A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment?
Abstract
:1. Introduction
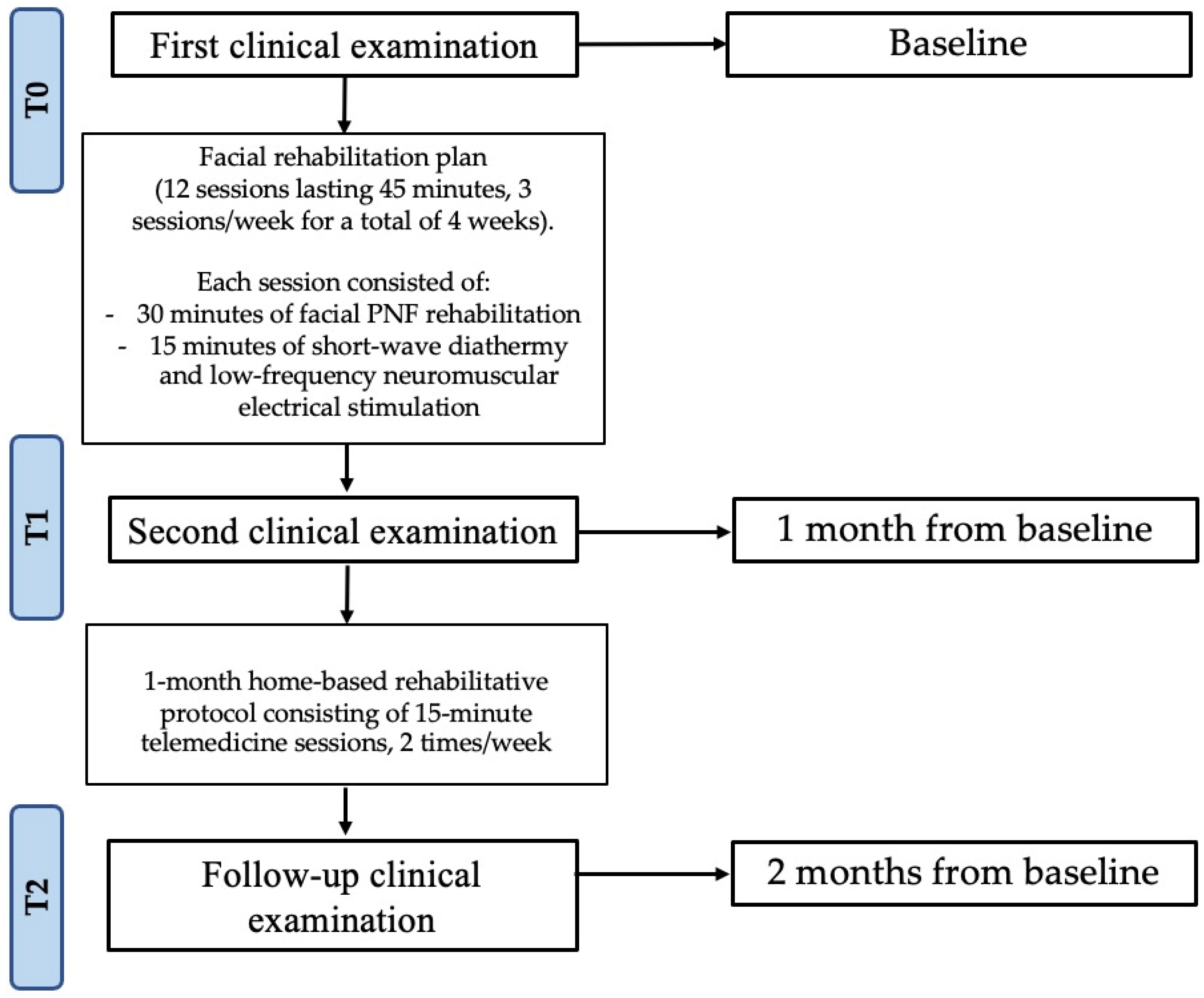
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabricius, J.; Kothari, S.F.; Kothari, M. Assessment and rehabilitation interventions for central facial palsy in patients with acquired brain injury: A systematic review. Brain Inj. 2021, 35, 511–519. [Google Scholar] [CrossRef]
- Barbara, M.; Antonini, G.; Vestri, A.; Volpini, L.; Monini, S. Role of Kabat physical rehabilitation in Bell’s palsy: A randomized trial. Acta Otolaryngol. 2010, 130, 167–172. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Santilli, V.; Paoloni, M.; Bernetti, A.; Saggini, R.; Paolucci, T. Idiopathic facial palsy: Umbrella review of systematic reviews and meta-analyses. J. Biol. Regul. Homeost. Agents 2020, 34, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Prud’hon, S.; Kubis, N. Bell’s palsy. Rev. Med. Interne 2019, 40, 28–37. [Google Scholar] [CrossRef]
- Paolucci, T.; Cardarola, A.; Colonnelli, P.; Ferracuti, G.; Gonnella, R.; Murgia, M.; Santilli, V.; Paoloni, M.; Bernetti, A.; Agostini, F.; et al. Give me a kiss!: An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur. J. Phys. Rehabil. Med. 2020, 56, 58–67. [Google Scholar] [CrossRef]
- Peitersen, E. Bell’s palsy: The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Oto-Laryngologica 2002, 122, 4–30. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef] [Green Version]
- Karp, E.; Waselchuk, E.; Landis, C.; Fahnhorst, J.; Lindgren, B.; Lyford-Pike, S. Facial Rehabilitation as Noninvasive Treatment for Chronic Facial Nerve Paralysis. Otol. Neurotol. 2019, 40, 241–245. [Google Scholar] [CrossRef]
- Coulson, S.E.; O’Dwyer, N.J.; Adams, R.D.; Croxson, G.R. Expression of emotion and quality of life after facial nerve paralysis. Otol. Neurotol. 2004, 25, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kleiss, I.J.; Hohman, M.H.; Susarla, S.M.; Marres, H.A.M.; Hadlock, T.A. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: A retrospective cohort study. Clin. Otolaryngol. 2015, 40, 651–656. [Google Scholar] [CrossRef]
- Walker, D.T.; Hallam, M.J.; Ni Mhurchadha, S.; Mccabe, P.; Nduka, C. The psychosocial impact of facial palsy: Our experience in one hundred and twenty six patients. Clin. Otolaryngol. 2012, 37, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Saadi, R.; Shokri, T.; Schaefer, E.; Hollenbeak, C.; Lighthall, J.G. Depression Rates after Facial Paralysis. Ann. Plast. Surg. 2019, 83, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A.Y.; Gurusinghe, A.D.R.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.C.; Slattery, W.H.; Snyder-Warwick, A.K. Facial nerve grading instruments: Systematic review of the literature and suggestion for uniformity. Plast. Reconstr. Surg. 2015, 135, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.G.; Fradet, G.; Nedzelski, J.M. Development of a sensitive clinical facial grading system. Eur. Arch. Otorhinolaryngol. 1996, 114, 380–386. [Google Scholar] [CrossRef]
- Andresen, N.S.; Zhu, V.; Lee, A.; Sebetka, W.; Kimura, J.; Hansen, M.R.; Gantz, B.J.; Sun, D.Q. Electrodiagnostic testing in acute facial palsy: Outcomes and comparison of methods. Laryngoscope Investig. Otolaryngol. 2020, 5, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Valls-Solé, J. Electrodiagnostic studies of the facial nerve in peripheral facial palsy and hemifacial spasm. Muscle and Nerve 2007, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Baugh, R.F.; Basura, G.J.; Ishii, L.E.; Schwartz, S.R.; Drumheller, C.M.; Burkholder, R.; Deckard, N.A.; Dawson, C.; Driscoll, C.; Gillespie, M.B.; et al. Clinical Practice Guideline: Bell’s Palsy. Otolaryngol. Neck Surg. 2013, 149, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Calder, H.B.; White, D.E. Facial nerve EMG monitoring. Neurodiagn. J. 1996, 36, 28–46. [Google Scholar] [CrossRef]
- Demeco, A.; Marotta, N.; Moggio, L.; Pino, I.; Marinaro, C.; Barletta, M.; Petraroli, A.; Palumbo, A.; Ammendolia, A. Quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J. Electromyogr. Kinesiol. 2021, 56, 102485. [Google Scholar] [CrossRef] [PubMed]
- Grosheva, M.; Wittekindt, C.; Guntinas-Lichius, O. Prognostic value of electroneurography and electromyography in facial palsy. Laryngoscope 2008, 118, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Sittel, C. Diagnostik von erkrankungen und der funktion des N. facialis. HNO 2004, 52, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Shafshak, T.S. The treatment of facial palsy from the point of view of physical and rehabilitation medicine. Eura. Medicophys. 2006, 42, 41–47. [Google Scholar] [PubMed]
- Gagyor, I.; Madhok, V.B.; Daly, F.; Sullivan, F. Antiviral treatment for bell’s palsy (Idiopathic facial paralysis). Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, W.; Zhang, Z.G.; Zhao, S.J. Combination of electric acupuncture grouping anticipation and facial nervous electromyogram in the treatment of infranuclear facial palsy in 80 patients. Chin. J. Clin. Rehabil. 2006, 10, 21–23. [Google Scholar]
- Hadlock, T.; Cheney, M.L. Facial reanimation: An invited review and commentary. Arch. Facial Plast. Surg. 2008, 10, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.J.; Soares, B.G.D.O.; Vieira, V.P. Physical therapy for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, K.; Ijaz Gondal, M.; Qamar, M.; Basharat, A.; Ahmad, W.; Ali, S. Comparison of efficacy of Kabat rehabilitation and facial exercises along with nerve stimulation in patients with Bell’s palsy. BLDE Univ. J. Heal. Sci. 2018, 3, 31. [Google Scholar] [CrossRef]
- Namura, M.; Motoyoshi, M.; Namura, Y.; Shimizu, N. The effects of PNF training on the facial profile. J. Oral Sci. 2008, 50, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Tharani, G.; Gopinath, Y.; Kamatchi, K. Comparision of pnf versus conventional exercises for facial symmetry and facial function in bell ’ s palsy. International Journal of Current Advanced Research. Rawal Med. J. 2018, 43, 543–546. [Google Scholar]
- Ordahan, B.; Karahan, A. yavuz Role of low-level laser therapy added to facial expression exercises in patients with idiopathic facial (Bell’s) palsy. Lasers Med. Sci. 2017, 32, 931–936. [Google Scholar] [CrossRef]
- Kandakurti, P.K.; Shanmugam, S.; Basha, S.A.; Amaravadi, S.K.; Suganthirababu, P.; Gopal, K.; George, G.S. The effectiveness of low-level laser therapy combined with facial expression exercises in patients with moderate-to-severe Bell’s palsy: A study protocol for a randomised controlled trial. Int. J. Surg. Protoc. 2020, 24, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.M.R.P.; Dharma, B.D.I.; Silakarma, I.G.N.M.D.; Samsarga, G.W. Multifaceted rehabilitation strategies for ramsay hunt syndrome: A case report and a review of literature. Bali Med. J. 2020, 9, 477–480. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Inzitari, M.T.; Caruso, M.G.; Ammendolia, A.; Enix, D. Neuromuscular electrical stimulation and shortwave diathermy in unrecovered Bell palsy: A randomized controlled study. Medicine (United States) 2020, 99, e19152. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.; Arnold, D.; Kuttenreich, A.-M.; Thielker, J.; Klingner, C.; Geitner, M.; Mastryukova, V.; Guntinas-Lichius, O. Implantable Facial Pacemaker: Definition of electrostimulation parameters for implantable solutions to treat facial palsy. Laryngo-Rhino-Otol. 2021, 100(S 02), S304–S305. [Google Scholar]
- Osthues, M.; Kuttenreich, A.M.; Volk, G.F.; Dobel, C.; Strauss, B.; Altmann, U.; Guntinas-Lichius, O. Continual rehabilitation motivation of patients with postparalytic facial nerve syndrome. Eur. Arch. Oto-Rhino-Laryngology 2022, 279, 481–491. [Google Scholar] [CrossRef]
- Vaughan, A.; Gardner, D.; Miles, A.; Copley, A.; Wenke, R.; Coulson, S. A systematic review of physical rehabilitation of facial palsy. Front. Neurol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Tan, J.R.; Coulson, S.; Keep, M. Face-to-face versus video assessment of facial paralysis: Implications for telemedicine. J. Med. Internet Res. 2019, 21, e11109. [Google Scholar] [CrossRef]
- Ten Harkel, T.C.; Speksnijder, C.M.; Van Der Heijden, F.; Beurskens, C.H.G.; Ingels, K.J.A.O.; Maal, T.J.J. Depth accuracy of the RealSense F200: Low-cost 4D facial imaging. Sci. Rep. 2017, 7, 16263. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the state-of-the-art and areas of application. JMIR Rehabil. Assist. Technol. 2017, 4, e7511. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Ammendolia, A. Why is telerehabilitation necessary? A pre-post COVID-19 comparative study of ICF activity and participation. J. Enabling Technol. 2021, 15, 117–121. [Google Scholar] [CrossRef]
- Neely, J.G.; Cherian, N.G.; Dickerson, C.B.; Nedzelski, J.M. Sunnybrook facial grading system: Reliability and criteria for grading. Laryngoscope 2010, 120, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Marotta, N.; Demeco, A.; Moggio, L.; Paola, P.; Marotta, M.; Iona, T.; Invernizzi, M.; Leigheb, M.; Ammendolia, A. Electromyographic assessment of anterior cruciate ligament injury risk in male tennis players: Which role for visual input? a proof-of-concept study. Diagnostics 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Demeco, A.; Marotta, N.; Spanò, R.; Curci, C.; Farì, G.; Fortunato, F.; Iona, T.; Lippi, L.; Paolucci, T.; et al. Neuromuscular Impairment of Knee Stabilizer Muscles in a COVID-19 Cluster of Female Volleyball Players: Which Role for Rehabilitation in the Post-COVID-19 Return-to-Play? Appl. Sci. 2022, 12, 557. [Google Scholar] [CrossRef]
- Sacco, I.C.N.; Gomes, A.A.; Otuzi, M.E.; Pripas, D.; Onodera, A.N. A method for better positioning bipolar electrodes for lower limb EMG recordings during dynamic contractions. J. Neurosci. Methods 2009, 180, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Blouin, J.S.; Dakin, C.J.; Dalton, B.H.; Blouin, J.S. Rectification is required to extract oscillatory envelope modulation from surface electromyographic signals. J. Neurophysiol. 2014, 112, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- D’Alessio, T.; Conforto, S. Extraction of the envelope from surface EMG signals. IEEE Eng. Med. Biol. Mag. 2001, 20, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pavese, C.; Cecini, M.; Caspani, P.; Monteleone, S.; Klersy, C.; Toffola, E.D. activity limitations and participation restrictions in patients with peripheral facial palsy: A cross-sectional study over a six-year period. Eur. J. Phys. Rehabil. Med. 2020, 56, 725–732. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Andrenelli, E.; Negrini, F.; Patrini, M.; Lazzarini, S.G.; Ceravolo, M.G.; Kiekens, C.; Arienti, C.; Ceravolo, M.G.; Côté, P.; et al. Rehabilitation and COVID-19: A rapid living systematic review by cochrane rehabilitation field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur. J. Phys. Rehabil. Med. 2021, 57, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Szczepura, A.; Holliday, N.; Neville, C.; Johnson, K.; Khan Khan, A.J.; Oxford, S.W.; Nduka, C. Raising the digital profile of facial palsy: National surveys of patients’ and clinicians’ experiences of changing UK treatment pathways and views on the future role of digital technology. J. Med. Internet Res. 2020, 22, e20406. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.; Liu, K.; Smith, M.; Nakamoto, M.; Mitchell, C.; Zhu, E.; Ma, E.; Morden, F.T.; Chong, A.; Van, N.; et al. Early Impact of the COVID-19 Pandemic on Outpatient Neurologic Care in Hawai’i. Hawai’i J. Health Soc. Welf. 2022, 81, 6–12. [Google Scholar]
- Pacheco, T.B.F.; Bezerra, D.A.; Silva, J.P.D.S.; Cacho, Ê.W.A.; De Souza, C.G.; Cacho, R.O. The Implementation of Teleconsultations in a Physiotherapy Service During COVID-19 Pandemic in Brazil: A Case Report. Int. J. Telerehabil. 2021, 13, e6368. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Ammendolia, A.; Marinaro, C.; Demeco, A.; Moggio, L.; Costantino, C. International classification of functioning, disability and health (ICF) and correlation between disability and finance assets in chronic stroke patients. Acta Biomed. 2020, 91, 1–4. [Google Scholar] [CrossRef]
- Robinson, M.W.; Baiungo, J. Facial Rehabilitation: Evaluation and Treatment Strategies for the Patient with Facial Palsy. Otolaryngol. Clin. N. Am. 2018, 51, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.E.; de Jongh, F.W.; Ingels, K.J.A.O.; Pouwels, S. e-Health and telemedicine implementation in facial paralysis: Challenges and pitfalls. Eur. J. Plast. Surg. 2021, 44, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Rogante, M.; Kairy, D.; Giacomozzi, C.; Grigioni, M. A quality assessment of systematic reviews on telerehabilitation: What does the evidence tell us? Ann. Ist. Super. Sanita 2015, 51, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Velayati, F.; Ayatollahi, H.; Hemmat, M. A Systematic Review of the Effectiveness of Telerehabilitation Interventions for Therapeutic Purposes in the Elderly. Methods Inf. Med. 2020, 59, 104–109. [Google Scholar] [CrossRef] [PubMed]

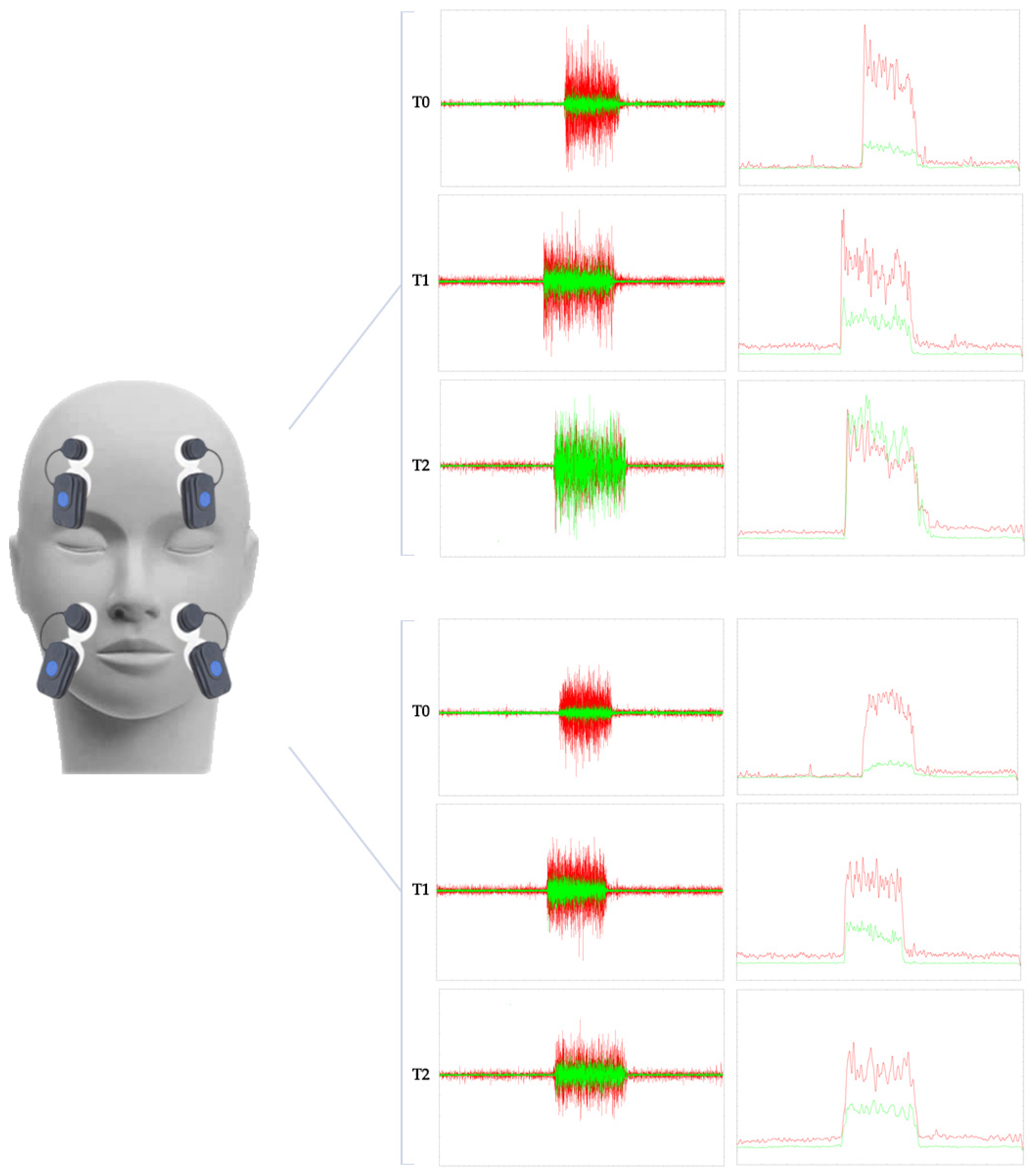
| Clinical Outcome Measures | T0 | T1 | T2 |
|---|---|---|---|
| Sunnybrook Facial Grading System | 25 | 51 | 73 |
| HB Scale | Grade 3: Moderate dysfunction | Grade 2: Mild dysfunction | Grade 2: Mild dysfunction |
| Facial Nerve Grading System 2.0 | Grade III: Moderate dysfunction | Grade II: Mild dysfunction | Grade II: Mild dysfunction |
| FaCE | 59 | 67 | 69 |
| SAQ Worksheet | 55.6% | 44.4% | 33.3% |
| VAS | 1 | 0 | 0 |
| BDI | 4 | 1 | 1 |
| HADS | |||
| HADS-A | 6 | 3 | 2 |
| HADS-D | 5 | 2 | 1 |
| SCL-90 | |||
| Somatization | 5 | 7 | 0 |
| Obsessive-Compulsive | 1 | 1 | 0 |
| Interpersonal Sensitivity | 1 | 0 | 0 |
| Depression | 3 | 1 | 0 |
| Anxiety | 4 | 1 | 0 |
| Hostility | 1 | 0 | 0 |
| Phobic anxiety | 0 | 0 | 0 |
| Paranoid ideation | 0 | 0 | 0 |
| Psychoticism | 0 | 0 | 0 |
| Sleep disorders | 4 | 6 | 2 |
| EQ-5D-3L | 0.689 | 1.000 | 1.000 |
| EQVAS | 70% | 90% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Marotta, N.; Agostini, F.; Drago Ferrante, V.; Demeco, A.; Ferrillo, M.; Inzitari, M.T.; Pellegrino, R.; Russo, I.; Ozyemisci Taskiran, O.; et al. A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment? J. Pers. Med. 2022, 12, 497. https://doi.org/10.3390/jpm12030497
de Sire A, Marotta N, Agostini F, Drago Ferrante V, Demeco A, Ferrillo M, Inzitari MT, Pellegrino R, Russo I, Ozyemisci Taskiran O, et al. A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment? Journal of Personalized Medicine. 2022; 12(3):497. https://doi.org/10.3390/jpm12030497
Chicago/Turabian Stylede Sire, Alessandro, Nicola Marotta, Francesco Agostini, Vera Drago Ferrante, Andrea Demeco, Martina Ferrillo, Maria Teresa Inzitari, Raffaello Pellegrino, Ilaria Russo, Ozden Ozyemisci Taskiran, and et al. 2022. "A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment?" Journal of Personalized Medicine 12, no. 3: 497. https://doi.org/10.3390/jpm12030497
APA Stylede Sire, A., Marotta, N., Agostini, F., Drago Ferrante, V., Demeco, A., Ferrillo, M., Inzitari, M. T., Pellegrino, R., Russo, I., Ozyemisci Taskiran, O., Bernetti, A., & Ammendolia, A. (2022). A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment? Journal of Personalized Medicine, 12(3), 497. https://doi.org/10.3390/jpm12030497














