Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measures
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 2006, 63, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M. Depressive symptoms with cognitive dysfunction increase the risk of cognitive impairment: Analysis of the Korean Longitudinal Study of Aging (KLoSA), 2006–2018. Int. Psychogeriatr. 2021, 33, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol 2011, 7, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, W.D.; Aizenstein, H.J.; Alexopoulo, G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry 2013, 18, 963–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieguez, S.; Staub, F.; Bruggimann, L.; Bogousslavsky, J. Is poststroke depression a vascular depression? J. Neurol. Sci. 2004, 226, 53–58. [Google Scholar] [CrossRef]
- Vinciguerra, L.; Lanza, G.; Puglisi, V.; Fisicaro, F.; Pennisi, M.; Bella, R.; Cantone, M. Update on the neurobiology of vascular cognitive impairment: From lab to clinic. Int. J. Mol. Sci. 2020, 21, 2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorca, G.E.; Castilla-Guerra, L.; Moreno, M.C.F.; Doblado, S.R.; Hernández, M.D.J. Post-stroke depression: An update. Neurol. Engl. Ed. 2015, 30, 23–31. [Google Scholar]
- Bartoli, F.; Di Brita, C.; Crocamo, C.; Clerici, M.; Carrà, G. Early post-stroke depression and mortality: Meta-analysis and meta-regression. Front. Psychiatry 2018, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Ayerbe, L.; Ayis, S.; Crichton, S.L.; Rudd, A.G. Wolfe CDA Explanatory factors for the increased mortality of stroke patients with depression. Neurology 2014, 83, 2007–2012. [Google Scholar] [CrossRef] [Green Version]
- Paolucci, S.; Iosa, M.; Coiro, P.; Venturiero, V.; Savo, A.; De Angelis, D.; Morone, G. Post-stroke depression increases disability more than 15% in ischemic stroke survivors: A case-control study. Front. Neurol. 2019, 10, 926. [Google Scholar] [CrossRef] [Green Version]
- Newberg, A.R.; Davydow, D.S.; Lee, H.B. Cerebrovascular disease basis of depression: Post-stroke depression and vascular depression. Int. Rev. Psychiatry 2006, 18, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Jorge, R.E. Post-stroke depression: A review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, R.E.; Acion, L.; Moser, D.; Adams, H.P.; Robinson, R.G. Escitalopram and enhancement of cognitive recovery following stroke. Arch. Gen. Psychiatry 2010, 67, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Narushima, K.; Robinson, R.G. The effect of early versus late antidepressant treatment on physical impairment associated with poststroke depression: Is there a time-related therapeutic window? J. Nerv. Ment. Dis. 2003, 191, 645–652. [Google Scholar] [CrossRef]
- Withers, H.; Plumbley-Jones, J.; Pyatt, E.; Williams, L.; Yule, L.; Kyte, D. The effectiveness of cognitive behavioural therapy versus antidepressants for treatment of post-stroke depression in adults. Br. Stud. Dr. J. 2021, 5, 5–17. [Google Scholar] [CrossRef]
- Chang, K.J.; Hong, C.H.; Roh, H.W.; Lee, K.S.; Lee, E.H.; Kim, J.; Lim, H.K.; Son, S.J. A 12-week multi-domain lifestyle modification to reduce depressive symptoms in older adults: A preliminary report. Psychiatry Investig. 2018, 15, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Mayman, N.A.; Tuhrim, S.; Jette, N.; Dhamoon, M.S.; Stein, L.K. Sex differences in post-stroke depression in the elderly. J. Stroke Cereb. Dis. 2021, 30, 105948. [Google Scholar] [CrossRef]
- Chang, W.H.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.G.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Han, E.Y.; et al. Korean Stroke Cohort for functioning and rehabilitation (KOSCO): Study rationale and protocol of a multi-centre prospective cohort study. BMC Neurol. 2015, 15, 42. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Kang, Y. A normative study of the Korean Mini-Mental State Examination (K-MMSE) in the elderly. Korean J. Psychol. 2006, 25, 1–12. [Google Scholar]
- Bae, J.N.; Cho, M.J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef]
- Mijajlović, M.D.; Pavlović, A.; Brainin, M.; Heiss, W.D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A. Post-stroke dementia–a comprehensive review. BMC Med. 2017, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.G.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Han, E.Y.; et al. Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke: The KOSCO study. Stroke 2020, 51, 99–107. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Rasquin, S.; Lodder, J.; Verhey, F. The association between psychiatric and cognitive symptoms after stroke: A prospective study. Cereb. Dis. 2005, 19, 309–316. [Google Scholar] [CrossRef]
- Lugtenburg, A.; Oude Voshaar, R.C.; Van Zelst, W.; Schoevers, R.A.; Enriquez-Geppert, S.; Zuidersma, M. The relationship between depression and executive function and the impact of vascular disease burden in younger and older adults. Age Ageing 2017, 46, 697–701. [Google Scholar] [CrossRef] [Green Version]
- Mahncke, H.W.; Bronstone, A.; Merzenich, M.M. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog. Brain Res. 2006, 157, 81–109. [Google Scholar]
- Narasimhalu, K.; Lee, J.; Leong, Y.-L.; Ma, L.; De Silva, D.A.; Wong, M.C.; Chang, H.M.; Chen, C. Inflammatory markers and their association with post stroke cognitive decline. Int. J. Stroke 2015, 10, 513–518. [Google Scholar] [CrossRef]
- Castilla-Guerra, L.; Fernandez Moreno, M.d.C.; Esparrago-Llorca, G.; Colmenero-Camacho, M.A. Pharmacological management of post-stroke depression. Expert Rev. Neurother 2020, 20, 157–166. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kong, W.; Li, X.; Hu, B. Abnormal core functional connectivity on the pathology of MDD and antidepressant treatment: A systematic review. J. Affect. Disord. 2022, 296, 622–634. [Google Scholar] [CrossRef]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef]

| Younger Adults (<65 Years), n = 1846 | Older Adults (≥65 Years), n = 1369 | |||||
|---|---|---|---|---|---|---|
| Variables | No-PSD | PSD | t/x2 | No-PSD | PSD | t/x2 |
| n | 1438 | 408 | N/A | 931 | 438 | N/A |
| Age, years | 51.83 (9.0) | 52.87 (8.9) | −2.05 * | 72.99 (5.6) | 73.91 (5.9) | −2.78 ** |
| Female sex | 438 (30.5) | 143 (35.0) | 3.11 | 351(37.7) | 214 (48.9) | 15.30 *** |
| Limited education (<9 years) | 131 (9.1) | 56 (13.7) | 7.44 ** | 387 (41.6) | 233 (53.2) | 16.26 *** |
| Initial NIHSS | 4.00 (5.4) | 3.58 (4.9) | 1.45 | 4.05 (5.5) | 3.47 (5.0) | 1.96 |
| Ischemic type | 1082 (75.2) | 295 (72.3) | 1.45 | 837 (89.9) | 392 (89.5) | 0.05 |
| Hypertension | 634 (44.1) | 170 (41.7) | 0.76 | 614 (66.0) | 284 (64.8) | 0.16 |
| Diabetes mellitus | 250 (17.4) | 81 (19.9) | 1.32 | 266 (28.6) | 121 (27.6) | 0.13 |
| Coronary heart disease | 65 (4.5) | 10 (2.5) | 3.49 | 91 (9.8) | 38 (8.7) | 0.42 |
| Atrial fibrillation | 65 (4.5) | 25 (6.1) | 1.77 | 126 (13.5) | 55 (12.6) | 0.25 |
| Left ventricular hypertrophy | 17 (1.2) | 4 (1.0) | 0.12 | 7 (0.8) | 6 (1.4) | 1.20 |
| Peripheral artery disease | 5 (0.3) | 1 (0.2) | 0.10 | 9 (1.0) | 4 (0.9) | 0.01 |
| Hyperlipidemia | 210 (14.6) | 58 (14.2) | 0.04 | 154 (16.5) | 69 (15.8) | 0.14 |
| Low cholesterol | 39 (2.7) | 17 (4.2) | 2.29 | 38 (4.1) | 15 (3.4) | 0.35 |
| Unruptured intracranial aneurysm | 21 (1.5) | 7 (1.7) | 0.14 | 9 (1.0) | 6 (1.4) | 0.45 |
| Arteriovenous malformation | 5 (0.3) | 2 (0.5) | 0.17 | 3 (0.3) | 1 (0.2) | 0.09 |
| Moyamoya disease | 14 (1.0) | 4 (1.0) | 0.00 | 2 (0.2) | 1 (0.2) | 0.00 |
| Obesity | 214 (14.9) | 50 (12.3) | 1.79 | 118 (12.7) | 54 (12.3) | 0.03 |
| Smoking | 699 (48.6) | 202 (49.5) | 0.10 | 296 (31.8) | 138 (31.5) | 0.01 |
| Alcohol consumption | 775 (53.9) | 207 (50.7) | 1.27 | 317 (34.0) | 132 (30.1) | 2.07 |
| K-GDS-SF at 3 months | 3.03 (2.0) | 10.65 (2.2) | −63.92 *** | 3.50 (2.1) | 10.75 (2.3) | −56.07 *** |
| K-MMSE at 3 months | 28.87 (1.4) | 28.44 (1.5) | 5.12 *** | 26.90 (2.8) | 25.29 (3.6) | 8.33 *** |
| Younger Adults (<65 Years), n = 1846 | Older Adults (≥65 Years), n = 1369 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Months | Groups | Normal | Cognitive Decline | Censored | Total | Normal | Cognitive Decline | Censored | Total |
| 3 | No-PSD | 1438 (100) | 0 (0) | 0 (0) | 1438 (100) | 931 (100) | 0 (0) | 0 (0) | 931 (100) |
| PSD | 408 (100) | 0 (0) | 0 (0) | 408 (100) | 438 (100) | 0 (0) | 0 (0) | 438 (100) | |
| Total | 1846 (100) | 0 (0) | 0 (0) | 1846 (100) | 1369 (100) | 0 (0) | 0 (0) | 1369 (100) | |
| 6 | No-PSD | 1250 (86.9) | 5 (0.3) | 183 (12.7) | 1438 (100) | 802 (86.1) | 7 (0.8) | 122 (13.1) | 931 (100) |
| PSD | 347 (85.0) | 5 (1.2) | 56 (13.7) | 408 (100) | 363 (82.9) | 9 (2.1) | 66 (15.1) | 438 (100) | |
| Total | 1597 (86.5) | 10 (0.5) | 239 (12.9) | 1846 (100) | 1165 (85.1) | 16 (1.2) | 188 (13.7) | 1369 (100) | |
| 12 | No-PSD | 1099 (87.9) | 5 (0.4) | 146 (11.7) | 1250 (100) | 690 (86.0) | 2 (0.2) | 110 (13.7) | 802 (100) |
| PSD | 307 (88.5) | 0 (0.0) | 40 (11.5) | 347 (100) | 289 (79.6) | 7 (1.9) | 67 (18.5) | 363 (100) | |
| Total | 1406 (88.0) | 5 (0.3) | 186 (11.6) | 1597 (100) | 979 (84.0) | 9 (0.8) | 177 (15.2) | 1165 (100) | |
| 18 | No-PSD | 993 (90.4) | 4 (0.4) | 102 (9.3) | 1099 (100) | 624 (90.4) | 4 (0.6) | 62 (9.0) | 690 (100) |
| PSD | 269 (87.6) | 1 (0.3) | 37 (12.1) | 307 (100) | 242 (83.7) | 6 (2.1) | 41 (14.2) | 289 (100) | |
| Total | 1262 (89.8) | 5 (0.4) | 139 (9.9) | 1406 (100) | 866 (88.5) | 10 (1.0) | 103 (10.5) | 979 (100) | |
| 24 | No-PSD | 902 (90.8) | 4 (0.4) | 87 (8.8) | 993 (100) | 579 (92.8) | 7 (1.1) | 38 (6.1) | 624 (100) |
| PSD | 249 (92.6) | 1 (0.4) | 19 (7.1) | 269 (100) | 214 (88.4) | 6 (2.5) | 22 (9.1) | 242 (100) | |
| Total | 1151 (91.2) | 5 (0.4) | 106 (8.4) | 1262 (100) | 793 (91.6) | 13 (1.5) | 60 (6.9) | 866 (100) | |
| 30 | No-PSD | 838 (92.9) | 2 (0.2) | 62 (6.9) | 902 (100) | 529 (91.4) | 9 (1.6) | 41 (7.1) | 579 (100) |
| PSD | 222 (89.2) | 0 (0.0) | 27 (10.8) | 249 (100) | 193 (90.2) | 2 (0.9) | 19 (8.9) | 214 (100) | |
| Total | 1060 (92.1) | 2 (0.2) | 89 (7.7) | 1151 (100) | 722 (91.0) | 11 (1.4) | 60 (7.6) | 793 (100) | |
| 36 | No-PSD | 783 (93.4) | 4 (0.5) | 51 (6.1) | 838 (100) | 494 (93.4) | 3 (0.6) | 32 (6.0) | 529 (100) |
| PSD | 211 (95.0) | 1 (0.5) | 10 (4.5) | 222 (100) | 175 (90.7) | 2 (1.0) | 16 (8.3) | 193 (100) | |
| Total | 994 (93.8) | 5 (0.5) | 61 (5.8) | 1060 (100) | 669 (92.7) | 5 (0.7) | 48 (6.6) | 722 (100) | |
| 48 | No-PSD | 751 (95.9) | 3 (0.4) | 29 (3.7) | 783 (100) | 445 (90.1) | 4 (0.8) | 45 (9.1) | 494 (100) |
| PSD | 197 (93.4) | 2 (0.9) | 12 (5.7) | 211 (100) | 155 (88.6) | 4 (2.3) | 16 (9.1) | 175 (100) | |
| Total | 948 (95.4) | 5 (0.5) | 41 (4.1) | 994 (100) | 600 (89.7) | 8 (1.2) | 61 (9.1) | 669 (100) | |
| 60 | No-PSD | 706 (94.0) | 4 (0.5) | 41 (5.5) | 751 (100) | 401(90.1) | 1(0.2) | 43(9.7) | 445(100) |
| PSD | 187 (94.9) | 1 (0.5) | 9 (4.6) | 197 (100) | 138(89.0) | 2(1.3) | 15(9.7) | 155(100) | |
| Total | 893 (94.2) | 5 (0.5) | 50 (5.3) | 948 (100) | 539(89.8) | 3(0.5) | 58(9.7) | 600(100) | |
| Total | No-PSD | 706 (49.1) | 31 (2.1) | 701 (48.7) | 1438 (100) | 401 (43.1) | 37 (4.0) | 493 (53.0) | 931 (100) |
| PSD | 187 (45.8) | 11 (2.7) | 210 (51.5) | 408 (100) | 138 (31.5) | 38 (8.7) | 262 (59.8) | 438 (100) | |
| Total | 893 (48.4) | 42 (2.3) | 911 (49.3) | 1846 (100) | 539 (39.4) | 75 (5.5) | 755 (55.1) | 1369 (100) | |
| Younger Adults (<65 Years), n = 1845 | Older Adults (≥65 Years), n = 1366 | |||
|---|---|---|---|---|
| Variables | Estimate (SE) | Hazard Ratio (95% CI) | Estimate (SE) | Hazard Ratio (95% CI) |
| Age | 0.40 (0.03) | 1.04 (0.99–1.09) | 0.00 (0.02) | 1.00 (0.96–1.05) |
| Limited education (<9 years) | 0.72 (0.39) | 2.04 (0.95–4.40) | −0.53 (0.30) | 0.59 (0.33–1.06) |
| Female sex | −0.25 (0.34) | 0.78 (0.40–1.53) | 0.30 (0.26) | 1.35 (0.81–2.26) |
| K-MMSE at 3 months | −0.31 (0.09) *** | 0.73 (0.61–0.88) | −0.13 (0.04) ** | 0.88 (0.81–0.95) |
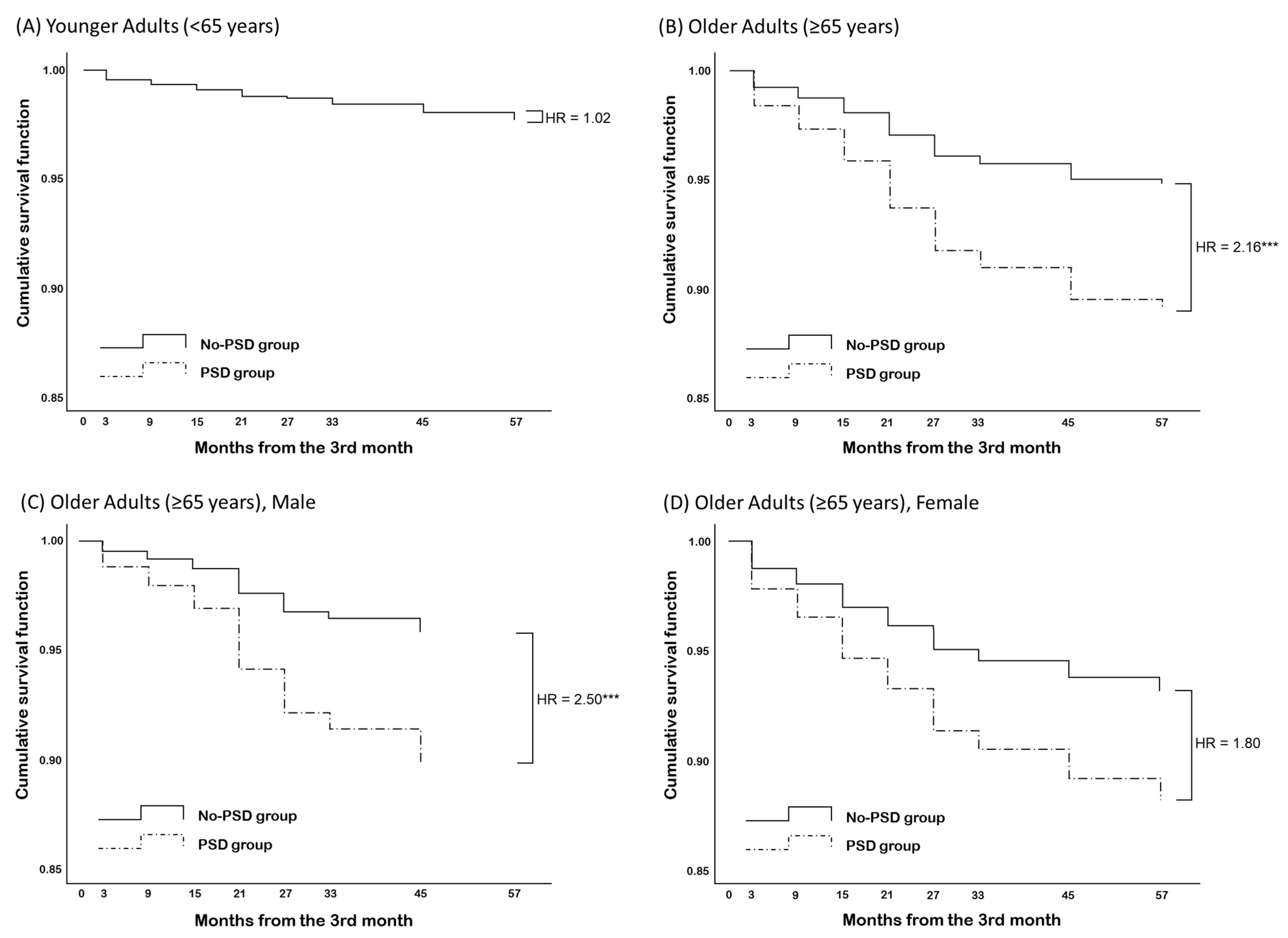
| PSD | 0.19 (0.36) | 1.02 (0.50–2.07) | 0.77 (0.25) ** | 2.16 (1.34–3.50) |
| Male, n = 803 | Female, n = 563 | |||
|---|---|---|---|---|
| Variables | Estimate (SE) | Hazard Ratio (95% CI) | Estimate (SE) | Hazard Ratio (95% CI) |
| Age | 0.04 (0.03) | 1.04 (0.97–1.11) | −0.03 (0.03) | 0.97 (0.91–1.03) |
| Limited education (<9 years) | −0.66 (0.44) | 0.52 (0.22–1.22) | −0.49 (0.42) | 0.61 (0.27–1.39) |
| K-MMSE at 3 months | −1.15 (0.07) * | 0.86 (0.75–1.00) | −0.16 (0.05) * | 0.86 (0.77–0.95) |
| K-GDS-SF, factor 1 | 0.29 (0.11) ** | 1.34 (1.09–1.65) | 0.05 (0.11) | 1.05 (0.86–1.29) |
| K-GDS-SF, factor 2 | 0.05 (0.17) | 1.05 (0.75–1.47) | 0.32 (0.17) | 1.37 (0.98–1.92) |
| K-GDS-SF, factor 3 | −0.23 (0.20) | 0.80 (0.54–1.17) | −0.33 (0.19) | 0.72 (0.49–1.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Shin, Y.-I.; Oh, G.-J.; Lee, Y.-S.; Joo, M.C.; Lee, S.Y.; Song, M.-K.; et al. Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study. J. Pers. Med. 2022, 12, 389. https://doi.org/10.3390/jpm12030389
Shin M, Sohn MK, Lee J, Kim DY, Shin Y-I, Oh G-J, Lee Y-S, Joo MC, Lee SY, Song M-K, et al. Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study. Journal of Personalized Medicine. 2022; 12(3):389. https://doi.org/10.3390/jpm12030389
Chicago/Turabian StyleShin, Minyoung, Min Kyun Sohn, Jongmin Lee, Deog Young Kim, Yong-Il Shin, Gyung-Jae Oh, Yang-Soo Lee, Min Cheol Joo, So Young Lee, Min-Keun Song, and et al. 2022. "Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study" Journal of Personalized Medicine 12, no. 3: 389. https://doi.org/10.3390/jpm12030389
APA StyleShin, M., Sohn, M. K., Lee, J., Kim, D. Y., Shin, Y.-I., Oh, G.-J., Lee, Y.-S., Joo, M. C., Lee, S. Y., Song, M.-K., Han, J., Ahn, J., Lee, Y.-H., Chang, W. H., Shin, S., Choi, S. M., Lee, S. K., & Kim, Y.-H. (2022). Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study. Journal of Personalized Medicine, 12(3), 389. https://doi.org/10.3390/jpm12030389









