Imaging Following Chemotherapy and Radiation Therapy for Gynecologic Malignancies. Radiographics 2010, 30, 1843–1856. [Google Scholar] [CrossRef]
- Parikh, J.H.; Barton, D.P.J.; Ind, T.E.J.; Sohaib, S.A. MR Imaging Features of Vaginal Malignancies. Radiographics 2008, 28, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Wakely, S.; Senior, E.; Lomas, D. MRI of Malignant Neoplasms of the Uterine Corpus and Cervix. Am. J. Roentgenol. 2007, 188, 1577–1587. [Google Scholar] [CrossRef] [Green Version]
- Gemignani, M.L.; Alektiar, K.M.; Leitao, M.; Mychalczak, B.; Chi, D.; Venkatraman, E.; Barakat, R.R.; Curtin, J.P. Radical Surgical Resection and High-Dose Intraoperative Radiation Therapy (HDR-IORT) in Patients with Recurrent Gynecologic Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 687–694. [Google Scholar] [CrossRef]
- Lakhman, Y.; Nougaret, S.; Miccò, M.; Scelzo, C.; Vargas, H.A.; Sosa, R.E.; Sutton, E.J.; Chi, D.S.; Hricak, H.; Sala, E. Role of MR Imaging and FDG PET/CT in Selection and Follow-up of Patients Treated with Pelvic Exenteration for Gynecologic Malignancies. Radiographics 2015, 35, 1295–1313. [Google Scholar] [CrossRef] [Green Version]
- Fulcher, A.S.; O’Sullivan, S.G.; Segreti, E.M.; Kavanagh, B.D. Recurrent Cervical Carcinoma: Typical and Atypical Manifestations. Radiographics 1999, 19, 103–116. [Google Scholar] [CrossRef]
- Choi, J.; Sim, J.S.; Lee, H.J. Recurrent Uterine Cervical Carcinoma. Korean J. Radiol. 2000, 1, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Pawlik, T.M.; Skibber, J.M.; Rodriguez-Bigas, M.A. Pelvic Exenteration for Advanced Pelvic Malignancies. Ann. Surg. Oncol. 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Iafrate, F.; Laghi, A.; Paolantonio, P.; Rengo, M.; Mercantini, P.; Ferri, M.; Ziparo, V.; Passariello, R. Preoperative Staging of Rectal Cancer with MR Imaging: Correlation with Surgical and Histopathologic Findings. Radiographics 2006, 26, 701–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, O.F.; Lakhman, Y.; Sala, E.; Burger, I.A.; Vargas, H.A.; Goldman, D.A.; Andikyan, V.; Park, K.J.; Chi, D.S.; Hricak, H. Role of Preoperative MR Imaging in the Evaluation of Patients with Persistent or Recurrent Gynaecological Malignancies before Pelvic Exenteration. Eur. Radiol. 2013, 23, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, V.; Carignan, L.; Bourdon, F.; Prosmanne, O. MR Imaging of Cervical Carcinoma: A Practical Staging Approach. Radiographics 2000, 20, 1539–1549. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Kang, H.K.; Chung, T.W.; Seo, J.J.; Park, J.G. Uterine Cervical Carcinoma after Therapy: CT and MR Imaging Findings. Radiographics 2003, 23, 969–980. [Google Scholar] [CrossRef]
- Pannu, H.K.; Fishman, E.K.; Russell Morgan, T.H. Second International Conference on Cervical Cancer Evaluation of Cervical Cancer by Computed Tomography: Current Status. Interdiscip. Int. J. Am. Cancer Soc. 2003, 98, 2039–2043. [Google Scholar] [CrossRef]
- Pannu, H.K.; Corl, F.M.; Fishman, E.K. CT Evaluation of Cervical Cancer: Spectrum of Disease. Radiographics 2001, 21, 1155–1168. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, Y.I.; Park, W.; Yang, K.; Kim, H.; Cha, H. Para-Aortic Lymph Node Recurrence after Curative Radiotherapy for Cervical Cancer. Int. J. Gynecol. Cancer 2019, 29, 1116–1120. [Google Scholar] [CrossRef]
- Malignancies, G.; Sebastià, C.; Paredes, P.; Salvador, R.; Buñesch, L.; Nicolau, C. Pathways of Lymphatic Spread In. RadioGraphics 2015, 35, 916–945. [Google Scholar]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Devine, C.; Viswanathan, C.; Faria, S.; Marcal, L.; Sagebiel, T.L. Imaging and Staging of Cervical Cancer. Semin. Ultrasound CT MRI 2019, 40, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.W.; Amendola, M.A.; Konerding, K.F.; Tisnado, J.; Hazra, T.A. Computed Tomographic Detection of Pelvic and Inguinal Lymph-Node Metastases from Primary and Recurrent Pelvic Malignant Disease. Radiology 1980, 137, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, S.; Rosado-de-Christenson, M.L.; Walker, C.M.; Kunin, J.R.; Betancourt, S.L.; Shoup, B.L.; Pettavel, P.P. Imaging Features of Thoracic Metastases from Gynecologic Neoplasms. Radiographics 2014, 34, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Havrilesky, L.J.; Wong, T.Z.; Secord, A.A.; Berchuck, A.; Clarke-Pearson, D.L.; Jones, E.L. The Role of PET Scanning in the Detection of Recurrent Cervical Cancer. Gynecol. Oncol. 2003, 90, 186–190. [Google Scholar] [CrossRef]



| CE-DW-MRI |
|---|
|
|
|
|
|
| Type of Recurrence | Imaging Findings |
|---|---|
| Local | |
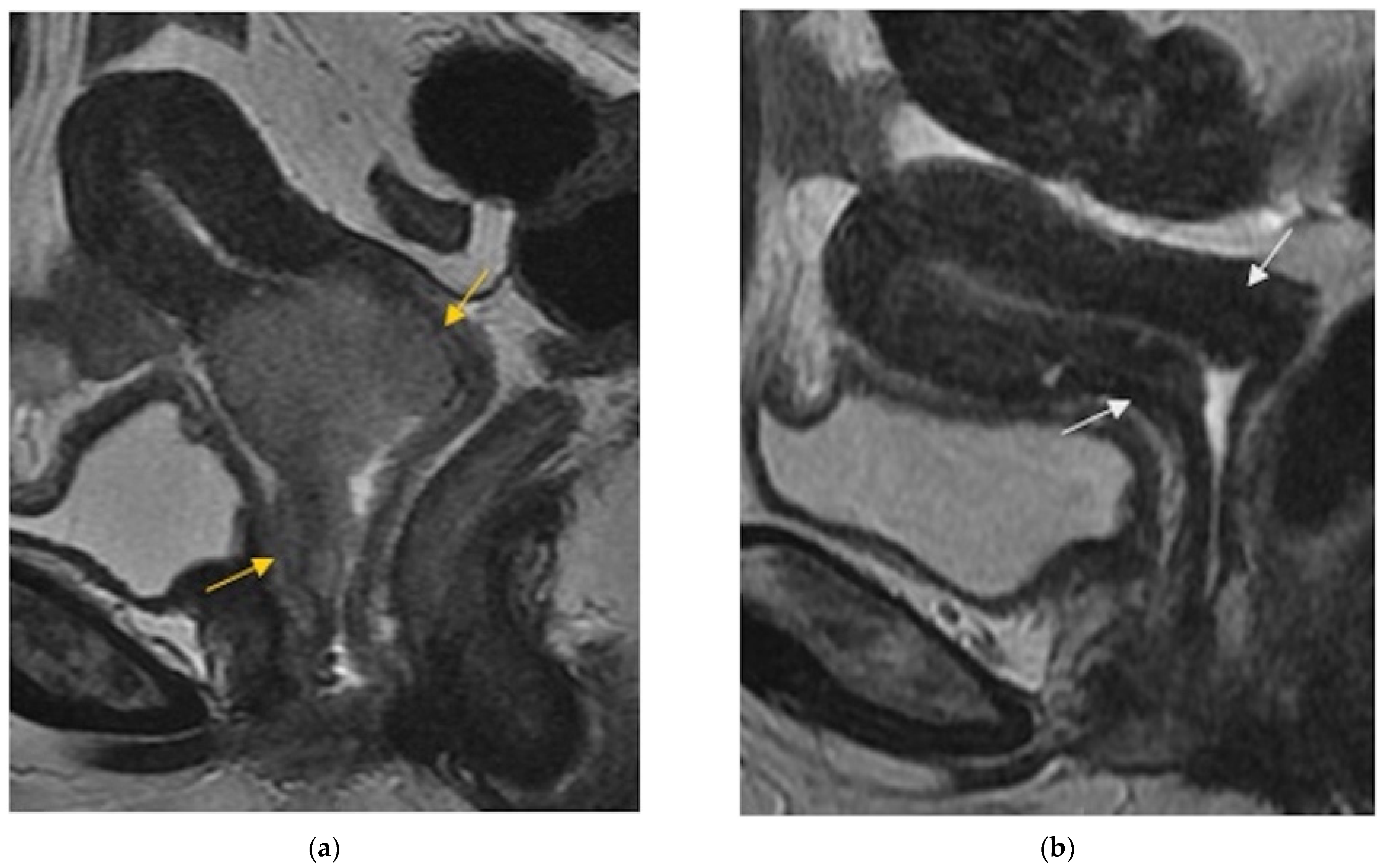
Cervix, parametria, or vaginal cuff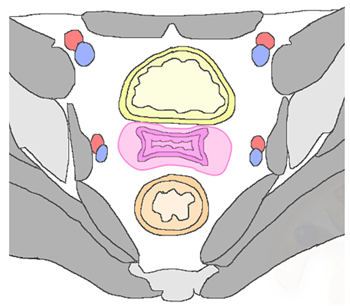 |
|
| Regional | |
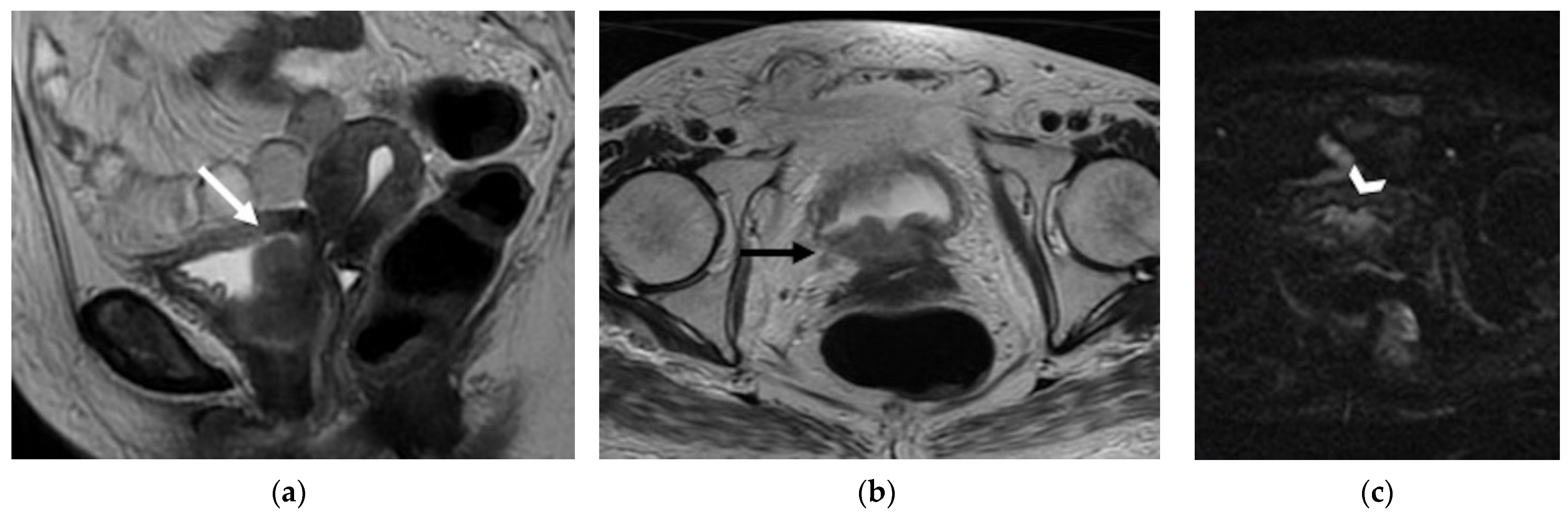
| Anterior Urinary bladder and urethra 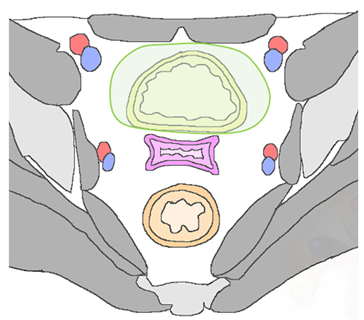 |
|
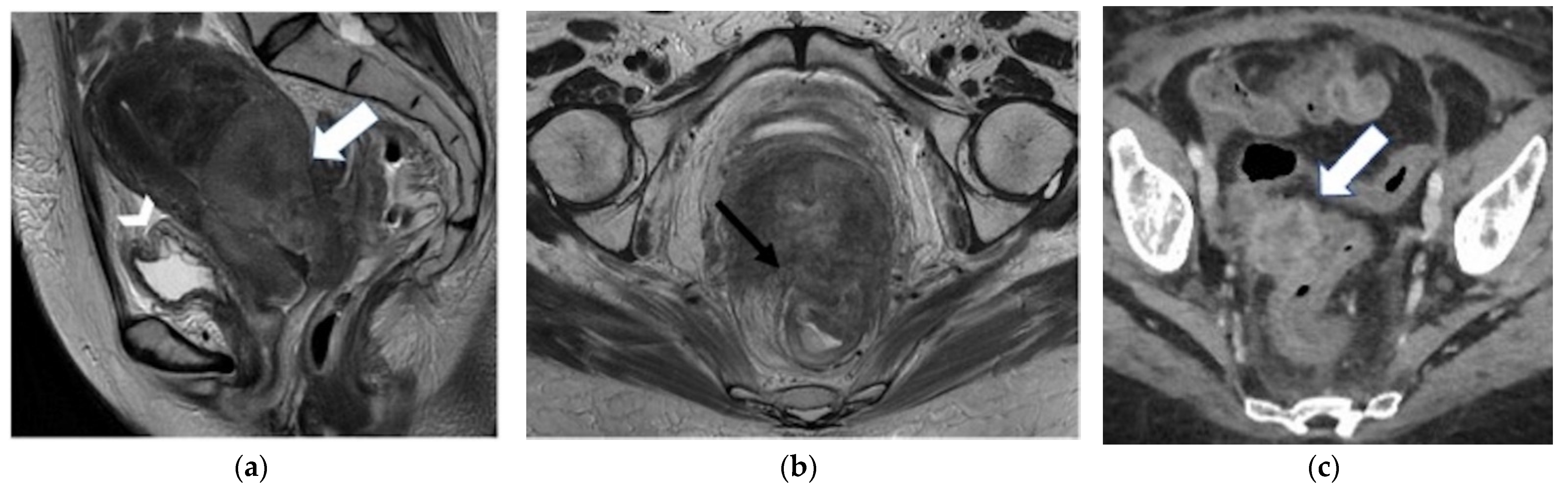
| Posterior Rectal or sigmoid colon 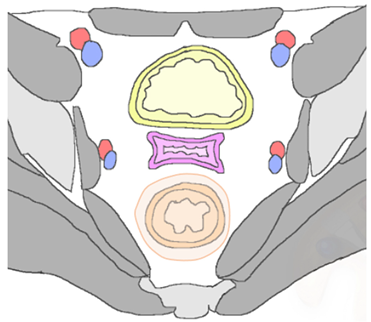 | An infiltrating, spiculated mass, causing rectal or sigmoid luminal narrowing.
|
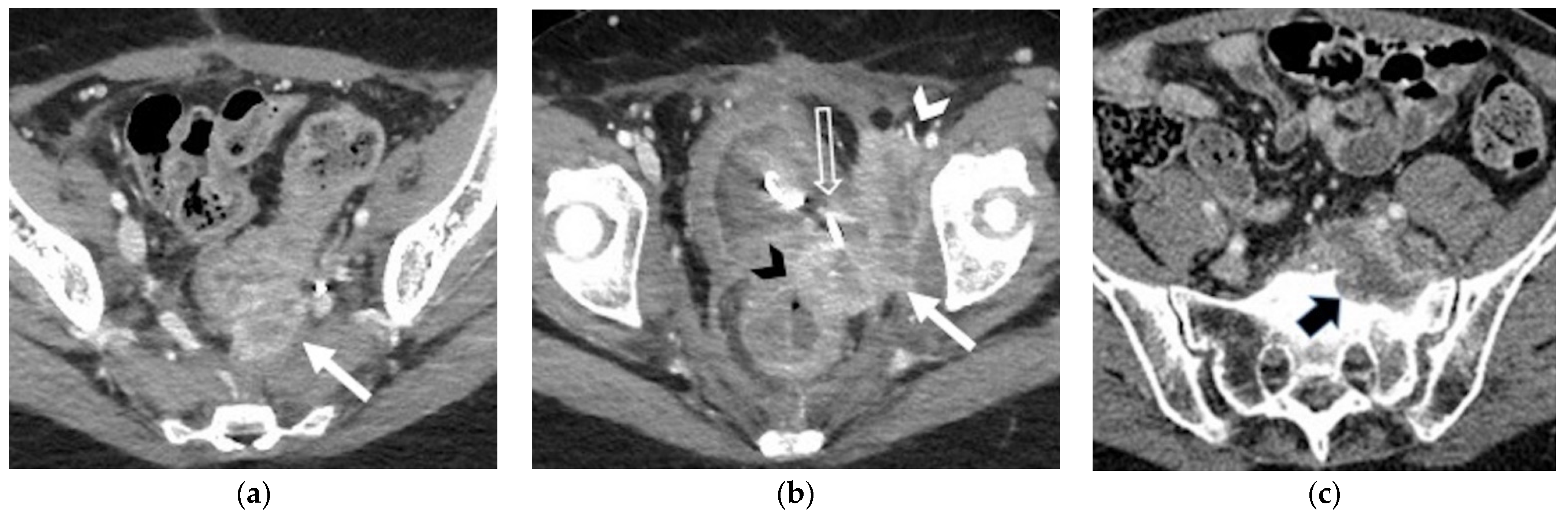
| Lateral Pelvic sidewall 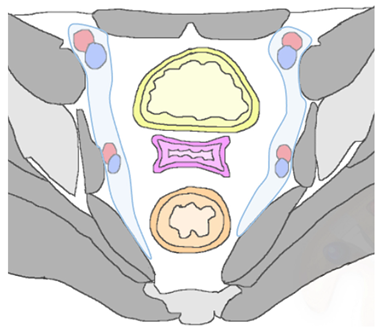 | Pelvic sidewall recurrence is defined as a tumor extending within 3 mm and abutting the obturator internus or piriformis muscles with concomitant loss of fat planes. Other findings include:
|
| Lymphadenopathies | |
| Paracervical, parametrial, internal and external iliac, obturator, sacral, common iliac and para-aortic lymph nodes. |
|
| Distant | |
| Supra-diaphragmatic lymph nodes |
|
| Abdominal and extra-abdominal recurrence |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miccò, M.; Lupinelli, M.; Mangialardi, M.; Gui, B.; Manfredi, R. Patterns of Recurrent Disease in Cervical Cancer. J. Pers. Med. 2022, 12, 755. https://doi.org/10.3390/jpm12050755
Miccò M, Lupinelli M, Mangialardi M, Gui B, Manfredi R. Patterns of Recurrent Disease in Cervical Cancer. Journal of Personalized Medicine. 2022; 12(5):755. https://doi.org/10.3390/jpm12050755
Chicago/Turabian StyleMiccò, Maura, Michela Lupinelli, Matteo Mangialardi, Benedetta Gui, and Riccardo Manfredi. 2022. "Patterns of Recurrent Disease in Cervical Cancer" Journal of Personalized Medicine 12, no. 5: 755. https://doi.org/10.3390/jpm12050755
APA StyleMiccò, M., Lupinelli, M., Mangialardi, M., Gui, B., & Manfredi, R. (2022). Patterns of Recurrent Disease in Cervical Cancer. Journal of Personalized Medicine, 12(5), 755. https://doi.org/10.3390/jpm12050755






