Risk of Dementia According to Surgery Type: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
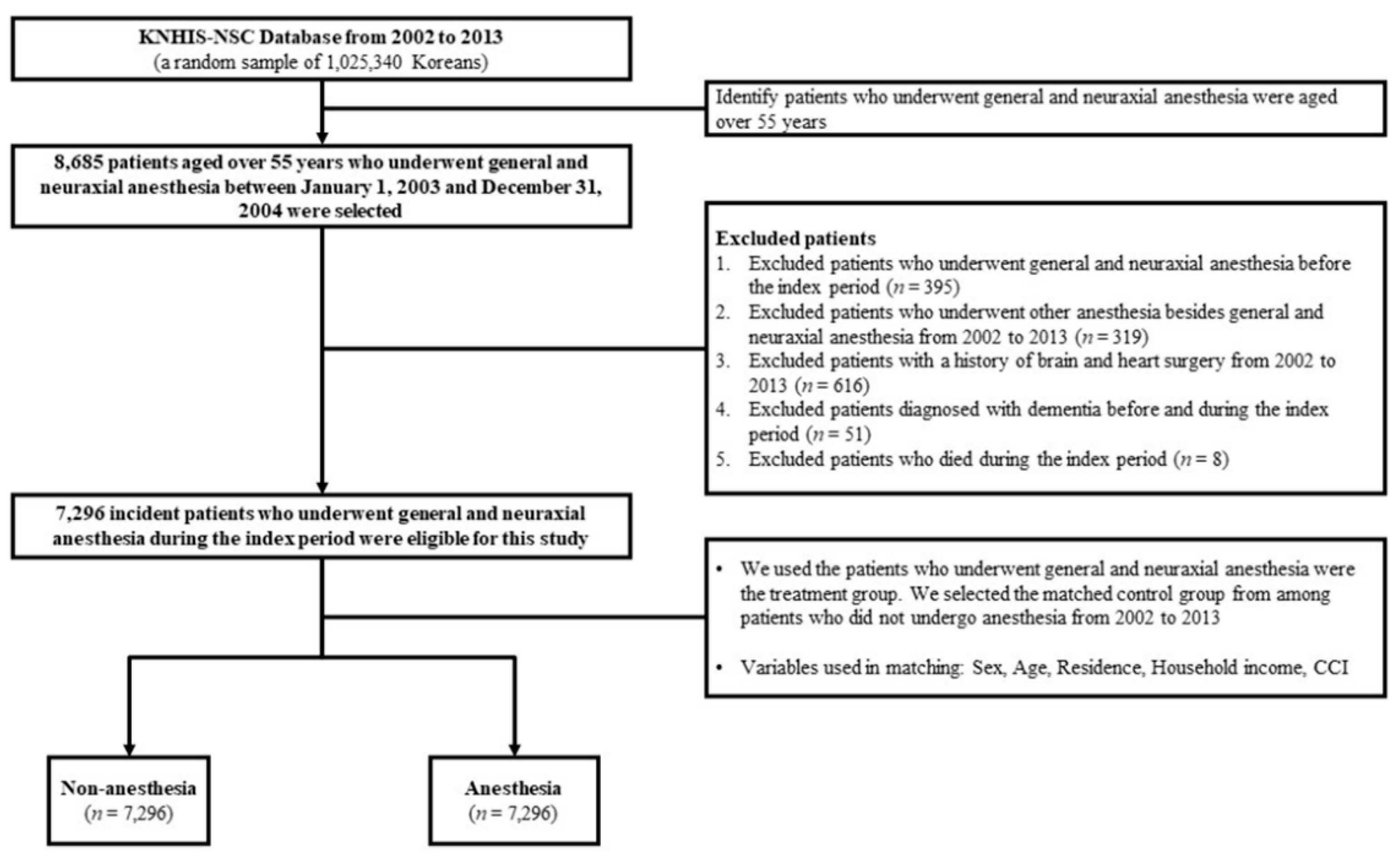
2.1. National Sample Cohort
2.2. Study Setting and Participants
2.3. Predictor and Outcome Variables
2.4. Statistical Analysis
3. Results
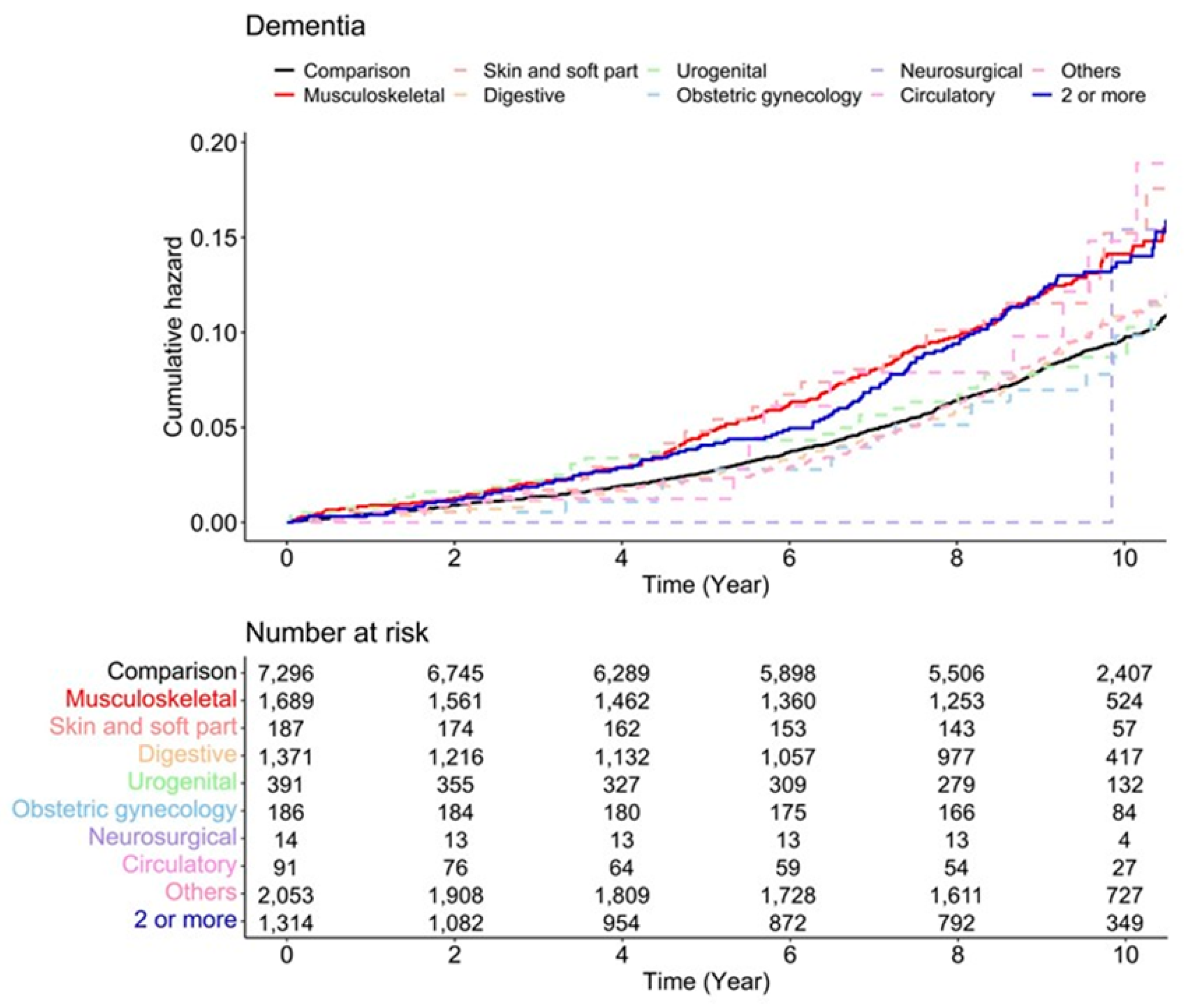
3.1. Hazard Ratios of Dementia According to Surgery Type
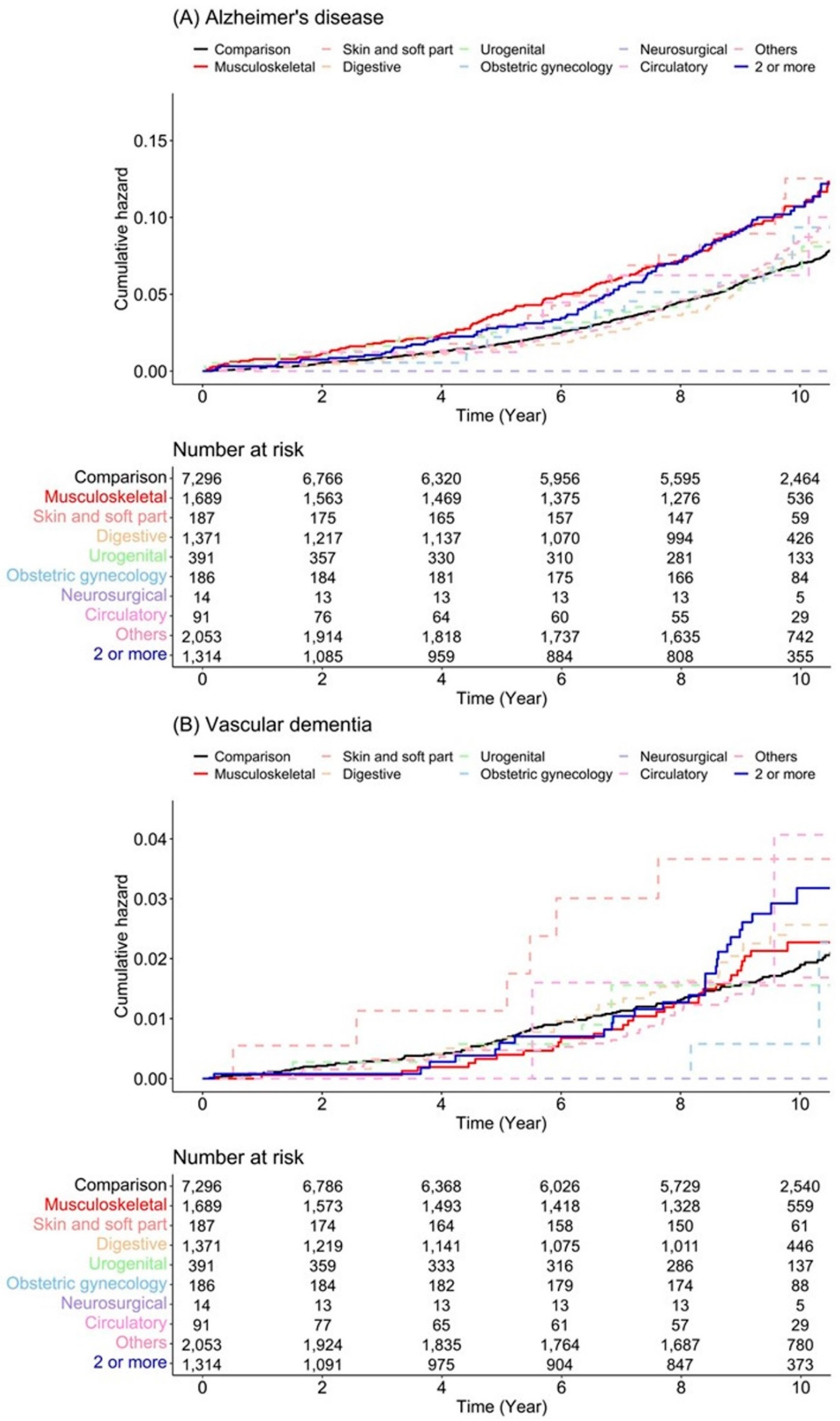
3.2. Subgroup Analysis According to Sex, Dementia Type, Anesthesia Type, and Scale of Surgery
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Code | Disease |
| F00 | Dementia in Alzheimer’s disease |
| G30 | Alzheimer’s disease |
| F01 | Vascular dementia |
| F02 | Dementia in other diseases classified elsewhere |
| F03 | Unspecified dementia |
| Comorbidities | International Classification of Disease, 10th Revision Code | Original Weight | Updated Weight |
|---|---|---|---|
| Myocardial infarction | I21.x, I22.x, I25.2 | 1 | 0 |
| Congestive heart failure | I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5-I42.9, I43.x, I50.x, P29.0 | 1 | 2 |
| Peripheral vascular disease | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 | 1 | 0 |
| Cerebrovascular disease | G45.x, G46.x, H34.0, I60.x-I69.x | 1 | 0 |
| Chronic pulmonary disease | I27.8, I27.9, J40.x-J47.x, J60.x-J67.x, J68.4, J70.1, J70.3 | 1 | 1 |
| Rheumatologic disease | M05.x, M06.x, M31.5, M32.x-M34.x, M35.1, M35.3, M36.0 | 1 | 1 |
| Peptic ulcer disease | K25.x-K28.x | 1 | 0 |
| Mild liver disease | B18.x, K70.0-K70.3, K70.9, K71.3-K71.5, K71.7, K73.x, K74.x, K76.0, K76.2-K76.4, K76.8, K76.9, Z94.4 | 1 | 2 |
| Diabetes without chronic complication | E10.0, E10.1, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 | 1 | 0 |
| Diabetes with chronic complication | E10.2-E10.5, E10.7, E11.2-E11.5, E11.7, E12.2-E12.5, E12.7, E13.2-E13.5, E13.7, E14.2-E14.5, E14.7 | 2 | 1 |
| Hemiplegia or paraplegia | G04.1, G11.4, G80.1, G80.2, G81.x, G82.x, G83.0-G83.4, G83.9 | 2 | 2 |
| Renal disease | I12.0, I13.1, N03.2-N03.7, N05.2-N05.7, N18.x, N19.x, N25.0, Z49.0-Z49.2, Z94.0, Z99.2 | 2 | 1 |
| Any malignancy including leukemia and lymphoma | C00.x-C26.x, C30.x-C34.x, C37.x-C41.x, C43.x, C45.x-C58.x, C60.x-C76.x, C81.x-C85.x, C88.x, C90.x-C97.x | 2 | 2 |
| Moderate or severe liver disease | I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 | 3 | 4 |
| Metastatic solid tumor | C77.x-C80.x | 6 | 6 |
| Acquired immune deficiency syndrome/human immunodeficiency virus | B20.x-B22.x, B24.x | 6 | 4 |
| Surgical type | The number of dementia events |
| Non-anesthesia | 615 |
| Musculoskeletal | 189 |
| Skin and soft part | 22 |
| Digestive | 107 |
| Urogenital | 29 |
| Obstetric gynecology | 16 |
| Neurosurgical | 1 |
| Circulatory | 9 |
| Others | 182 |
| 2 or more | 125 |
| Surgical type | The number of censored cases (no event) |
| Non-anesthesia | 6681 |
| Musculoskeletal | 1500 |
| Skin and soft part | 165 |
| Digestive | 1264 |
| Urogenital | 362 |
| Obstetric gynecology | 170 |
| Neurosurgical | 13 |
| Circulatory | 82 |
| Others | 1871 |
| 2 or more | 1189 |
| Surgical type | The number of termination cases |
| Non-anesthesia | 5063 |
| Musculoskeletal | 1154 |
| Skin and soft part | 131 |
| Digestive | 874 |
| Urogenital | 257 |
| Obstetric gynecology | 153 |
| Neurosurgical | 12 |
| Circulatory | 48 |
| Others | 1485 |
| 2 or more | 713 |
| Surgical type | The number of follow-up loss or drop-out cases |
| Non-anesthesia | 1618 |
| Musculoskeletal | 346 |
| Skin and soft part | 34 |
| Digestive | 390 |
| Urogenital | 105 |
| Obstetric gynecology | 17 |
| Neurosurgical | 1 |
| Circulatory | 34 |
| Others | 386 |
| 2 or more | 476 |
| Sex | Male | Female | ||
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Surgical Site | ||||
| Comparison | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Musculoskeletal | 1.47 (1.10–1.96) ** | 1.56 (1.17–2.09) ** | 1.43 (1.17–1.74) *** | 1.38 (1.13–1.69) ** |
| Skin and soft part | 1.48 (0.70–3.14) | 1.61 (0.76–3.43) | 1.51 (0.90–2.53) | 1.46 (0.87–2.45) |
| Digestive | 1.20 (0.90–1.62) | 1.11 (0.83–1.50) | 1.02 (0.76–1.36) | 0.95 (0.71–1.28) |
| Urogenital | 1.06 (0.67–1.68) | 0.79 (0.50–1.24) | 1.26 (0.65–2.44) | 1.31 (0.68–2.54) |
| Obstetric gynecology | – | – | 0.84 (0.51–1.38) | 0.89 (0.54–1.47) |
| Neurosurgical | 1.67 (0.23–11.91) | 1.98 (0.28–14.18) | 0.00 (0-Inf) | 0.00 (0-Inf) |
| Circulatory | 2.07 (0.92–4.65) | 2.06 (0.91–4.64) | 1.16 (0.37–3.61) | 1.67 (0.54–5.22) |
| Others | 0.93 (0.67–1.29) | 0.98 (0.70–1.36) | 1.13 (0.93–1.37) | 1.21 (1.00–1.47) |
| 2 or more | 1.45 (1.05–2.00) * | 1.35 (0.97–1.86) | 1.52 (1.19–1.93) *** | 1.47 (1.16–1.87) ** |
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Alzheimer’s disease | ||||||
| Comparison | 7296 | 450 | 7.01 | 1.00 (ref) | 1.00 (ref) | |
| Musculoskeletal | 1689 | 148 | 10.46 | 1.57 (1.31–1.90) ** | 1.55 (1.28–1.86) ** | <0.001 |
| Skin and soft part | 187 | 17 | 10.63 | 1.59 (0.98–2.58) | 1.59 (0.98–2.58) | 0.061 |
| Digestive | 1371 | 76 | 6.87 | 1.03 (0.81–1.32) | 1.01 (0.79–1.29) | 0.918 |
| Urogenital | 391 | 22 | 6.88 | 1.04 (0.68–1.59) | 0.95 (0.62–1.47) | 0.827 |
| Obstetric gynecology | 186 | 14 | 7.93 | 1.15 (0.67–1.95) | 1.06 (0.62–1.80) | 0.839 |
| Neurosurgical | 14 | 0 | 0 | 0.00 (0-Inf) | 0.00 (0-Inf) | 0.984 |
| Circulatory | 91 | 5 | 7.62 | 1.17 (0.49–2.83) | 1.51 (0.62–3.64) | 0.363 |
| Others | 2053 | 138 | 7.76 | 1.15 (0.95–1.39) | 1.19 (0.98–1.44) | 0.078 |
| 2 or more | 1314 | 97 | 10.27 | 1.58 (1.27–1.97) ** | 1.50 (1.21–1.88) ** | <0.001 |
| Vascular dementia | ||||||
| Comparison | 7296 | 122 | 1.88 | 1.00 (ref) | 1.00 (ref) | |
| Musculoskeletal | 1689 | 30 | 2.07 | 1.14 (0.76–1.70) | 1.13 (0.76–1.69) | 0.553 |
| Skin and soft part | 187 | 6 | 3.72 | 2.04 (0.90–4.62) | 2.02 (0.89–4.60) | 0.092 |
| Digestive | 1371 | 26 | 2.33 | 1.29 (0.84–1.97) | 1.25 (0.81–1.91) | 0.312 |
| Urogenital | 391 | 5 | 1.54 | 0.85 (0.35–2.09) | 0.70 (0.28–1.73) | 0.441 |
| Obstetric gynecology | 186 | 2 | 1.11 | 0.59 (0.15–2.39) | 0.62 (0.15–2.51) | 0.501 |
| Neurosurgical | 14 | 0 | 0 | 0.00 (0-Inf) | 0.00 (0-Inf) | 0.990 |
| Circulatory | 91 | 2 | 3.01 | 1.70 (0.42–6.87) | 1.91 (0.47–7.75) | 0.366 |
| Others | 2053 | 30 | 1.66 | 0.90 (0.61–1.35) | 0.94 (0.63–1.41) | 0.772 |
| 2 or more | 1314 | 27 | 2.80 | 1.57 (1.04–2.39) * | 1.49 (0.98–2.26) | 0.064 |
| Non-Matching | ||||||
|---|---|---|---|---|---|---|
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
| Dementia | ||||||
| General anesthesia | 1004 | 108 | 12.55 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 64 | 15.06 | 1.22 (0.90–1.66) | 0.98 (0.71–1.35) | 0.884 |
| Alzheimer’s disease | ||||||
| General anesthesia | 1004 | 82 | 9.45 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 53 | 12.37 | 1.44 (1.00–2.07) | 1.08 (0.75–1.54) | 0.690 |
| Vascular dementia | ||||||
| General anesthesia | 1004 | 19 | 2.14 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 7 | 1.59 | 0.77 (0.32–1.83) | 0.77 (0.31–1.87) | 0.557 |
| Matching | ||||||
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
| Dementia | ||||||
| General anesthesia | 552 | 64 | 14.14 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 64 | 15.06 | 1.07 (0.76–1.52) | 1.06 (0.75–1.52) | 0.733 |
| Alzheimer’s disease | ||||||
| General anesthesia | 552 | 49 | 10.74 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 53 | 12.37 | 1.16 (0.79–1.71) | 1.18 (0.79–1.75) | 0.426 |
| Vascular dementia | ||||||
| General anesthesia | 552 | 10 | 2.13 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 552 | 7 | 1.59 | 0.76 (0.29–1.99) | 0.79 (0.30–2.12) | 0.640 |
| Non-Matching | ||||||
|---|---|---|---|---|---|---|
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
| Dementia | ||||||
| General anesthesia | 791 | 64 | 12.29 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 23 | 20.19 | 1.64 (1.02–2.64) | 1.11 (0.67–1.84) | 0.697 |
| Alzheimer’s disease | ||||||
| General anesthesia | 791 | 47 | 8.92 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 18 | 15.78 | 1.78 (1.03–3.07) | 1.29 (0.73–2.30) | 0.386 |
| Vascular dementia | ||||||
| General anesthesia | 791 | 17 | 3.17 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 3 | 2.54 | 0.79 (0.23–2.70) | 0.43 (0.12–1.53) | 0.192 |
| Matching | ||||||
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
| Dementia | ||||||
| General anesthesia | 158 | 23 | 23.12 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 23 | 20.19 | 0.86 (0.48–1.53) | 0.99 (0.54–1.82) | 0.970 |
| Alzheimer’s disease | ||||||
| General anesthesia | 158 | 19 | 18.72 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 18 | 15.78 | 0.83 (0.44–1.59) | 1.06 (0.54–2.08) | 0.866 |
| Vascular dementia | ||||||
| General anesthesia | 158 | 8 | 7.62 | 1.00 (ref) | 1.00 (ref) | |
| Neuraxial anesthesia | 158 | 3 | 2.54 | 0.33 (0.09–1.26) | 0.45 (0.11–1.78) | 0.253 |
| CCI | 0 | 1 | ≥2 | |||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Dementia | ||||||
| Comparison | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Minor | 1.43 (1.03–1.98) * | 1.51 (1.09–2.09) * | 1.45 (0.92–2.30) | 1.90 (1.19–3.04) ** | 1.22 (0.69–2.15) | 1.25 (0.71–2.20) |
| Major | 1.84 (1.24–2.74) ** | 1.64 (1.10–2.45) * | 1.59 (0.91–2.77) | 1.93 (1.09–3.40) * | 1.26 (0.59–2.70) | 1.16 (0.54–2.51) |
| Variables | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Alzheimer’s disease | ||||||
| Comparison | 1689 | 105 | 6.97 | 1.00 (ref) | 1.00 (ref) | |
| Minor | 1167 | 97 | 9.75 | 1.46 (1.10–1.92) * | 1.60 (1.21–2.11) ** | <0.001 |
| Major | 522 | 51 | 12.14 | 1.83 (1.31–2.56) ** | 1.68 (1.20–2.35) * | 0.003 |
| Vascular dementia | ||||||
| Comparison | 1689 | 22 | 1.44 | 1.00 (ref) | 1.00 (ref) | |
| Minor | 1167 | 22 | 2.17 | 1.52 (0.84–2.75) | 1.62 (0.90–2.94) | 0.109 |
| Major | 522 | 8 | 1.85 | 1.30 (0.58–2.93) | 1.20 (0.53–2.70) | 0.661 |
References
- WHO. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 2 March 2022).
- Cornellà, N.; Sancho, J.; Sitges-Serra, A. Short and Long-Term Outcomes after Surgical Procedures Lasting for More than Six Hours. Sci. Rep. 2017, 7, 9221. [Google Scholar] [CrossRef] [PubMed]
- Shavit, Y.; Weidenfeld, J.; DeKeyser, F.G.; Fish, G.; Wolf, G.; Mayburd, E.; Meerson, Y.; Beilin, B. Effects of surgical stress on brain prostaglandin E2 production and on the pituitary–Adrenal axis: Attenuation by preemptive analgesia and by central amygdala lesion. Brain Res. 2005, 1047, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Aiello Bowles, E.J.; Larson, E.B.; Pong, R.P.; Walker, R.L.; Anderson, M.L.; Yu, O.; Gray, S.L.; Crane, P.K.; Dublin, S. Anesthesia Exposure and Risk of Dementia and Alzheimer’s Disease: A Prospective Study. J. Am. Geriatr. Soc. 2016, 64, 602–607. [Google Scholar] [CrossRef]
- Basques, B.; Bohl, D.; Golinvaux, N.; Samuel, A.; Grauer, J. General versus spinal anaesthesia for patients aged 70 years and older with a fracture of the hip. Bone Jt. J. 2015, 97, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Lin, C.-C.; Chen, K.-B.; Kuo, Y.-C.; Li, C.-Y.; Chung, C.-J. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case–control study. Alzheimer’s Dement. 2014, 10, 196–204. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Kim, J.W.; Woo, Y.C. Relationship between general anesthesia and Alzheimer disease: A protocol for a systematic review and meta-analysis. Medicine 2017, 96, e9314. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, M.; Vanacore, N.; Schiaffini, C.; Brusa, L.; Panella, M.; Talarico, G.; Bruno, G.; Meco, G.; Lenzi, G. A case-control study on Alzheimer’s disease and exposure to anesthesia. Neurol. Sci. 2002, 23, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.T.; Myung, W.; Lewis, M.; Lee, H.; Kim, S.E.; Lee, K.; Lee, C.; Choi, J.; Kim, H.; Carroll, B.J. Exposure to general anesthesia and risk of dementia: A nationwide population-based cohort study. J. Alzheimer’s Dis. 2018, 63, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, X.; Dong, Y.; Xu, Z.; Zhang, Y.; Xie, Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naïve and Alzheimer disease transgenic mice. Anesthesiology 2010, 112, 1404–1416. [Google Scholar] [CrossRef]
- Seitz, D.P.; Shah, P.S.; Herrmann, N.; Beyene, J.; Siddiqui, N. Exposure to general anesthesia and risk of Alzheimer’s disease: A systematic review and meta-analysis. BMC Geriatr. 2011, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Sprung, J.; Jankowski, C.J.; Roberts, R.O.; Weingarten, T.N.; Aguilar, A.L.; Runkle, K.J.; Tucker, A.K.; McLaren, K.C.; Schroeder, D.R.; Hanson, A.C.; et al. Anesthesia and Incident Dementia: A Population-Based, Nested, Case-Control Study. Mayo Clin. Proc. 2013, 88, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Edmondson, M.; Sakamoto, A.; Ma, D. Anesthesia, surgical stress, and “long-term” outcomes. Acta Anaesthesiol. Taiwanica 2015, 53, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- CDC. International Classification of Diseases, Tenth Revision (ICD-10). Available online: https://www.cdc.gov/nchs/icd/icd10.htm (accessed on 3 March 2022).
- KOICD. Available online: https://www.kcdcode.kr/browse/main/ (accessed on 3 March 2022).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Avidan, M.S.; Evers, A.S. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J. Alzheimer’s Dis. 2011, 24, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-L.; Yang, C.-W.; Tseng, Y.-K.; Sun, W.-Z.; Wang, J.-L.; Wang, S.-J.; Oyang, Y.-J.; Fuh, J.-L. Risk of dementia after anaesthesia and surgery. Br. J. Psychiatry 2014, 204, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Oyang, Y.J.; Lin, T.Y.; Sun, W.Z. Risk assessment of dementia after hysterectomy: Analysis of 14-year data from the National Health Insurance Research Database in Taiwan. J. Chin. Med. Assoc. 2020, 83, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Berger, M.; Eckenhoff, R.G.; Seitz, D.P. General anesthetic and the risk of dementia in elderly patients: Current insights. Clin. Interv. Aging 2014, 9, 1619–1628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, J.; Dong, Y.; Huang, W.; Bao, M. General anesthesia exposure and risk of dementia: A meta-analysis of epidemiological studies. Oncotarget 2017, 8, 59628. [Google Scholar] [CrossRef]
- Lee, J.J.; Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Shin, H.Y.; Park, Y.H.; Woo, Y.C. Relationship between Surgery under General Anesthesia and the Development of Dementia: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 3234013. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.-H.; Lee, J.J.; Lee, S.-H.; Kim, C.; Yu, H.; Kwon, Y.-S.; Kim, D.-K. Longitudinal Study of the Association between General Anesthesia and Increased Risk of Developing Dementia. J. Pers. Med. 2021, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.; Rasmussen, L.S. Anesthesia and the risk of dementia in the elderly. La Presse Médicale 2018, 47, e45–e51. [Google Scholar] [CrossRef] [PubMed]
- Velkers, C.; Berger, M.; Gill, S.S.; Eckenhoff, R.; Stuart, H.; Whitehead, M.; Austin, P.C.; Rochon, P.A.; Seitz, D. Association between exposure to general versus regional anesthesia and risk of dementia in older adults. J. Am. Geriatr. Soc. 2021, 69, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Fuh, J.-L. Exposure to general anesthesia and the risk of dementia. J. Pain Res. 2015, 8, 711. [Google Scholar]
- Bianchi, S.L.; Tran, T.; Liu, C.; Lin, S.; Li, Y.; Keller, J.M.; Eckenhoff, R.G.; Eckenhoff, M.F. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol. Aging 2008, 29, 1002–1010. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Xia, W.; Marcantonio, E.R.; Culley, D.J.; Crosby, G.; Tanzi, R.E.; Xie, Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases β-amyloid protein levels. Arch. Neurol. 2009, 66, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ishiguro, K.; Fujita, S.C. Ether stress-induced Alzheimer-like tau phosphorylation in the normal mouse brain. FEBS Lett. 2007, 581, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Dong, Y.; Maeda, U.; Alfille, P.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid β protein levels. Arch. Neurol. 2006, 104, 988–994. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, Y.; Maeda, U.; Moir, R.; Inouye, S.K.; Culley, D.J.; Crosby, G.; Tanzi, R.E. Isoflurane-induced apoptosis: A potential pathogenic link between delirium and dementia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1300–1306. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, Y.; Maeda, U.; Moir, R.D.; Xia, W.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J. Neurosci. 2007, 27, 1247–1254. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Zhang, G.; Moir, R.D.; Xia, W.; Yue, Y.; Tian, M.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The inhalation anesthetic desflurane induces caspase activation and increases amyloid β-protein levels under hypoxic conditions. J. Biol. Chem. 2008, 283, 11866–11875. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J.F.; Mackey, D.C.; Wasnick, J.D. Morgan & Mikhail’s Clinical Anesthesiology; McGraw-Hill: New York, NY, USA, 2013; Volume 15. [Google Scholar]
- Tannenbaum, C.; Paquette, A.; Hilmer, S.; Holroyd-Leduc, J.; Carnahan, R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging 2012, 29, 639–658. [Google Scholar] [PubMed]
- Knopman, D.; Petersen, R.; Cha, R.; Edland, S.; Rocca, W. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology 2005, 65, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.A.; Wolozin, B.; Weiss, K.B.; Bednar, M.M. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty 1. J. Alzheimer’s Dis. 2005, 7, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S.; Benjamin, H.J.; Asplund, C.A. Splints and casts: Indications and methods. Am. Fam. Phys. 2009, 80, 491–499. [Google Scholar]
- Magaziner, J.; Simonsick, E.M.; Kashner, T.M.; Hebel, J.R.; Kenzora, J.E. Predictors of functional recovery one year following hospital discharge for hip fracture: A prospective study. J. Gerontol. 1990, 45, M101–M107. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jo, J.Y.; Jung, J.S.; Kim, S.J. Prognostic Factors Predicting Early Recovery of Pre-fracture Functional Mobility in Elderly Patients With Hip Fracture. Ann. Rehabil. Med. 2014, 38, 827–835. [Google Scholar] [CrossRef]
- Velagapudi, R.; Subramaniyan, S.; Xiong, C.; Porkka, F.; Rodriguiz, R.M.; Wetsel, W.C.; Terrando, N. Orthopedic Surgery Triggers Attention Deficits in a Delirium-Like Mouse Model. Front. Immunol. 2019, 10, 2675. [Google Scholar] [CrossRef]
- Cognitive Neuropathology Group Medical Research Council. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001, 357, 169–175. [Google Scholar] [CrossRef]
- Adav, S.S.; Sze, S.K. Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling. Mol. Brain 2016, 9, 1–22. [Google Scholar] [CrossRef]
- Leandro, P.; Gomes, C.M. Protein misfolding in conformational disorders: Rescue of folding defects and chemical chaperoning. Mini Rev. Med. Chem. 2008, 8, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Ha-Duong, T. Exploring the Alzheimer amyloid-β peptide conformational ensemble: A review of molecular dynamics approaches. Peptides 2015, 69, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. 2017, 131, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Sardahaee, F.S.; Anderssen, S.; Ballard, C.; the Alzheimer’s Society Systematic Review Group. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment. Health 2010, 14, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; Weiss, J.P.; Crandall, B.G.; May, H.T.; Bair, T.L.; Osborn, J.S.; Anderson, J.L.; Muhlestein, J.B.; Horne, B.D.; Lappe, D.L. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm 2010, 7, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Johansson, B.; Nilsson, S.E.; Berg, S.; Pedersen, N.L.; Gatz, M.; McClearn, G. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: A population-based study of the oldest old. Int. Psychogeriatr. 2002, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hébert, R.; Lindsay, J.; Verreault, R.; Rockwood, K.; Hill, G.; Dubois, M.-F. Vascular dementia: Incidence and risk factors in the Canadian study of health and aging. Stroke 2000, 31, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Launer, L.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.; Copeland, J.; Dartigues, J.; Jagger, C.; Martinez-Lage, J. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 2000, 54, S4. [Google Scholar] [PubMed]
- Reitz, C.; Tang, M.-X.; Luchsinger, J.; Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004, 61, 705–714. [Google Scholar] [CrossRef]
- Rusanen, M.; Kivipelto, M.; Quesenberry, C.P.; Zhou, J.; Whitmer, R.A. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch. Intern. Med. 2011, 171, 333–339. [Google Scholar] [CrossRef]
- Sharp, S.I.; Aarsland, D.; Day, S.; Sønnesyn, H.; Alzheimer’s Society Vascular Dementia Systematic Review Group; Ballard, C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Atti, A.; Gatz, M.; Pedersen, N.; Johansson, B.; Fratiglioni, L. Midlife overweight and obesity increase late-life dementia risk: A population-based twin study. Neurology 2011, 76, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.F.; Zaric, D.; Boysen, G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol. Scand. 1988, 32, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
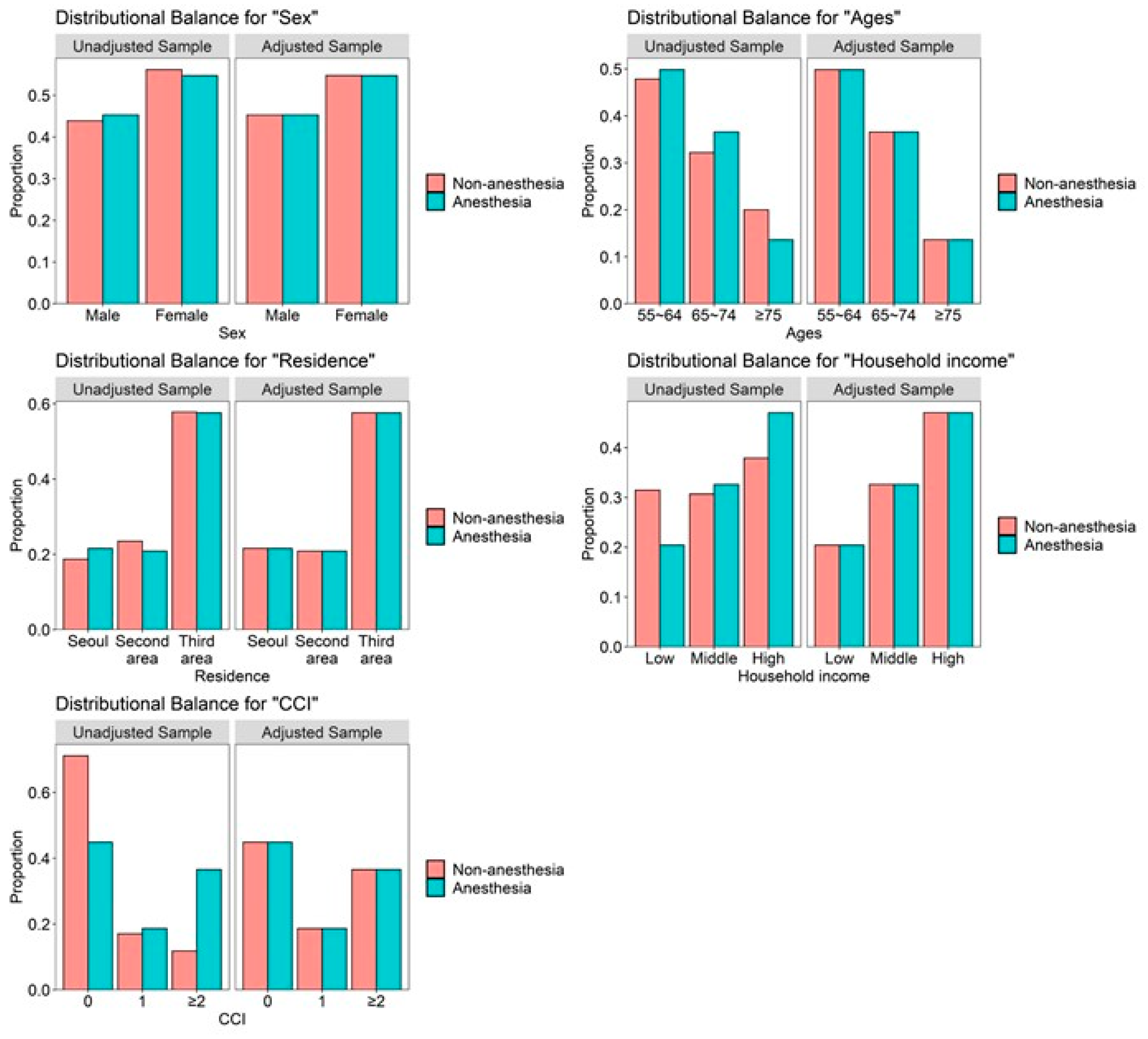
| Variables | Comparison (n = 7296) | Surgery under Anesthesia (n = 7296) | p-Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 3303 (45.3%) | 3304 (45.3%) | |
| Female | 3993 (54.7%) | 3992 (54.7%) | |
| Age (years) | 1.000 | ||
| 55–64 | 3636 (49.8%) | 3635 (49.8%) | |
| 65–74 | 2667 (36.6%) | 2668 (36.6%) | |
| ≥75 | 993 (13.6%) | 993 (13.6%) | |
| Residence | 1.000 | ||
| Seoul | 1574 (21.6%) | 1574 (21.6%) | |
| Second area | 1522 (20.9%) | 1522 (20.9%) | |
| Third area | 4200 (57.6%) | 4200 (57.6%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 1489 (20.4%) | 1489 (20.4%) | |
| Middle (30–70%) | 2377 (32.6%) | 2377 (32.6%) | |
| High (70–100%) | 3430 (47.0%) | 3430 (47.0%) | |
| CCI | 1.000 | ||
| 0 | 3273 (44.9%) | 3273 (44.9%) | |
| 1 | 1358 (18.6%) | 1358 (18.6%) | |
| ≥2 | 2665 (36.5%) | 2665 (36.5%) |
| Surgical Site | N | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Comparison | 7296 | 615 | 9.66 | 1.00 (ref) | 1.00 (ref) | |
| Musculoskeletal | 1689 | 189 | 13.47 | 1.46 (1.24–1.72) * | 1.44 (1.22–1.70) * | <0.001 |
| Skin and soft part | 187 | 22 | 13.99 | 1.51 (0.99–2.31) | 1.51 (0.99–2.32) | 0.057 |
| Digestive | 1371 | 107 | 9.75 | 1.06 (0.86–1.30) | 1.03 (0.84–1.27) | 0.776 |
| Urogenital | 391 | 29 | 9.13 | 0.99 (0.68–1.44) | 0.89 (0.61–1.30) | 0.561 |
| Obstetric gynecology | 186 | 16 | 9.08 | 0.95 (0.58–1.56) | 0.89 (0.54–1.47) | 0.653 |
| Neurosurgical | 14 | 1 | 7.80 | 0.82 (0.11–5.81) | 1.42 (0.20–10.10) | 0.727 |
| Circulatory | 91 | 9 | 13.87 | 1.55 (0.80–2.99) | 1.92 (0.99–3.72) | 0.052 |
| Others | 2053 | 182 | 10.32 | 1.10 (0.94–1.30) | 1.14 (0.97–1.35) | 0.114 |
| 2 or more | 1314 | 125 | 13.36 | 1.48 (1.22–1.80) * | 1.42 (1.17–1.72) * | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.S.; Lee, S.-H.; Kim, C.; Yu, H.; Sohn, J.-H.; Lee, J.J.; Kim, D.-K. Risk of Dementia According to Surgery Type: A Nationwide Cohort Study. J. Pers. Med. 2022, 12, 468. https://doi.org/10.3390/jpm12030468
Kwon YS, Lee S-H, Kim C, Yu H, Sohn J-H, Lee JJ, Kim D-K. Risk of Dementia According to Surgery Type: A Nationwide Cohort Study. Journal of Personalized Medicine. 2022; 12(3):468. https://doi.org/10.3390/jpm12030468
Chicago/Turabian StyleKwon, Young Suk, Sang-Hwa Lee, Chulho Kim, Hyunjae Yu, Jong-Hee Sohn, Jae Jun Lee, and Dong-Kyu Kim. 2022. "Risk of Dementia According to Surgery Type: A Nationwide Cohort Study" Journal of Personalized Medicine 12, no. 3: 468. https://doi.org/10.3390/jpm12030468
APA StyleKwon, Y. S., Lee, S.-H., Kim, C., Yu, H., Sohn, J.-H., Lee, J. J., & Kim, D.-K. (2022). Risk of Dementia According to Surgery Type: A Nationwide Cohort Study. Journal of Personalized Medicine, 12(3), 468. https://doi.org/10.3390/jpm12030468






