Vitamin D and Histological Features of Breast Cancer: Preliminary Data from an Observational Retrospective Italian Study
Abstract
:1. Introduction
2. Materials and Methods
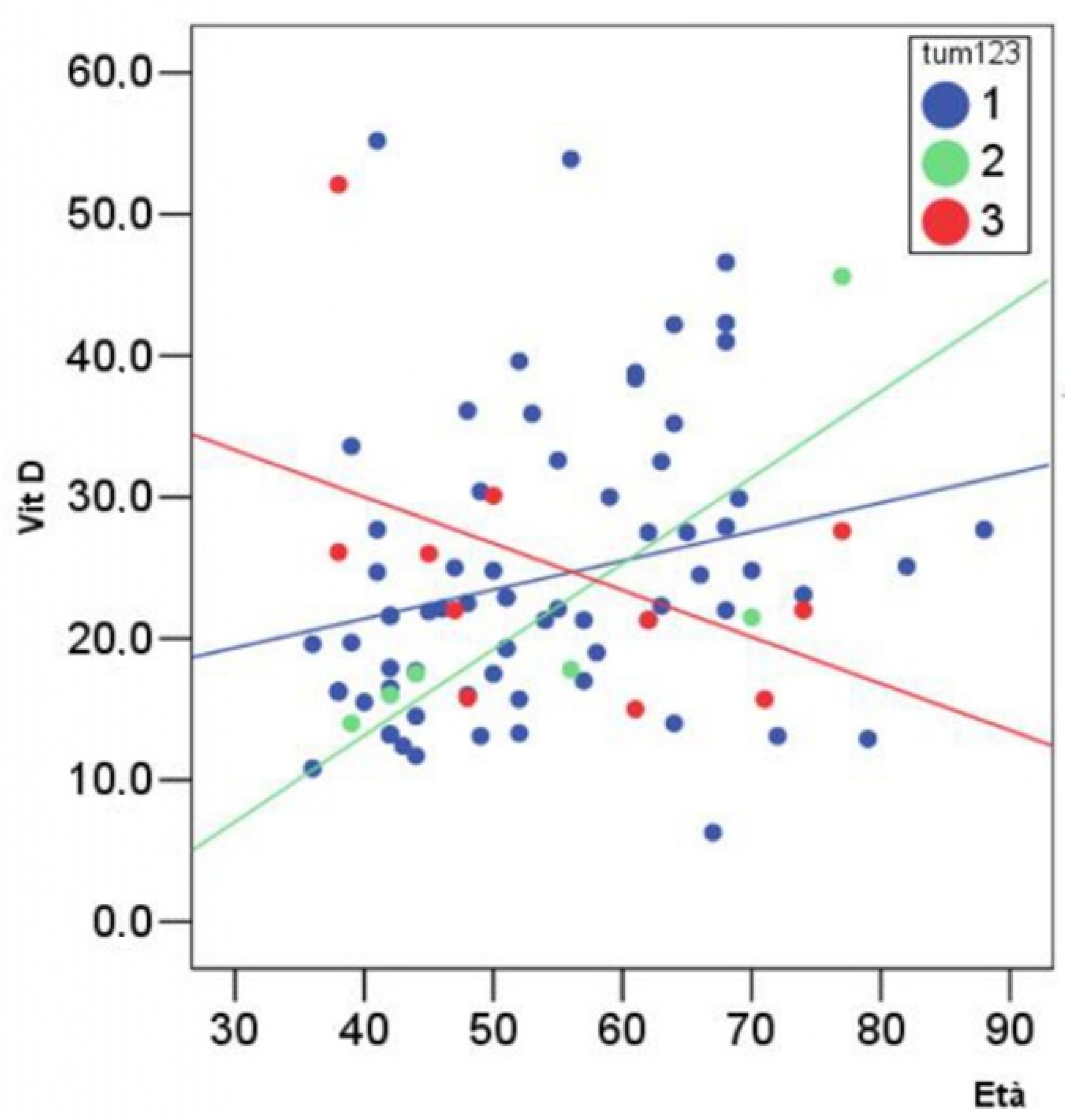
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumy, L.; Harrissart, G.; Dewaele, P.; Aljaber, A.; Bonneau, C.; Rouzier, R.; Eliès, A. Impact des régimes alimentaires sur la mortalité et le risque de récidive de cancer du sein: Revue de la littérature [Impact of nutrition on breast cancer mortality and risk of recurrence, a review of the evidence]. Bull. Cancer 2020, 107, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930, Erratum in J. Clin. Endocrinol. Metab. 2011, 96, 3908. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [Green Version]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open. 2020, 3, e2025850, Erratum in JAMA Netw. Open. 2020, 3, e2032460. [Google Scholar] [CrossRef]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1592–1599. [Google Scholar]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Puente-Yagüe, M.; Cuadrado-Cenzual, M.A.; Ciudad-Cabañas, M.J.; Hernández-Cabria, M.; Collado-Yurrita, L. Vitamin D: And its role in breast cancer. Kaohsiung J. Med. Sci. 2018, 34, 423–427. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Abbas, S.; Chang-Claude, J.; Linseisen, J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int. J. Cancer 2009, 124, 250–255. [Google Scholar] [CrossRef]
- Brown, R.B. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine 2019, 65, 238–243. [Google Scholar] [CrossRef]
- Scaranti, M.; Júnior Gde, C.; Hoff, A.O. Vitamin D and cancer: Does it really matter? Curr. Opin. Oncol. 2016, 28, 205–209. [Google Scholar] [CrossRef]
- Alipour, S.; Hadji, M.; Hosseini, L.; Omranipour, R.; Saberi, A.; Seifollahi, A.; Bayani, L.; Shirzad, N. Levels of serum 25-hydroxy-vitamin d in benign and malignant breast masses. Asian Pac. J. Cancer Prev. 2014, 15, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Ward, E.M.; DeSantis, C.E.; Lin, C.C.; Kramer, J.L.; Jemal, A.; Kohler, B.; Brawley, O.W.; Gansler, T. Cancer statistics: Breast cancer in situ. CA Cancer J. Clin. 2015, 65, 481–495. [Google Scholar] [CrossRef]
- Dossus, L.; Benusiglio, P.R. Lobular breast cancer: Incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015, 17, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppone, L.J.; Rickles, A.S.; Janelsins, M.C.; Insalaco, M.R.; Skinner, K.A. The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann. Surg. Oncol. 2012, 19, 2590–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsadakis, I.A. Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef]
- Peila, R.; Xue, X.; Cauley, J.A.; Chlebowski, R.; Manson, J.E.; Nassir, R.; Saquib, N.; Shadyab, A.H.; Zhang, Z.; Wassertheil-Smoller, S.; et al. A Randomized Trial of Calcium Plus Vitamin D Supplementation and Risk of Ductal Carcinoma In Situ of the Breast. JNCI Cancer Spectr. 2021, 5, pkab072. [Google Scholar] [CrossRef]
- Gissel, T.; Rejnmark, L.; Mosekilde, L.; Vestergaard, P. Intake of vitamin D and risk of breast cancer–A me-ta-analysis. J. Steroid Biochem. Mol. Biol. 2008, 111, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Gonzalez, F.J. The Evolution of Carcinogenesis. Toxicol. Sci. 2018, 165, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef]
- Verma, A.; Schwartz, Z.; Boyan, B.D. 24R,25-dihydroxyvitamin D3 modulates tumorigenicity in breast cancer in an estrogen receptor-dependent manner. Steroids 2019, 150, 108447. [Google Scholar] [CrossRef]
| Tum | n | Mean | SD | Min | Max | ANOVA | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 70 | 54.2 | 12.0 | 36 | 88 | Between Groups | 16,861 | 2 | 8430 | 0.054 | 0.948 |
| 2 | 6 | 54.7 | 15.8 | 39 | 77 | Within Groups | 13,218,403 | 84 | 157,362 | |||
| 3 | 11 | 55.6 | 14.1 | 38 | 77 | Total | 13,235,264 | 86 | ||||
| Total | 87 | 54.4 | 12.4 | 36 | 88 | |||||||
| Vit D | 1 | 70 | 24.3 | 10.2 | 6.3 | 55.2 | Between Groups | 33,251 | 2 | 16,625 | 0.155 | 0.856 |
| 2 | 6 | 22.1 | 11.8 | 14.0 | 45.6 | Within Groups | 8,984,555 | 84 | 106,959 | |||
| 3 | 11 | 24.9 | 10.4 | 15.0 | 52.1 | Total | 9,017,806 | 86 | ||||
| Total | 87 | 24.2 | 10.2 | 6.3 | 55.2 | |||||||
| yT | 1 | 70 | 13.6 | 16.0 | 0 | 105 | Between Groups | 1,034,492 | 2 | 517,246 | 2.094 | 0.130 |
| (mm) | 2 | 6 | 14.0 | 13.0 | 1 | 35 | Within Groups | 20,752,675 | 84 | 247,056 | ||
| 3 | 11 | 24.0 | 15.3 | 4 | 60 | Total | 21,787,167 | 86 | ||||
| Total | 87 | 14.9 | 15.9 | 0 | 105 | |||||||
| yN | 1 | 66 | 0.5 | 0.9 | 0 | 3 | Between Groups | 1184 | 2 | 0.592 | 0.843 | 0.435 |
| 2 | 3 | 0.0 | 0.0 | 0 | 0 | Within Groups | 51,985 | 74 | 0.702 | |||
| 3 | 8 | 0.3 | 0.7 | 0 | 2 | Total | 53,169 | 76 | ||||
| Total | 77 | 0.5 | 0.8 | 0 | 3 | |||||||
| ER | 1 | 70 | 57.8 | 40.7 | 0 | 100 | Between Groups | 5,156,117 | 2 | 2,578,058 | 1.687 | 0.191 |
| 2 | 6 | 58.5 | 37.7 | 1 | 90 | Within Groups | 128,387,286 | 84 | 1,528,420 | |||
| 3 | 11 | 81.0 | 26.8 | 1 | 95 | Total | 133,543,402 | 86 | ||||
| Total | 87 | 60.8 | 39.4 | 0 | 100 | |||||||
| PR | 1 | 70 | 28.9 | 37.6 | 0 | 98 | Between Groups | 7,798,377 | 2 | 3,899,189 | 2.677 | 0.075 |
| 2 | 6 | 44.3 | 48.2 | 0 | 90 | Within Groups | 122,346,542 | 84 | 1,456,506 | |||
| 3 | 11 | 56.1 | 36.4 | 1 | 90 | Total | 130,144,920 | 86 | ||||
| Total | 87 | 33.4 | 38.9 | 0 | 98 | |||||||
| Ki67 | 1 | 70 | 34.5 | 25.7 | 1 | 80 | Between Groups | 3,001,866 | 2 | 1,500,933 | 2.552 | 0.084 |
| 2 | 1 | 20.0 | . | 20 | 20 | Within Groups | 46,465,122 | 79 | 588,166 | |||
| 3 | 11 | 17.2 | 9.7 | 5 | 35 | Total | 49,466,988 | 81 | ||||
| Total | 82 | 32.0 | 24.7 | 1 | 80 | |||||||
| HER2 | 1 | 70 | 1.4 | 1.1 | 0 | 3 | Between Groups | 5001 | 2 | 2500 | 1.964 | 0.147 |
| 2 | 6 | 1.5 | 1.4 | 0 | 3 | Within Groups | 106,953 | 84 | 1273 | |||
| 3 | 11 | 0.7 | 0.9 | 0 | 2 | Total | 111,954 | 86 | ||||
| Total | 87 | 1.4 | 1.1 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lello, S.; Capozzi, A.; Scardina, L.; Ionta, L.; Sorge, R.; Scambia, G.; Franceschini, G. Vitamin D and Histological Features of Breast Cancer: Preliminary Data from an Observational Retrospective Italian Study. J. Pers. Med. 2022, 12, 465. https://doi.org/10.3390/jpm12030465
Lello S, Capozzi A, Scardina L, Ionta L, Sorge R, Scambia G, Franceschini G. Vitamin D and Histological Features of Breast Cancer: Preliminary Data from an Observational Retrospective Italian Study. Journal of Personalized Medicine. 2022; 12(3):465. https://doi.org/10.3390/jpm12030465
Chicago/Turabian StyleLello, Stefano, Anna Capozzi, Lorenzo Scardina, Lucia Ionta, Roberto Sorge, Giovanni Scambia, and Gianluca Franceschini. 2022. "Vitamin D and Histological Features of Breast Cancer: Preliminary Data from an Observational Retrospective Italian Study" Journal of Personalized Medicine 12, no. 3: 465. https://doi.org/10.3390/jpm12030465
APA StyleLello, S., Capozzi, A., Scardina, L., Ionta, L., Sorge, R., Scambia, G., & Franceschini, G. (2022). Vitamin D and Histological Features of Breast Cancer: Preliminary Data from an Observational Retrospective Italian Study. Journal of Personalized Medicine, 12(3), 465. https://doi.org/10.3390/jpm12030465








