The Role of Preoperative Platelet-to-Lymphocyte Ratio as a Predictor for Incisional Hernias after Hand-Assisted Laparoscopic Liver Surgery for Metastatic Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
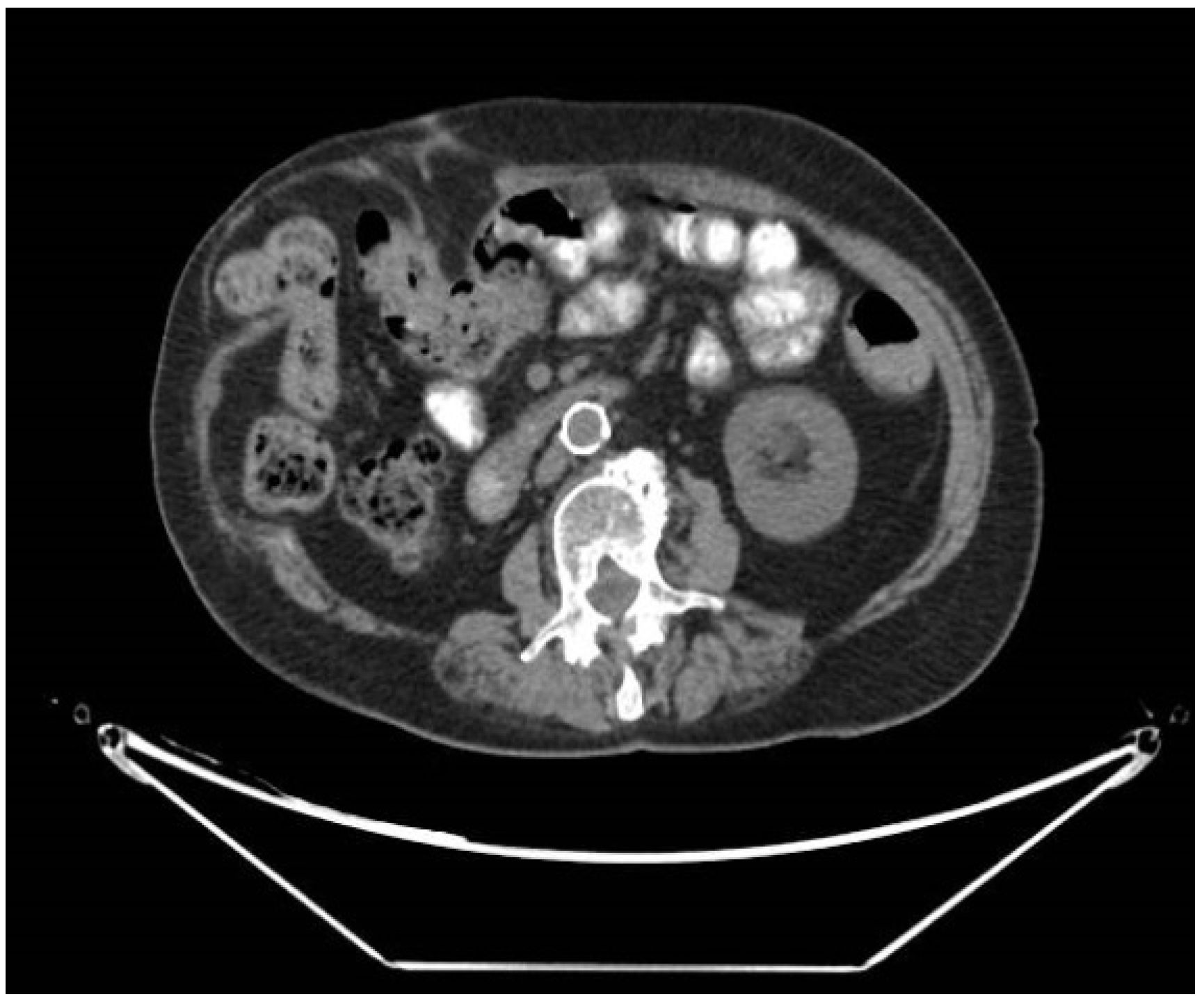
2.1. Radiological Assessment of Incisional Hernia
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Capussotti, L.; Ferrero, A.; Viganò, L.; Ribero, D.; Lo Tesoriere, R.; Polastri, R. Major liver resections synchronous with colorectal surgery. Ann. Surg. Oncol. 2007, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Wakabayashi, G. First quarter century of laparoscopic liver resection. World J. Gastroenterol. 2017, 23, 3581–3588. [Google Scholar] [CrossRef]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The southampton consensus guidelines for laparoscopic liver surgery: From indication to implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, J.; Young, C.J. Thirteen-year experience with hand-assisted laparoscopic surgery in colorectal patients. ANZ J. Surg. 2020, 90, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2008, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [PubMed]
- Sadot, E.; Goldberg, N.; Damoni, E.; Aranovich, D.; Kashtan, H.; Bitterman, A.; Haddad, R. Laparoscopic hand-assisted liver resection for tumours in the left lateral section. J. Minim. Access Surg. 2020, 16, 35–40. [Google Scholar] [PubMed]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Primavesi, F.; Stättner, S.; Jäger, T.; Göbel, G.; Presl, J.; Tomanová, K.; Buchner, S.; Maglione, M.; Resch, T.; Hutter, J.; et al. Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer. Cancers 2019, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Wabitsch, S.; Schulz, P.; Fröschle, F.; Kästner, A.; Fehrenbach, U.; Benzing, C.; Haber, P.K.; Denecke, T.; Pratschke, J.; Fikatas, P.; et al. Incidence of incisional hernia after laparoscopic liver resection. Surg. Endosc. 2021, 35, 1108–1115. [Google Scholar] [CrossRef]
- Kazaryan, A.M.; Marangos, I.P.; Røsok, B.I.; Rosseland, A.R.; Villanger, O.; Fosse, E.; Mathisen, Ø.; Edwin, B. Laparoscopic resection of colorectal liver metastases: Surgical and long-term oncologic outcome. Ann. Surg. 2010, 252, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu, J.; Bessa-Melo, R.; Graça, L.; Costa-Maia, J. Incisional hernia in hepatobiliary and pancreatic surgery: Incidence and risk factors. Hernia 2019, 23, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pans, A.; Elen, P.; Dewé, W.; Desaive, C. Long-term results of polyglactin mesh for the prevention of incisional hernias in obese patients. World J. Surg. 1998, 22, 479–482. [Google Scholar] [CrossRef]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Itatsu, K.; Yokoyama, Y.; Sugawara, G.; Kubota, H.; Tojima, Y.; Kurumiya, Y.; Kono, H.; Yamamoto, H.; Ando, M.; Nagino, M. Incidence of and risk factors for incisional hernia after abdominal surgery. J. Br. Surg. 2014, 101, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.D.; Ravn, P.; Winslet, M.; Chester, K. Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br. J. Surg. 2006, 93, 1456–1463. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Neal, C.P.; Cairns, V.; Jones, M.J.; Masood, M.M.; Nana, G.R.; Mann, C.D.; Garcea, G.; Dennison, A.R. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med. Oncol. 2015, 32, 144. [Google Scholar] [CrossRef]
- Rotem, R.; Erenberg, M.; Rottenstreich, M.; Segal, D.; Yohay, Z.; Idan, I.; Yohay, D.; Weintraub, A.Y. Early prediction of post cesarean section infection using simple hematological biomarkers: A case control study. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 245, 84–88. [Google Scholar] [CrossRef]
- Jones, H.G.; Qasem, E.; Dilaver, N.; Egan, R.; Bodger, O.; Kokelaar, R.; Evans, M.D.; Davies, M.; Beynon, J.; Harris, D. Inflammatory cell ratios predict major septic complications following rectal cancer surgery. Int. J. Colorectal Dis. 2018, 33, 857–862. [Google Scholar] [CrossRef]
- Mohri, Y.; Tanaka, K.; Toiyama, Y.; Ohi, M.; Yasuda, H.; Inoue, Y.; Kusunoki, M. Impact of preoperative neutrophil to lymphocyte ratio and postoperative infectious complications on survival after curative gastrectomy for gastric cancer: A single institutional cohort study. Medicine 2016, 95, e3125. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.Y.; Lin, Z.X.; Li, Y.; Jiang, N.; Li, X.; Wu, D.H.; Wang, T.T.; Chen, J.; Lin, Q.; Wu, X.Y. Poor oncologic outcomes of hepatocellular carcinoma patients with intra-abdominal infection after hepatectomy. World J. Gastroenterol. 2015, 21, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Lavand’homme, P.; Ambrosoli, A.L.; Cappelleri, G.; Saccani Jotti, G.M.; Meschi, T.; Fanelli, G.; Allegri, M. Effect of Preoperative Inflammatory Status and Comorbidities on Pain Resolution and Persistent Postsurgical Pain after Inguinal Hernia Repair. Med. Inflamm. 2016, 2016, 5830347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Ruan, X.; Shao, X.; Huang, X.; Fang, G.; Zheng, X. Clinical value of the neutrophil/lymphocyte ratio in diagnosing adult strangulated inguinal hernia. Int. J. Surg. 2016, 36, 76–80. [Google Scholar] [CrossRef]
- Xie, X.; Feng, S.; Tang, Z.; Chen, L.; Huang, Y.; Yang, X. Neutrophil-to-Lymphocyte Ratio Predicts the Severity of Incarcerated Groin Hernia. Med. Sci. Monit. 2017, 23, 5558–5563. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y.; Inoue, K.; Mori, K.; Gorai, K.; Shimamoto, R.; Onitsuka, T.; Iguchi, H.; Okazaki, M.; Nakagawa, M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as predictors of wound healing failure in head and neck reconstruction. Acta Otolaryngol. 2017, 137, 106–110. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Harker, L.A.; Roskos, L.K.; Marzec, U.M.; Carter, R.A.; Cherry, J.K.; Sundell, B.; Cheung, E.N.; Terry, D.; Sheridan, W. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood 2000, 95, 2514–2522. [Google Scholar] [CrossRef]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeg, H.J. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1319–1339. [Google Scholar] [CrossRef]
- Wolber, E.-M.; Jelkmann, W. Thrombopoietin: The novel hepatic hormone. Physiology 2002, 17, 6–10. [Google Scholar] [CrossRef]
- Gouin-Thibault, I.; Achkar, A.; Samama, M.M. The thrombophilic state in cancer patients. Acta Haematol. 2001, 106, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.A.; Hao, S.; Irish, W.; Zervos, E.E.; Tuttle-Newhall, J.E.; Parikh, A.A. Thirty-Day Morbidity after Simultaneous Resection of Colorectal Cancer and Colorectal Liver Metastasis: American College of Surgeons NSQIP Analysis. J. Am. Coll. Surg. 2020, 230, 617–627.e9. [Google Scholar] [CrossRef] [PubMed]
| All Cohort (n = 89) | Patients with Incisional Hernia (n = 7) | Patients without Incisional Hernia (n = 82) | p | |
|---|---|---|---|---|
| Age | 65.1 ± 12.2 | 67.6 ± 15.4 | 64.9 ± 11.9 | 0.59 |
| Gender | 0.46 | |||
| Male | 50 (56.2%) | 3 (6%) | 47 (94%) | |
| Female | 39 (43.8%) | 4 (10.3%) | 35 (89.7%) | |
| Diabetes mellitus | 0.86 | |||
| Yes | 23 (25.8%) | 2 (8.7%) | 21 (91.3%) | |
| No | 66 (74.2%) | 5 (7.6%) | 61 (92.4%) | |
| Smoking | 0.38 | |||
| Yes | 15 (16.8%) | 2 (13.3%) | 13 (86.7%) | |
| No | 74 (83.2%) | 5 (6.8%) | 69 (93.750 | |
| BMI | 0.037 | |||
| <25 | 33 (37.1%) | 0 (0%) | 33 (100%) | |
| >25 | 56 (62.9%) | 7 (12.5%) | 49 (87.5%) | |
| ASA | 0.49 | |||
| 2-1 | 63 (70.8%) | 6 (9.5%) | 57 (90.5%) | |
| 4-3 | 26 (29.2%) | 1 (3.8%) | 25 (96.2%) | |
| No. of liver metastases (mean ± SD) | 1.76 ± 1.22 | 1.14 ± 0.38 | 1.81 ± 1.25 | 0.16 |
| Size (mean ± SD) | 31 ± 4 | 23.77 ± 10 | 32.14 ± 4 | 0.12 |
| Neoadjuvant chemotherapy | 0.67 | |||
| Yes | ||||
| No | 58 (65.2%) | 4 (6.9%) | 54 (93.1%) | |
| 31 (34.8%) | 3 (9.7%) | 28 (90.3%) | ||
| Avastin | 0.91 | |||
| Yes | 27 (30.3%) | 2 (7.4%) | 25 (92.6%) | |
| No | 62 (69.7%) | 5 (8.1%) | 57 (91.9%) | |
| Simultaneous colon resection | 0.016 | |||
| Yes | ||||
| No | 6 (6.7%) | 2 (33.3%) | 4 (66.7%) | |
| 83 (93.3%) | 5 (6%) | 78 (94%) | ||
| EBL(mL) (mean ± SD) | 337.2 ± 327.84 | 385.71 ± 484.83 | 332.9+324.81 | 0.68 |
| Blood transfusion | 0.97 | |||
| Yes | 13 (14.6%) | 1 (7.7%) | 12 (92.3%) | |
| No | 76 85.4%) | 6 (7.9%) | 70 (92.1%) | |
| Postop complication | 0.79 | |||
| Yes | 16 (18%) | 1 (6.3%) | 15 (93.8%) | |
| No | 73 (82%) | 6 (8.2%) | 67 (91.8%) |
| Entire Cohort (n = 89) | Patients with Incisional Hernia (n = 7) | Patients without Incisional Hernia (n = 82) | p | |
|---|---|---|---|---|
| Albumin | 0.45 | |||
| <3.5 | 6 (6.7%) | 0 (0%) | 6 (100%) | |
| >3.5 | 83 (93.2%) | 7 (8.4%) | 76 (81.5%) | |
| NLR | 0.46 | |||
| <2.5 | 52 (58.4%) | 5 (9.6%) | 47 (90.4%) | |
| >2.5 | 37 (41.6%) | 2 (5.4%) | 35 (95.6%) | |
| PLR | 0.09 | |||
| <200 | 80 (89.9%) | 5 (6.3%) | 75 (93.8%) | |
| >200 | 9 (10.1%) | 2 (22.2%) | 7 (77.8%) | |
| PNI | 0.46 | |||
| <52 | 52 (58.4%) | 5 (9.6%) | 47 (90.4%) | |
| >52 | 37 (41.6%) | 2 (5.4%) | 35 (94.6%) | |
| SSI | 0.27 | |||
| <600 | 76 (85.4%) | 5 (6.6%) | 71 (93.4%) | |
| >600 | 13 (14.6%) | 2 (15.4%) | 11 (84.6%) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p | HR | p | HR | |
| Diabetes mellitus No vs. yes | 0.98 | 1.01 (0.18–5.59) | ||
| Smoking No vs. yes | 0.16 | 3.43 (0.61–19.31) | ||
| BMI <25 vs. >25 | 0.15 | 0.011 (0.00–5.62) | ||
| ASA 1–2 vs. 3–4 | 0.5 | 2.08 (0.24–17.97) | ||
| Simultaneous colon No vs. yes | 0.004 | 0.052 (0.007–0.38) | 0.03 | 0.1 (0.01–0.83) |
| PLR <200 vs. >200 | 0.029 | 0.13 (0.02–0.81) | 0.04 | 0.11(0.01–0.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamid, A.; Abu-Zaydeh, O.; Sawaied, M.; Goldberg, N.; Haddad, R. The Role of Preoperative Platelet-to-Lymphocyte Ratio as a Predictor for Incisional Hernias after Hand-Assisted Laparoscopic Liver Surgery for Metastatic Colorectal Cancer. J. Pers. Med. 2022, 12, 492. https://doi.org/10.3390/jpm12030492
Mahamid A, Abu-Zaydeh O, Sawaied M, Goldberg N, Haddad R. The Role of Preoperative Platelet-to-Lymphocyte Ratio as a Predictor for Incisional Hernias after Hand-Assisted Laparoscopic Liver Surgery for Metastatic Colorectal Cancer. Journal of Personalized Medicine. 2022; 12(3):492. https://doi.org/10.3390/jpm12030492
Chicago/Turabian StyleMahamid, Ahmad, Omar Abu-Zaydeh, Muneer Sawaied, Natalia Goldberg, and Riad Haddad. 2022. "The Role of Preoperative Platelet-to-Lymphocyte Ratio as a Predictor for Incisional Hernias after Hand-Assisted Laparoscopic Liver Surgery for Metastatic Colorectal Cancer" Journal of Personalized Medicine 12, no. 3: 492. https://doi.org/10.3390/jpm12030492
APA StyleMahamid, A., Abu-Zaydeh, O., Sawaied, M., Goldberg, N., & Haddad, R. (2022). The Role of Preoperative Platelet-to-Lymphocyte Ratio as a Predictor for Incisional Hernias after Hand-Assisted Laparoscopic Liver Surgery for Metastatic Colorectal Cancer. Journal of Personalized Medicine, 12(3), 492. https://doi.org/10.3390/jpm12030492







