Relation of the ‘Atrial Fibrillation Better Care (ABC) Pathway’ to the Quality of Anticoagulation in Atrial Fibrillation Patients Taking Vitamin K Antagonists
Abstract
:1. Introduction
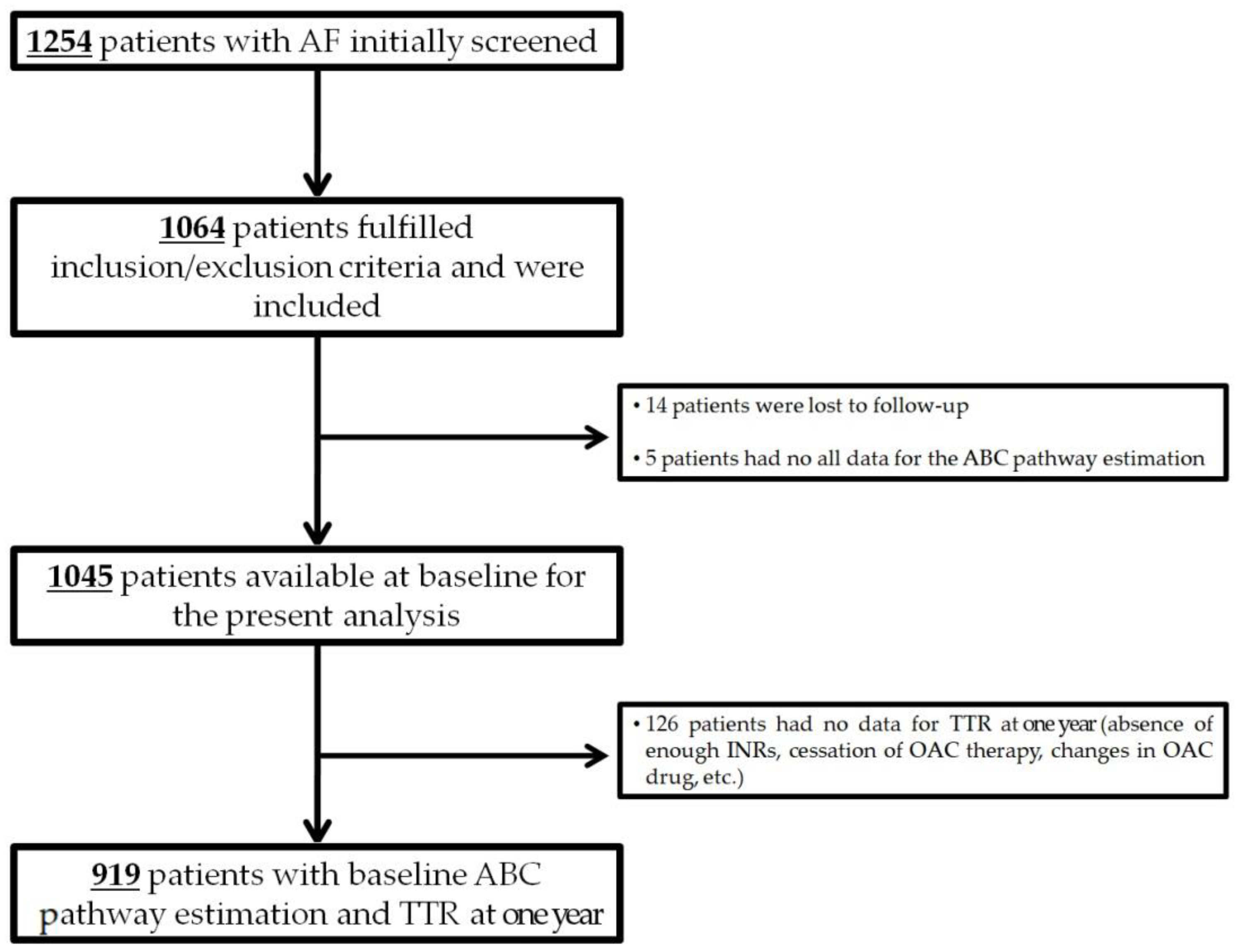
2. Materials and Methods
2.1. ABC (Atrial Fibrillation Better Care) Pathway Assessment
2.2. Follow-Up and Study Outcomes
2.3. Statistical Analyses
3. Results
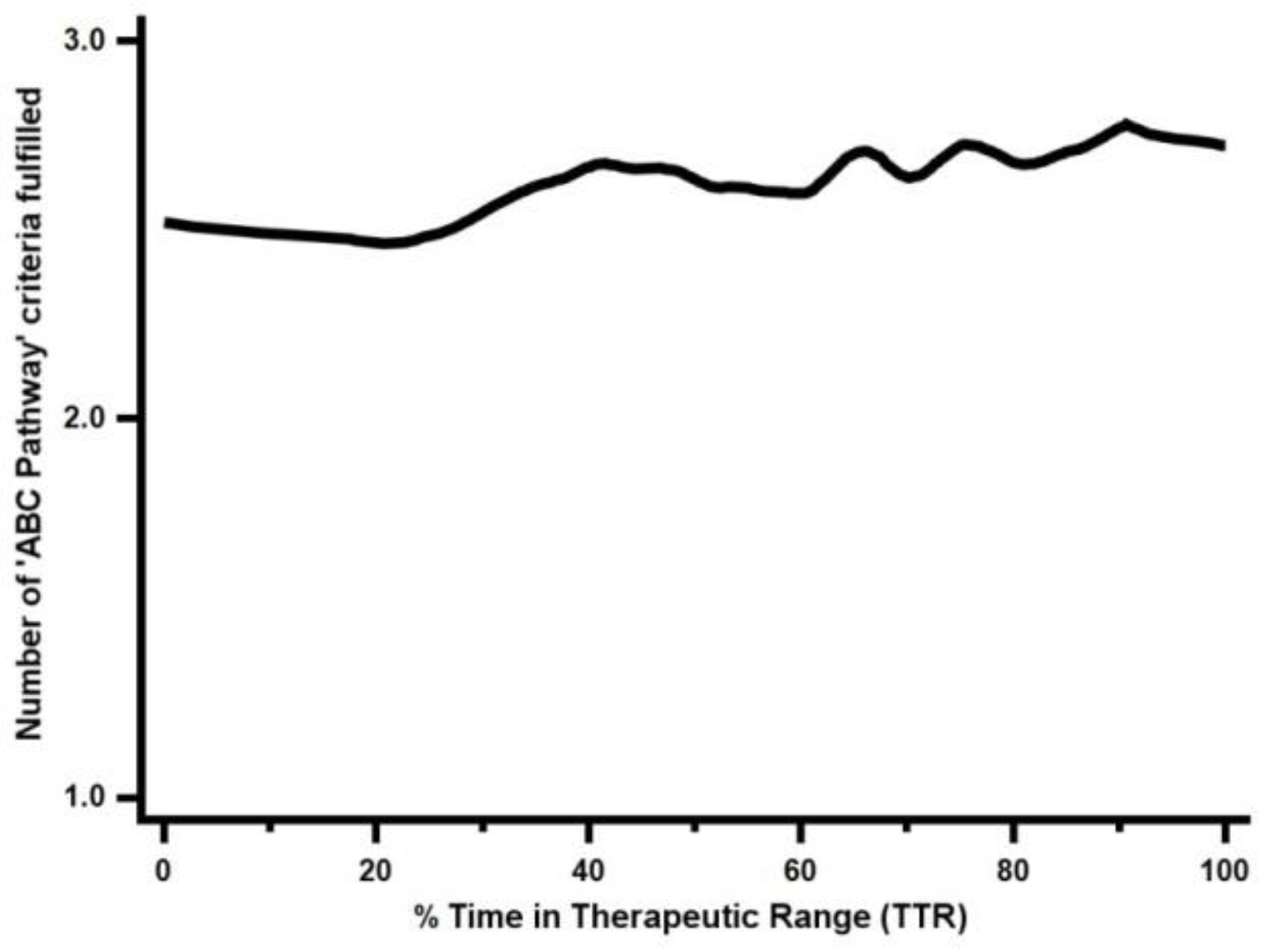
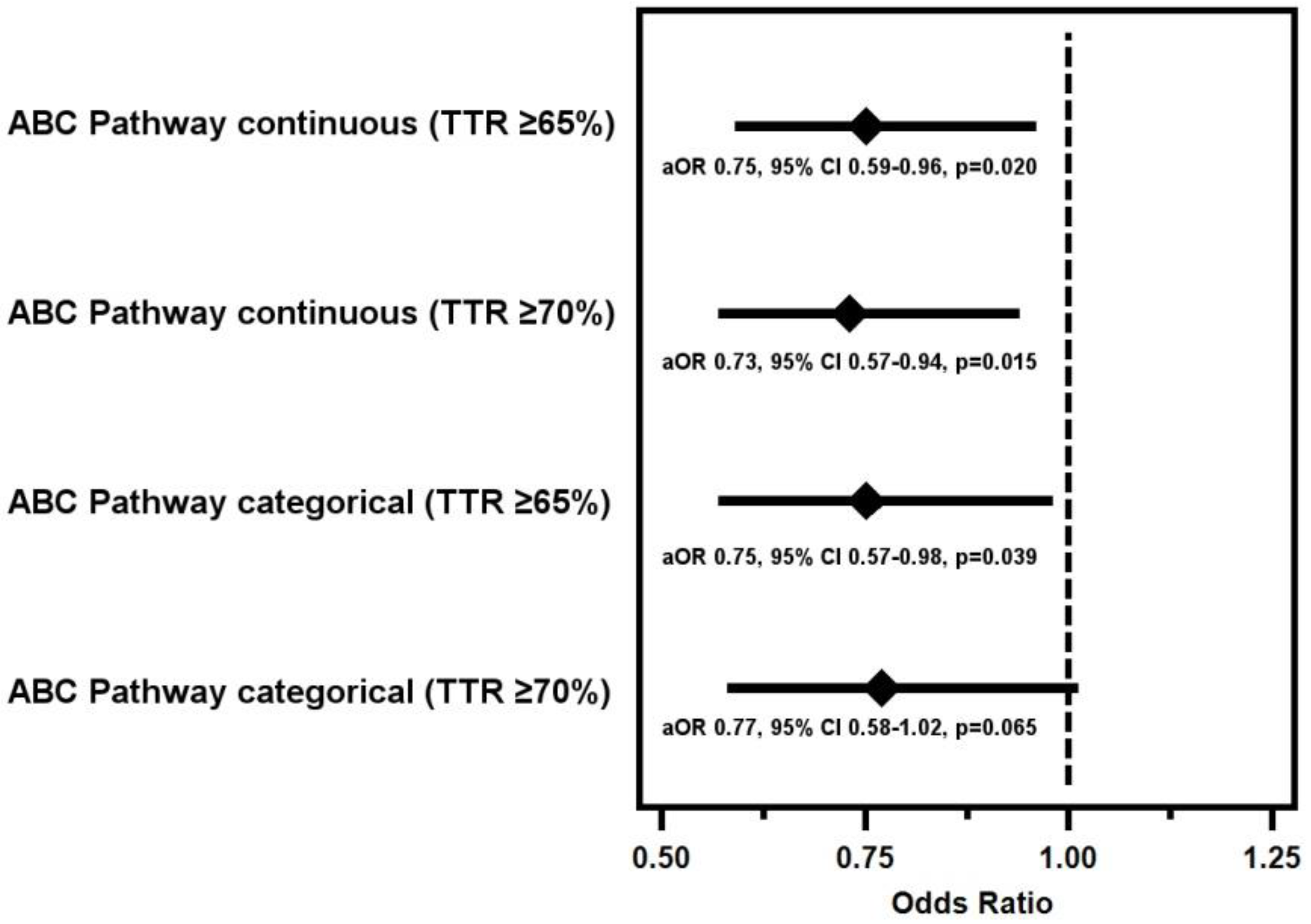
3.1. ABC Pathway Adherence and Quality of Anticoagulation Control
3.2. ABC Pathway Compliance and PINRR
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Lip, G.Y.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Caravaca, J.M.; Roldán, V.; Esteve-Pastor, M.A.; Valdés, M.; Vicente, V.; Lip, G.Y.H.; Marín, F. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb. Haemost. 2017, 117, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Sobieraj-Teague, M.; Eikelboom, J.W. Direct oral anticoagulants: Evidence and unresolved issues. Lancet 2020, 396, 1767–1776. [Google Scholar] [CrossRef]
- Stevens, D.; Harrison, S.L.; Kolamunnage-Dona, R.; Lip, G.Y.H.; Lane, D.A. The Atrial Fibrillation Better Care pathway for managing atrial fibrillation: A review. Europace 2021, 23, 1511–1527. [Google Scholar] [CrossRef]
- Gallagher, C.; Hendriks, J.M.; Nyfort-Hansen, K.; Sanders, P.; Lau, D.H. Integrated care for atrial fibrillation: The heart of the matter. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Lip, G.Y.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Chao, T.-F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.-K.; Chan, Y.-H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb. Haemost. 2021, 122, 20–47. [Google Scholar] [CrossRef]
- Van den Ham, H.A.; Klungel, O.H.; Leufkens, H.G.M.; Van Staa, T.P.; Van Staa, T. The patterns of anticoagulation control and the risk of stroke, bleeding and mortality in patients with non-valvular atrial fibrillation. J. Thromb. Haemost. 2013, 11, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pignatelli, P.; Cribari, F.; Carnevale, R.; Saliola, M.; Violi, F.; Lip, G.Y. Time to therapeutic range (TtTR), anticoagulation control, and cardiovascular events in vitamin K antagonists–naive patients with atrial fibrillation. Am. Heart J. 2018, 200, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Caravaca, J.; Roldán, V.; Esteve-Pastor, M.A.; Valdés, M.; Vicente, V.; Marin, F.; Lip, G.Y. Reduced Time in Therapeutic Range and Higher Mortality in Atrial Fibrillation Patients Taking Acenocoumarol. Clin. Ther. 2018, 40, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Hausner, C.; Nagler, M.; Göbel, S.; Eggebrecht, L.; Panova-Noeva, M.; Arnold, N.; Lauterbach, M.; Bickel, C.; Michal, M.; et al. Subtherapeutic Anticoagulation Control under Treatment with Vitamin K-Antagonists—Data from a Specialized Coagulation Service. Thromb. Haemost. 2019, 119, 1347–1357. [Google Scholar] [CrossRef]
- Rivera-Caravaca, J.M.; Marín, F.; Esteve-Pastor, M.A.; Gálvez, J.; Lip, G.Y.; Vicente, V.; Roldán, V. Murcia atrial fibrillation project II: Protocol for a prospective observational study in patients with atrial fibrillation. BMJ Open. 2019, 9, e033712. [Google Scholar] [CrossRef] [Green Version]
- Apostolakis, S.; Sullivan, R.M.; Olshansky, B.; Lip, G.Y. Factors Affecting Quality of Anticoagulation Control Among Patients With Atrial Fibrillation on Warfarin: The SAMe-TT2R2 score. Chest 2013, 144, 1555–1563. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Cannegieter, S.C.; Van Der Meer, F.J.; Briët, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.-H.; Li, W.-H.; Hai, J.-J.; Chan, E.W.; Wong, I.C.; Tse, H.-F.; Lip, G.Y.; Siu, C.-W. Time in Therapeutic Range and Percentage of International Normalized Ratio in the Therapeutic Range as a Measure of Quality of Anticoagulation Control in Patients with Atrial Fibrillation. Can. J. Cardiol. 2016, 32, 1247.e23–1247.e28. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Setakis, E.; Plumb, J.M.; Clemens, A.; van Staa, T.-P. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb. Haemost. 2011, 106, 968–977. [Google Scholar] [CrossRef]
- Garcia, D.A.; Lopes, R.D.; Hylek, E.M. New-onset atrial fibrillation and warfarin initiation: High risk periods and implications for new antithrombotic drugs. Thromb. Haemost. 2010, 104, 1099–1105. [Google Scholar]
- Marcatto, L.R.; Sacilotto, L.; Tavares, L.C.; Facin, M.; Olivetti, N.; Strunz, C.M.C.; Darrieux, F.; Scanavacca, M.; Krieger, J.E.; Pereira, A.C.; et al. Pharmaceutical Care Increases Time in Therapeutic Range of Patients with Poor Quality of Anticoagulation with Warfarin. Front. Pharmacol. 2018, 9, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, M.; Lane, D.A. The Compelling Issue of Nonvitamin K Antagonist Oral Anticoagulant Adherence in Atrial Fibrillation Patients: A Systematic Need for New Strategies. Thromb. Haemost. 2020, 120, 369–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Caravaca, J.M.; Esteve-Pastor, M.A.; Roldán, V.; Marín, F.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants: Impact of non-adherence and discontinuation. Expert Opin. Drug Saf. 2017, 16, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, M.C.; Lee, L.H.; Ng, H.J.; Ko, Y. Knowledge, satisfaction, and concerns regarding warfarin therapy and their association with warfarin adherence and anticoagulation control. Thromb. Res. 2014, 133, 550–554. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.S.; Kim, E.J.; Park, S.; Chung, N.; Chu, S.H. Factors Affecting Medication Adherence and Anticoagulation Control in Korean Patients Taking Warfarin. J. Cardiovasc. Nurs. 2011, 26, 466–474. [Google Scholar] [CrossRef]
- Gallego, P.; Roldán, V.; Marin, F.; Gálvez, J.; Valdés, M.; Vicente, V.; Lip, G.Y. SAMe-TT2R2 Score, Time in Therapeutic Range, and Outcomes in Anticoagulated Patients with Atrial Fibrillation. Am. J. Med. 2014, 127, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Clarkesmith, D.; Pattison, H.; Lip, G.Y.H.; Lane, D.A. Educational Intervention Improves Anticoagulation Control in Atrial Fibrillation Patients: The TREAT Randomised Trial. PLoS ONE 2013, 8, e74037. [Google Scholar] [CrossRef]
- Lane, D.A.; Ponsford, J.; Shelley, A.; Sirpal, A.; Lip, G.Y. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: Effects of an educational intervention programme: The West Birmingham Atrial Fibrillation Project. Int. J. Cardiol. 2006, 110, 354–358. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Menichelli, D.; Violi, F.; Lip, G.Y. Integrated Care Management of Patients with Atrial Fibrillation and Risk of Cardiovascular Events: The ABC (Atrial fibrillation Better Care) Pathway in the ATHERO-AF Study Cohort. Mayo Clin. Proc. 2019, 94, 1261–1267. [Google Scholar] [CrossRef]
- Proietti, M.; Lip, G.Y.H.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.-A.; Kalarus, Z.; Tavazzi, L.; et al. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2021, 23, 174–183. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.-S.; Guo, Y.; et al. Adherence to the ‘Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes—A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb. Haemost. 2021, 122, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Heneghan, C.; Perera, R.; Roberts, N.; Hollowell, J.; Glasziou, P.; Bankhead, C.; Xu, Y. Anticoagulation Control and Prediction of Adverse Events in Patients with Atrial Fibrillation: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2008, 1, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastori, D.; Lip, G.Y.H.; Poli, D.; Antonucci, E.; Rubino, L.; Menichelli, D.; Saliola, M.; Violi, F.; Palareti, G.; Pignatelli, P.; et al. Determinants of low-quality warfarin anticoagulation in patients with mechanical prosthetic heart valves. The nationwide PLECTRUM study. Br. J. Haematol. 2020, 190, 588–593. [Google Scholar] [CrossRef]
- Van Walraven, C.; Jennings, A.; Oake, N.; Fergusson, D.; Forster, A.J. Effect of Study Setting on Anticoagulation Control: A systematic review and metaregression. Chest 2006, 129, 1155–1166. [Google Scholar] [CrossRef]
| N = 1045 | |
|---|---|
| Demographic | |
| Male sex, n (%) | 506 (48.4) |
| Age (years), median (IQR) | 77 (70–83) |
| BMI (kg/m2), median (IQR) | 30.0 (26.8–33.3) |
| Comorbidities, n (%) | |
| Hypertension | 874 (83.6) |
| Diabetes mellitus | 393 (37.6) |
| Heart failure | 261 (25.0) |
| History of stroke/TIA/thromboembolism | 162 (15.5) |
| Renal impairment | 197 (18.9) |
| Coronary artery disease | 190 (18.2) |
| Peripheral artery disease | 66 (6.3) |
| Hypercholesterolemia | 608 (58.2) |
| Current smoking habit | 157 (15.0) |
| Current alcohol consumption | 71 (6.8) |
| History of previous bleeding | 173 (16.6) |
| COPD/OSAH | 230 (22.0) |
| Hepatic disease | 68 (6.5) |
| Concomitant malignant disease | 150 (14.4) |
| Concomitant treatment, n (%) | |
| Antiarrhythmics | 214 (20.5) |
| Calcium antagonist | 317 (30.3) |
| Beta-blockers | 723 (69.2) |
| Statins | 555 (53.1) |
| Diuretics | 571 (54.6) |
| Antiplatelet therapy | 256 (24.5) |
| ACE inhibitors | 255 (24.4) |
| Angiotensin II receptor blockers | 456 (43.6) |
| Oral antidiabetics/insulin | 279 (26.7) |
| CHA2DS2-VASc, median (IQR) | 4 (3–5) |
| HAS-BLED, median (IQR) | 2 (2–3) |
| SAMe-TT2R2, median (IQR) | 1 (1–2) |
| TTR < 65% | TTR < 70% | |||
|---|---|---|---|---|
| aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| ABC pathway (1 criterion) | Ref. | Ref. | ||
| ABC pathway (2 criteria) | 0.51 (0.22–1.20) | 0.125 | 0.30 (0.10–0.88) | 0.028 |
| ABC pathway (3 criteria) | 0.41 (0.18–0.95) | 0.038 | 0.25 (0.08–0.72) | 0.010 |
| ABC pathway (A criterion) | 0.56 (0.24–1.33) | 0.191 | 0.42 (0.16–1.15) | 0.092 |
| ABC pathway (B criterion) | 1.02 (0.70–1.48) | 0.923 | 0.99 (0.68–1.46) | 0.974 |
| ABC pathway (C criterion) | 0.67 (0.49–0.91) | 0.010 | 0.66 (0.48–0.92) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roldán, V.; Martínez-Montesinos, L.; López-Gálvez, R.; García-Tomás, L.; Lip, G.Y.H.; Rivera-Caravaca, J.M.; Marín, F. Relation of the ‘Atrial Fibrillation Better Care (ABC) Pathway’ to the Quality of Anticoagulation in Atrial Fibrillation Patients Taking Vitamin K Antagonists. J. Pers. Med. 2022, 12, 487. https://doi.org/10.3390/jpm12030487
Roldán V, Martínez-Montesinos L, López-Gálvez R, García-Tomás L, Lip GYH, Rivera-Caravaca JM, Marín F. Relation of the ‘Atrial Fibrillation Better Care (ABC) Pathway’ to the Quality of Anticoagulation in Atrial Fibrillation Patients Taking Vitamin K Antagonists. Journal of Personalized Medicine. 2022; 12(3):487. https://doi.org/10.3390/jpm12030487
Chicago/Turabian StyleRoldán, Vanessa, Lorena Martínez-Montesinos, Raquel López-Gálvez, Lucía García-Tomás, Gregory Y. H. Lip, José Miguel Rivera-Caravaca, and Francisco Marín. 2022. "Relation of the ‘Atrial Fibrillation Better Care (ABC) Pathway’ to the Quality of Anticoagulation in Atrial Fibrillation Patients Taking Vitamin K Antagonists" Journal of Personalized Medicine 12, no. 3: 487. https://doi.org/10.3390/jpm12030487
APA StyleRoldán, V., Martínez-Montesinos, L., López-Gálvez, R., García-Tomás, L., Lip, G. Y. H., Rivera-Caravaca, J. M., & Marín, F. (2022). Relation of the ‘Atrial Fibrillation Better Care (ABC) Pathway’ to the Quality of Anticoagulation in Atrial Fibrillation Patients Taking Vitamin K Antagonists. Journal of Personalized Medicine, 12(3), 487. https://doi.org/10.3390/jpm12030487







