The Many Faces of Huntington’s Chorea Treatment: The Impact of Sudden Withdrawal of Tiapride after 40 Years of Use and a Systematic Review
Abstract
:1. Introduction
2. Case Descriptions
3. Materials and Methods
4. Results
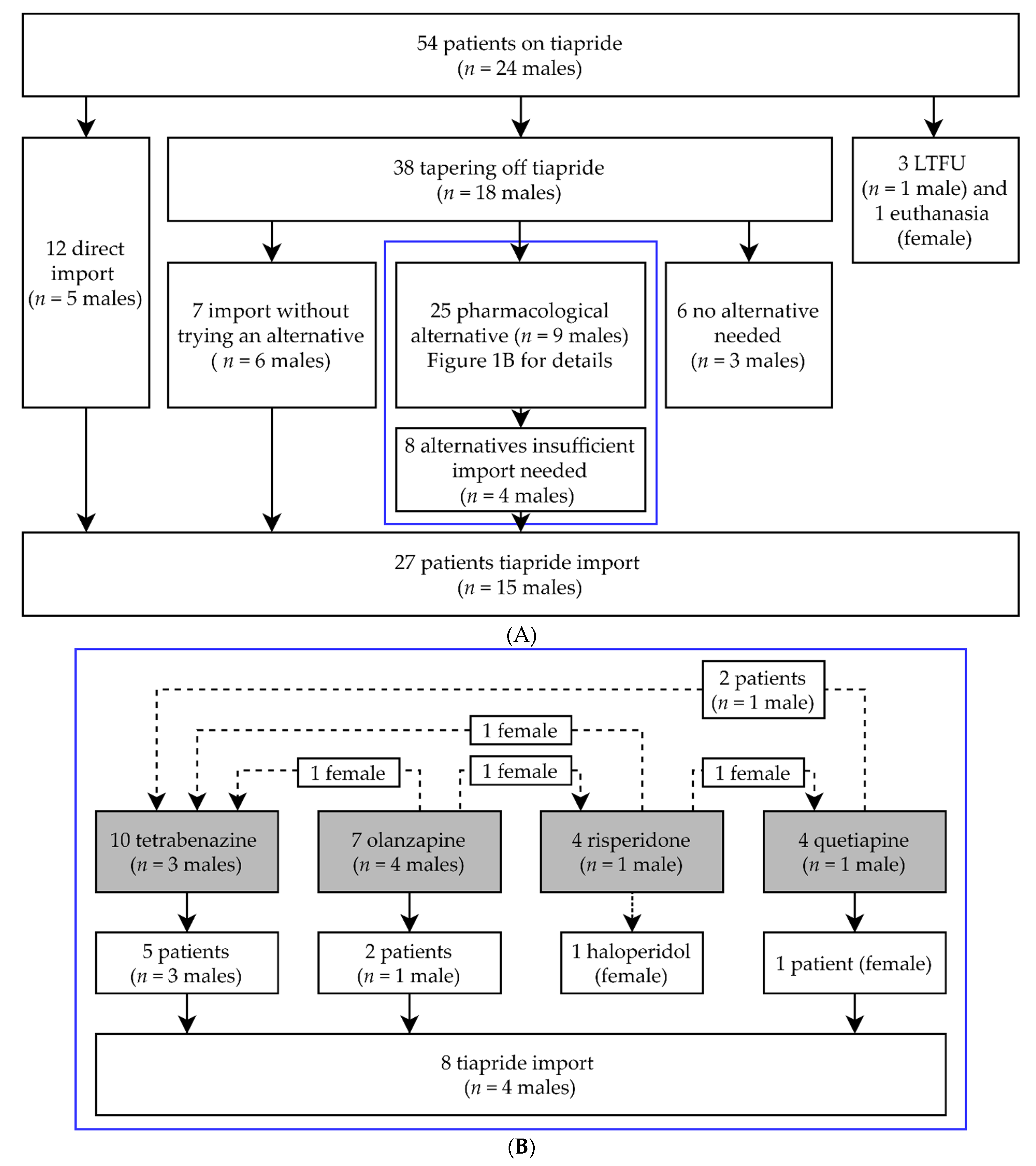
4.1. Evaluation of Medical Records
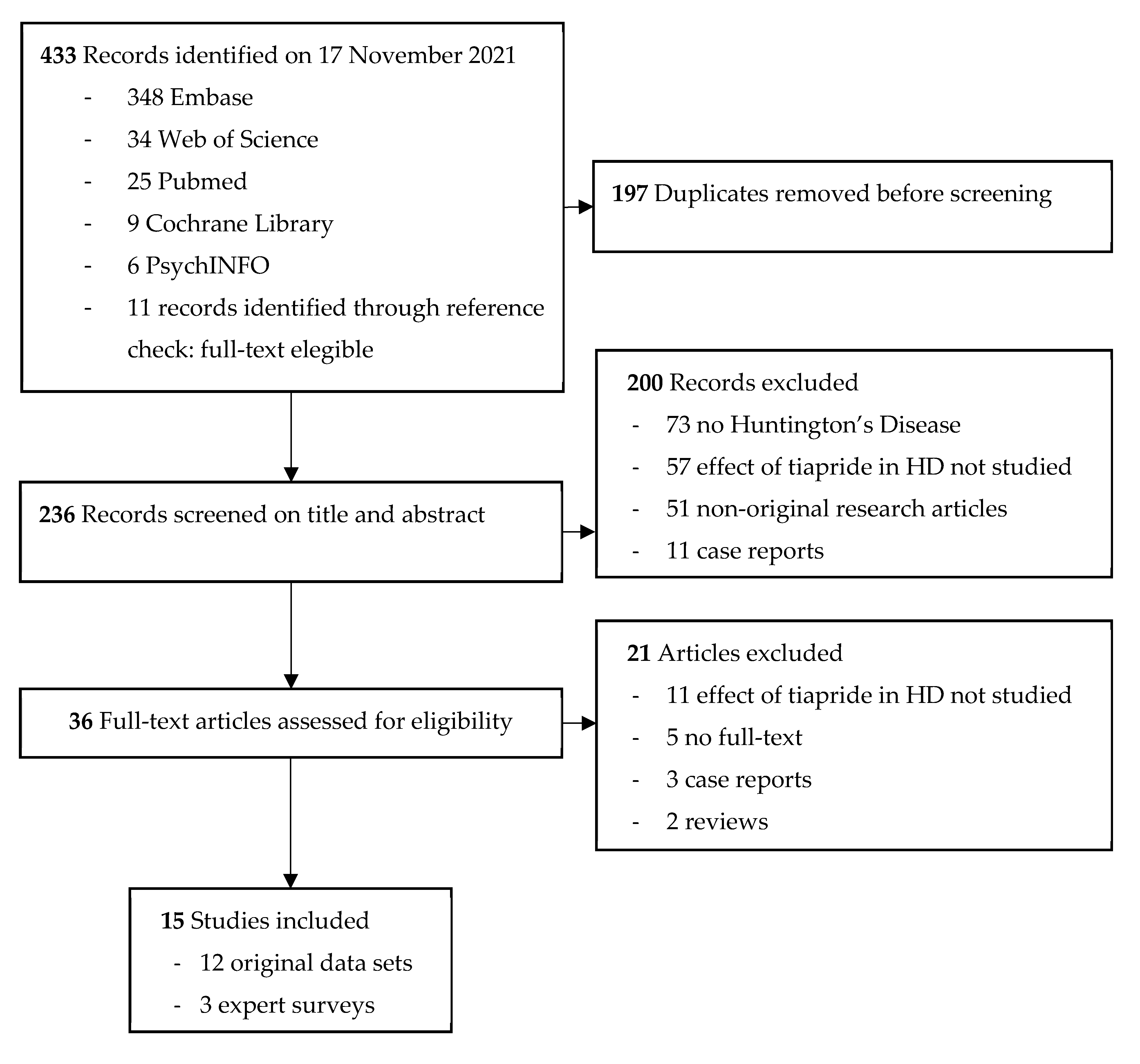
4.2. Systematic Literature Review on Tiapride in Huntington’s Disease
4.3. Safety and Side-Effect Profile of Tiapride
4.4. Expert Opinions
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Caron, N.S.; Wright, G.E.B.; Hayden, M.R. Huntington Disease. In GeneReviews(®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Folstein, S.E.; Folstein, M.F. Psychiatric features of Huntington’s disease: Recent approaches and findings. Psychiatr. Dev. 1983, 1, 193–205. [Google Scholar] [PubMed]
- Roos, R.A.C. Clinical neurology. In Huntington’s Disease, 4th ed.; Bates, G.P., Tabrizi, S.J., Jones, L., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 25–35. [Google Scholar]
- Baake, V.; Reijntjes, R.; Dumas, E.M.; Thompson, J.C.; Roos, R.A.C. Cognitive decline in Huntington’s disease expansion gene carriers. Cortex 2017, 95, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs 2022. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Rodrigues, F.B.; Duarte, G.S.; Mestre, T.A.; Bachoud-Levi, A.C.; Bentivoglio, A.R.; Burgunder, J.M.; Cardoso, F.; Claassen, D.O.; Landwehrmeyer, G.B.; et al. An MDS Evidence-Based Review on Treatments for Huntington’s Disease. Mov. Disord. 2022, 37, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Coppen, E.M.; Roos, R.A. Current Pharmacological Approaches to Reduce Chorea in Huntington’s Disease. Drugs 2017, 77, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Bilney, B.; Morris, M.E.; Perry, A. Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: A systematic review. Neurorehabil. Neural Repair 2003, 17, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.A. Comprehensive Care. In Huntington’s Disease, 4th ed.; Bates, G.P., Tabrizi, S.J., Jones, L., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 393–409. [Google Scholar]
- Quinn, L.; Kegelmeyer, D.; Kloos, A.; Rao, A.K.; Busse, M.; Fritz, N.E. Clinical recommendations to guide physical therapy practice for Huntington disease. Neurology 2020, 94, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Zarotti, N.; Dale, M.; Eccles, F.; Simpson, J. Psychological Interventions for People with Huntington’s Disease: A Call to Arms. J. Huntington’s Dis. 2020, 9, 231–243. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Bashir, H.; Jankovic, J. Treatment options for chorea. Expert Rev. Neurother. 2018, 18, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Buis, C.; Flohil, J.M. CLINICAL EXPERIENCES WITH HALOPERIDOL (SERENASE). Ned. Tijdschr. Geneeskd. 1964, 108, 796–800. [Google Scholar] [PubMed]
- Koller, W.C.; Trimble, J. The gait abnormality of Huntington’s disease. Neurology 1985, 35, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.J.; Mones, R.J. Modification of choreiform activity by Haloperidol. JAMA 1971, 216, 675–676. [Google Scholar] [CrossRef]
- Chouza, C.; Romero, S.; Lorenzo, J. Clinical trial of tiapride in patients with dyskinesia. Sem. Hop. 1982, 58, 725–733. [Google Scholar]
- Deroover, J.; Baro, F.; Bourguignon, R.P.; Smets, P. Tiapride versus placebo: A double-blind comparative study in the management of Huntington’s chorea. Curr. Med. Res. Opin. 1984, 9, 329–338. [Google Scholar] [CrossRef]
- Roos, R.A.C.; de Haas, E.J.M.; Buruma, O.J.S.; de Wolff, F.A. Pharmacokinetics of tiapride in patients with tardive dyskinesia and Huntington’s disease. Eur. J. Clin. Pharmacol. 1986, 31, 191–194. [Google Scholar] [CrossRef]
- Eggers, C.; Rothenberger, A.; Berghaus, U. Clinical and neurobiological findings in children suffering from tic disease following treatment with tiapride. Eur. Arch. Psychiatry Neurol. Sci. 1988, 237, 223–229. [Google Scholar] [CrossRef]
- Lnĕnicka, J.; Stará, V. The therapeutic effect of tiapride in the treatment of dyskinetic forms of cerebral palsy in children. Cesk. Pediatr. 1992, 47, 670–672. [Google Scholar]
- Tiapride. Available online: https://www.drugs.com/international/tiapride.html (accessed on 11 February 2022).
- Farmacotherapeutisch Kompas. Tiapride. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/t/tiapride (accessed on 20 July 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5467, Tiapride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tiapride (accessed on 4 September 2020).
- Dose, M.; Lange, H.W. The benzamide tiapride: Treatment of extrapyramidal motor and other clinical syndromes. Pharmacopsychiatry 2000, 33, 19–27. [Google Scholar] [CrossRef]
- Steele, J.W.; Faulds, D.; Sorkin, E.M. Tiapride. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in geriatric agitation. Drugs Aging 1993, 3, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Burgunder, J.M.; Guttman, M.; Perlman, S.; Goodman, N.; van Kammen, D.P.; Goodman, L. An International Survey-based Algorithm for the Pharmacologic Treatment of Chorea in Huntington’s Disease. PLoS Curr. 2011, 3, RRN1260. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.; van Duijn, E.; Anderson, K.; Craufurd, D.; Edmondson, M.C.; Goodman, N.; van Kammen, D.P.; Goodman, L. An International survey-based algorithm for the pharmacologic treatment of irritability in Huntington’s disease. PLoS Curr. 2011, 3, RRN1259. [Google Scholar] [CrossRef] [PubMed]
- Bachoud-Lévi, A.C.; Ferreira, J.; Massart, R.; Youssov, K.; Rosser, A.; Busse, M.; Craufurd, D.; Reilmann, R.; de Michele, G.; Rae, D.; et al. International Guidelines for the Treatment of Huntington’s Disease. Front. Neurol. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ark, T. Regulation of the Minister for Medical Care of January 22, 2021, Reference 217289-1815434-Z, Amending Annexes 1 and 2 of the Health Insurance Scheme in Connection with the Change in the Entitlement to Registered Medicines; Staatscourant: The Hague, The Netherlands, 2021; p. 17. [Google Scholar]
- Zorginstituut Nederland. Reimbursement per DDD 2016-2020 for ATC-subgroup N05AL03: Tiapride. Available online: https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&tabel_h_00-totaal=B_01-basis&geg=vg&spec=vg_ddd&item=N05AL03 (accessed on 16 March 2022).
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- [Patient Organisation Huntington’s Disease in the Netherlands] Vereniging van Huntington. [CALL TO ACTION! Tiapride has been taken off-market]. Available online: https://www.huntington.nl/nieuws/682-oproep-tot-actie-tiapridal-is-van-de-markt-gehaald.html (accessed on 16 March 2022).
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Mov. Disord. 1996, 11, 136–142. [Google Scholar] [CrossRef]
- Sanofi Winthrop Industrie. Tiapridal (Tiapride) [Package Leaflet: Information for the User]; Sanofi Winthrop Industrie: Quetigny, France, 2013. [Google Scholar]
- Uppsala Monitoring Centre. Caveat Document; Statement of Reservations, Limitations and Conditions Relating to Data Released from VigiBase, the WHO Global Database of Reported Potential Side Effects of Medicinal Products; Uppsala Monitoring Centre: Uppsala, Sweden, 2021; p. 1. [Google Scholar]
- Brion, S.; Guerin, R. Action d’une molécule neurotrope originale dans certains syndromes neurologiques (mouvements anormaux et al.gies diverses). Sem. Hop. 1977, 53, 40–44. [Google Scholar]
- Costall, B.; Naylor, R.J. Démonstration neupharmacologique de l’effet antidyskinétique du Tiapride. Sem. Hop. 1977, 53, 72–76. [Google Scholar]
- Emile, J.; Bastard, J.; Truelle, J.L. Utilisation du tiapride en neurologie; Resultats préliminaires. Sem. Hop. 1977, 53, 16–20. [Google Scholar]
- Lhermitte, F.; Signoret, J.L.; Agid, Y. Etude des effets d’une molécule originale, le Tiapride, dans le traitement des mouvements anormaux d’origine extrapyramidale. Sem. Hop. 1977, 53, 9–15. [Google Scholar]
- Trillet, M.; Joyeux, O.; Masson, R. Tiapride et mouvements anormaux. Sem. Hop. 1977, 53, 21–27. [Google Scholar]
- Csanda, E.; Tarczy, M.; Jelencsik, I. Tiapride treatment of abnormal movements and painful conditions. Sem. Hop. 1984, 60, 3006–3008. [Google Scholar]
- Girotti, F.; Carella, F.; Scigliano, G.; Grassi, M.P.; Soliveri, P.; Giovannini, P.; Parati, E.; Caraceni, T. Effect of neuroleptic treatment on involuntary movements and motor performances in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1984, 47, 848–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grass, H.; Gottschaldt, M. Pharmakologische Therapiemöglichkeit extrapyramidaler Hyperkinesen mit Tiaprid. Psychiatr. Neurol. Med. Psychol. 1983, 35, 222–229. [Google Scholar]
- Mathe, J.F.; Cler, J.M.; Venisse, J.L. Therapeutic use of tiapride in movement disorders. Sem. Hop. 1978, 54, 517–520. [Google Scholar]
- Petit, H. Les indications du Tiapride en pathologie extrapyramidale. Lille Med. 1979, 24, 339–344. [Google Scholar] [PubMed]
- Quinn, N.; Marsden, C.D. Tiapride in 12 Huntington’s disease patients. J. Neurol. Neurosurg. Psychiatry 1985, 48, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, R.A.C.; Buruma, O.J.S.; Bruyn, G.W. Tiapride in the treatment of Huntington’s chorea. Acta Neurol. Scand. 1982, 65, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cichecki, F.; Witkowski, G.; Zielonka, D.; Sienkiewicz-Jarosz, H.; Stepniak, I.; Ziora-Jakutowicz, K. Treatment of chorea and its efficacy in huntington’s disease (HD) patients. J. Neurol. Neurosurg. Psychiatry 2016, 87, A72. [Google Scholar] [CrossRef]
- Desamericq, G.; Dolbeau, G.; Verny, C.; Charles, P.; Durr, A.; Youssov, K.; Simonin, C.; Azulay, J.P.; Tranchant, C.; Goizet, C.; et al. Effectiveness of anti-psychotics and related drugs in the Huntington french-speaking group cohort. PLoS ONE 2014, 9, e85430. [Google Scholar] [CrossRef]
- Konvalinkova, R.; Dusek, P.; Doleckova, K.; Uhrova, T.; Roth, J.; Klempir, J. Does the risperidone influence weight in Huntington’s disease? J. Neurol. Neurosurg. Psychiatry 2018, 89, A86. [Google Scholar]
- Sung, V.W.; Iyer, R.G.; Gandhi, S.K.; Shah-Manek, B.; DiBonaventura, M.; Abler, V.; Claassen, D.O. Physician perceptions of pharmacologic treatment options for chorea associated with Huntington disease in the United States. Curr. Med. Res. Opin. 2018, 34, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Meisel, A.; Winter, C.; Zschenderlein, R.; Arnold, G. Tourette syndrome: Efficient treatment with ziprasidone and normalization of body weight in a patient with excessive weight gain under tiapride. Mov. Disord. 2004, 19, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; LaPensee, C.R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shoulson, I.; Fahn, S. Huntington disease: Clinical care and evaluation. Neurology 1979, 29, 1–3. [Google Scholar] [CrossRef]
- Anderson, K.E.; van Duijn, E.; Craufurd, D.; Drazinic, C.; Edmondson, M.; Goodman, N.; van Kammen, D.; Loy, C.; Priller, J.; Goodman, L.V. Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntingtons Dis. 2018, 7, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, A.C.; Nopoulos, P.C.; Schultz, J.L. Sleep disturbances by disease type and stage in Huntington’s disease. Parkinsonism Relat. Disord. 2021, 91, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sun Pharmaceutical Industries Europe B.V. Package Leaflet: Information for the User. Tetrabenazine 25 mg Tablets; Ranbaxy (UK) Limited a Sun Pharmaceutical Company: Uxbridge, UK, 2021; p. 6. [Google Scholar]
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology 2006, 66, 366–372. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on Drug Review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- Klawans, H.L., Jr. The pharmacology of tardive dyskinesias. Am. J. Psychiatry 1973, 130, 82–86. [Google Scholar] [CrossRef]
- Leenders, K.L.; Blauth-Eckmeyer, E. PET study with the benzamide tiapride. Eur. Psychiatry 1996, 11, 416s. [Google Scholar] [CrossRef]
- Guy, W. Abnormal Involuntary Movement Scale (AIMS); ECDEU Assessment Manual for Psychopharmacology; U.S. Department of Health Education and Welfare: Washington, DC, USA, 1976; pp. 534–537. [Google Scholar]
- Armstrong, M.J.; Miyasaki, J.M. Evidence-based guideline: Pharmacologic treatment of chorea in Huntington disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2012, 79, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuroleptic and Huntington Disease Comparison of: Olanzapine, la Tetrabenazine and Tiapride (NEUROHD) NCT00632645. Available online: https://ClinicalTrials.gov/show/NCT00632645 (accessed on 11 February 2022).
| Reference | n HD Patients (n of Males) | Type of Research | HD Symptom | Tiapride Dosage | Time Frame/Follow-Up | Outcome | Side Effects of Tiapride |
|---|---|---|---|---|---|---|---|
| Chouza et al., 1982 [18] | 2 (uk) | Experimental, double-blind, placebo-controlled | Chorea | Build-up scheme, max 900 mg/day | 13 weeks | Subjective moderate–important improvement of chorea by patient, caregiver, and observer | Psychomotor excitation and suicidal thoughts at 900 mg/day in one patient |
| Cichecki et al., 2016 [50] | 20 (uk) | Observational, no control group | Chorea | Varies, unknown. Each patient had a stable dosage for 52 weeks. | 52 weeks | Non-significant difference in UHDRS-TMS at BL 36.9 ± 3.7 and at FU 40.3 ± 4 (p > 0.05) | None reported |
| Csanda et al., 1984 [43] | 15 (uk) | Experimental | Chorea | 600–900 mg/day | 52 weeks | Significant improvement in AIMS score (BL ±11 and FU ±5.5, p-value not given) | None reported |
| Deroover et al., 1984 [19] | 23 (11) | Experimental, cross-over randomized, double-blind, placebo-controlled | Chorea, Depression, Anxiety, Irritability | 3000 mg/day | 9 weeks, of which tiapride was administered twice for 3 weeks at a time | Chorea significantly improved on Likert 4-point scale (p < 0.05). Leeds psychomotor test for reaction time significantly faster with tiapride (p < 0.01). Insufficient data to draw conclusions on psychiatric symptoms | Mild sedation was common, some patients experienced extrapyramidal effects |
| Desamericq et al., 2014 [51] | 347 patients of which 43 (26) on tiapride | Observational, tiapride compared to dibenzodiazepines (olanzapine), risperidone, and tetrabenazine | Chorea, Functionality (UHDRS-TFC), Weight loss | Varies, unknown | Mean 122 weeks, max 8 years | No significant difference on UHDRS-TMS. More decline in functionality compared to olanzapine (p < 0.05). Less weight gain on tiapride (p < 0.05) compared to olanzapine or risperidone | None reported |
| Girotti et al., 1984 [44] | 18 (11), of which 12 (uk) received tiapride | Experimental, cross-over, tiapride compared to pimozide 12–16 mg/day and/or haloperidol 6–9 mg/day | Chorea | 600–800 mg/day | 13 weeks, of which 4 weeks tiapride | No significant improvement in chorea on the AIMS score with tiapride, while pimozide (p < 0.01) and haloperidol (p < 0.05) significantly improved the AIMS score. | Fewer side effects of tiapride compared to pimozide and haloperidol (both more somnolence, rigidity and dysarthria) |
| Grass et al., 1983 [45] | 4 (uk) | Experimental, no blinding | Chorea | Build-up scheme, max 600 mg/day | 2 weeks | Subjective good–very good improvement in chorea by observer. Supported by EMG measurements | None reported |
| Konvalinkova et al., 2018 [52] | 49 (uk) on tiapride, 260 (uk) without tiapride | Observational, cross-sectional | Weight loss | Unknown | NA | No significant weight difference between those with tiapride and those without | None reported |
| Mathe et al., 1978 [46] | 6 (uk) | Experimental, no blinding | Chorea | Varies, unknown | Varies, at least 1 week, details unknown | Notable improvement in chorea and in more severely diseased patients some improvement in functionality (e.g., writing) | Temporary somnolence, galactorrhea and/or blood pressure increase in single patients |
| Petit et al., 1979 [47] | 22 (13) | Experimental, no blinding | Chorea | 300–600 mg/day | Mean 31 weeks, max 26 months | Subjective moderate–good improvement on chorea | Weight increase in some patients, apathy, and/or somnolence |
| Quinn et al., 1985 [48] | 7 (uk) | Experimental, placebo-controlled | Chorea | 600–1200 mg/day | Unknown | Median abnormal movement count improved by 42%, chorea score by 6.5%, and functional score by 18.4% | None reported |
| Roos et al., 1982 [49] | 22 (9) | Experiment, cross-over, double-blind | Chorea | 300 mg/day | 4 weeks, of which 2 weeks tiapride | No significant improvement in involuntary movement count | In minority of patients, mild drowsiness |
| Reference | n Respondents | Practice Based in | HD Symptom | Initial Monotherapy without Comorbidities Present | Side Effects of Tiapride |
|---|---|---|---|---|---|
| Sung et al., 2018 [53] | 200 | 100% USA | Chorea | Respondents preferred tetrabenazine (50%). Antipsychotics (26%) were considered off-label alternatives. | NA * |
| Burgunder et al., 2011 [28] | 52 | 42% EU 54% USA/Canada 4% Australia | Chorea | Worldwide respondents preferred antipsychotics (58%) as a first choice, and tetrabenazine (56%) as an alternative monotherapy. Fifty percent of EU respondents preferred tiapride. * Risperidone (43%) and olanzapine (39%) were also preferred. × | Sedation, Parkinsonism |
| Groves et al., 2011 [29] | 55 | 47% EU 49% USA/Canada 4% Australia | Irritability | Respondents preferred an SSRI (57%) as first choice, and antipsychotics (31%), mirtazapine (28%), or anti-epileptic drugs (27%) as alternative monotherapy. Twenty-seven percent of EU respondents preferred tiapride. * | None reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feleus, S.; van Schaijk, M.; Roos, R.A.C.; de Bot, S.T. The Many Faces of Huntington’s Chorea Treatment: The Impact of Sudden Withdrawal of Tiapride after 40 Years of Use and a Systematic Review. J. Pers. Med. 2022, 12, 589. https://doi.org/10.3390/jpm12040589
Feleus S, van Schaijk M, Roos RAC, de Bot ST. The Many Faces of Huntington’s Chorea Treatment: The Impact of Sudden Withdrawal of Tiapride after 40 Years of Use and a Systematic Review. Journal of Personalized Medicine. 2022; 12(4):589. https://doi.org/10.3390/jpm12040589
Chicago/Turabian StyleFeleus, Stephanie, Malu van Schaijk, Raymund A. C. Roos, and Susanne T. de Bot. 2022. "The Many Faces of Huntington’s Chorea Treatment: The Impact of Sudden Withdrawal of Tiapride after 40 Years of Use and a Systematic Review" Journal of Personalized Medicine 12, no. 4: 589. https://doi.org/10.3390/jpm12040589
APA StyleFeleus, S., van Schaijk, M., Roos, R. A. C., & de Bot, S. T. (2022). The Many Faces of Huntington’s Chorea Treatment: The Impact of Sudden Withdrawal of Tiapride after 40 Years of Use and a Systematic Review. Journal of Personalized Medicine, 12(4), 589. https://doi.org/10.3390/jpm12040589






