Inflection-Point Nutrition Support Determined by Oral Mucosal Apoptosis Rate Is a Novel Assessment Strategy for Personalized Nutrition: A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
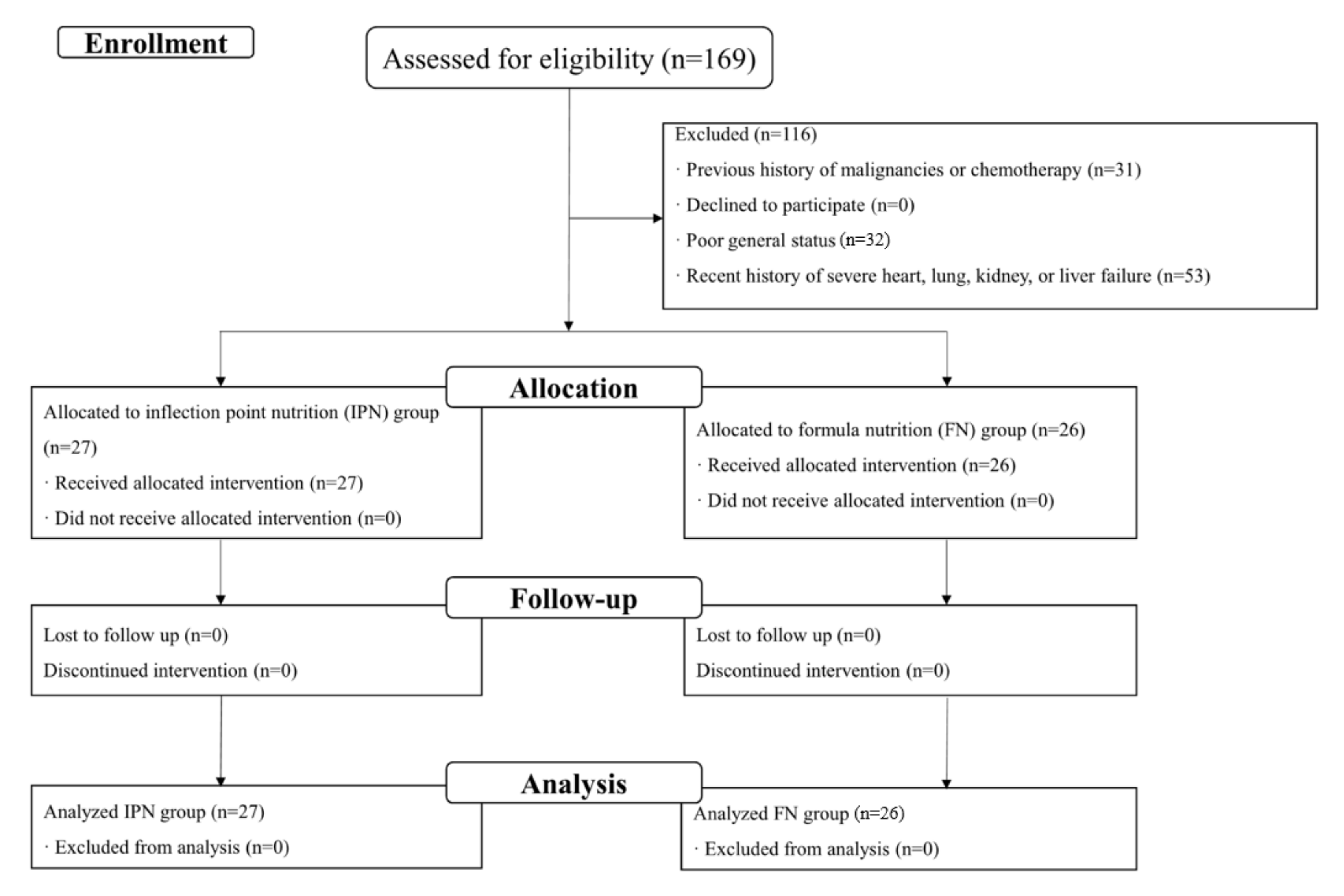
2.1. Study Design and Participants
2.2. Allocation of Patients
2.3. Monitoring of Apoptosis Rate of EOMECs
2.4. Clinical Management and Nutritional Support
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Included Patients
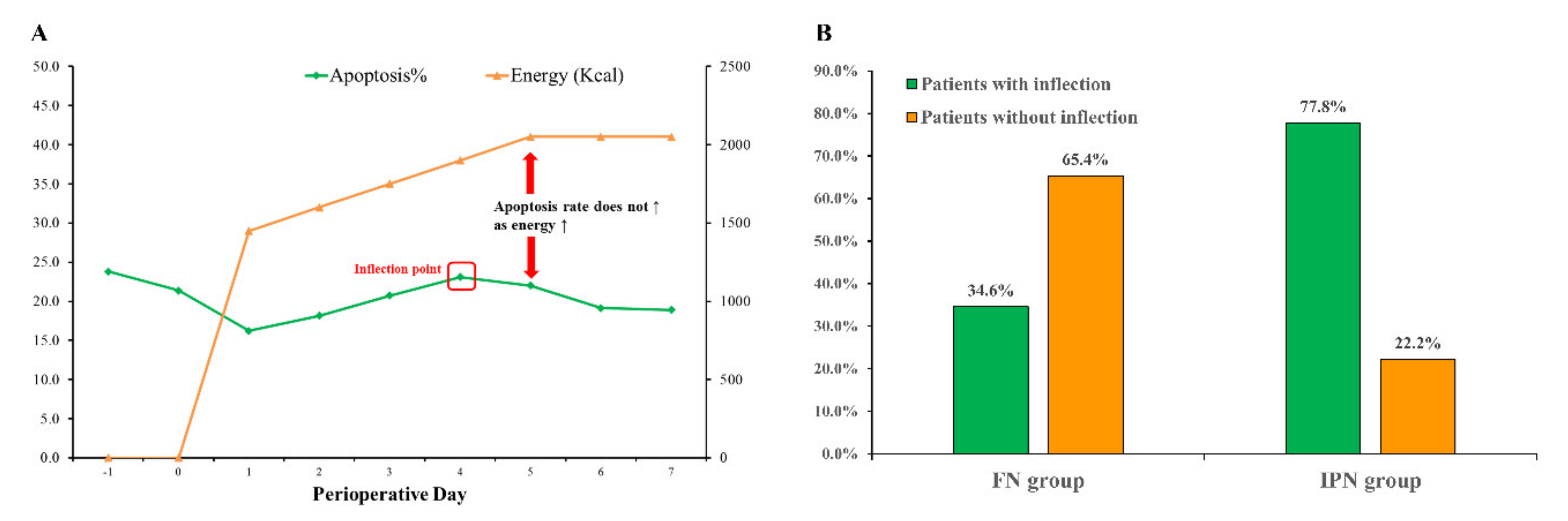
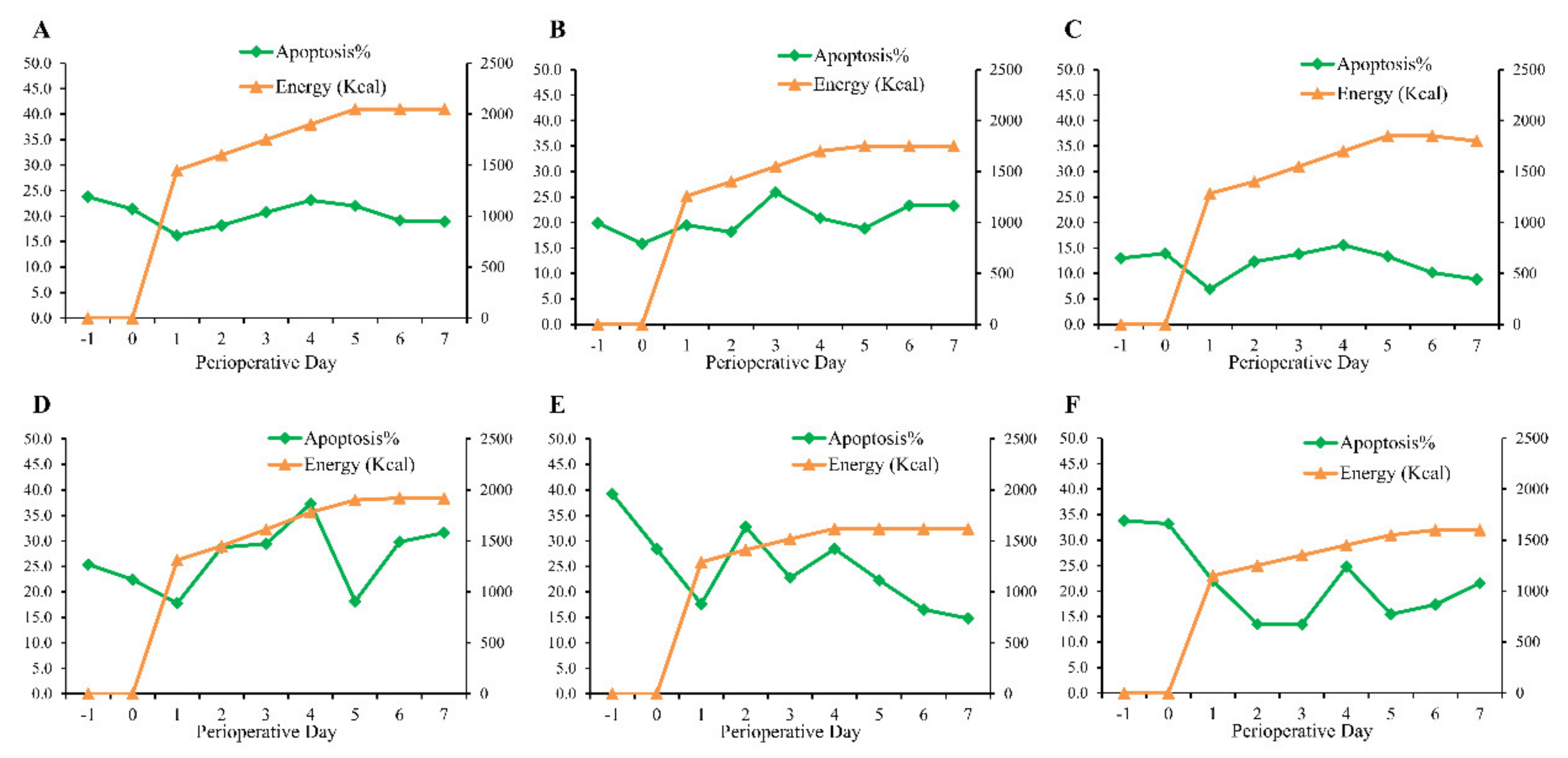
3.2. Prevalence and Description of Inflection-Point Phenomenon
3.3. Predictive Factors Associated with Inflection Phenomenon
3.4. Inflection-Point Nutrition Supply and Postoperative Recovery
4. Discussion
5. Clinical Implication and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Fuse, N.; Bando, H.; Chin, K.; Ito, S.; Yoshikawa, T.; Tsuburaya, A.; Tsuburaya, A.; Terashima, M.; Kawashima, Y.; Fukunaga, T.; et al. Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: A phase II study. Gastric Cancer 2017, 20, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Gastric Cancer Version 3.2020. Available online: https://www.nccn.org/ (accessed on 9 January 2022).
- Baiocchi, G.L.; Giacopuzzi, S.; Reim, D.; Piessen, G.; Costa, P.M.; Reynolds, J.V.; Meyer, H.J.; Morgagni, P.; Gockel, I.; Santos, L.L.; et al. Incidence and Grading of Complications After Gastrectomy for Cancer Using the GASTRODATA Registry: A European Retrospective Observational Study. Ann. Surg. 2020, 272, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, F.J.F.; de Jesus, V.H.F.; Franco, C.P.; Calsavara, V.F.; Ribeiro, H.S.C.; Diniz, A.L.; de Godoy, A.L.; de Farias, I.C.; Riechelmann, R.P.; Begnami, M.D.F.; et al. Predicting overall and major postoperative morbidity in gastric cancer patients. J. Surg. Oncol. 2019, 120, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Miyake, M.; Hama, N.; Miyamoto, A.; Ikeda, M.; et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann. Surg. Oncol. 2015, 22, S778–S785. [Google Scholar] [CrossRef]
- Kanda, M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg. Today 2020, 50, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Schiesser, M.; Kirchhoff, P.; Müller, M.K.; Schäfer, M.; Clavien, P.A. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery 2009, 145, 519–526. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K.; Kondrup, J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Aoyama, T.; Yoshikawa, T.; Sato, T.; Hayashi, T.; Yamada, T.; Ogata, T.; Cho, H. Equivalent feasibility and safety of perioperative care by ERAS in open and laparoscopy-assisted distal gastrectomy for gastric cancer: A single-institution ancillary study using the patient cohort enrolled in the JCOG0912 phase III trial. Gastric Cancer 2019, 22, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Lee, Y.; Min, S.H.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.-H. Multimodal Enhanced Recovery After Surgery (ERAS) Program is the Optimal Perioperative Care in Patients Undergoing Totally Laparoscopic Distal Gastrectomy for Gastric Cancer: A Prospective, Randomized, Clinical Trial. Ann. Surg. Oncol. 2018, 25, 3231–3238. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Correia, M.I. Nutritional assessment in the hospitalized patient. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 531–538. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Muth, E.R.; Rowe, W.A. The Harris-Benedict studies of human basal metabolism: History and limitations. J. Am. Diet. Assoc. 1998, 98, 439–445. [Google Scholar] [CrossRef]
- Boullata, J.; Williams, J.; Cottrell, F.; Hudson, L.; Compher, C. Accurate determination of energy needs in hospitalized patients. J. Am. Diet. Assoc. 2007, 107, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, Y.; Tao, D.; Yu, Y.; Hu, J.; Qiu, F.; Kulkarni, H.; Gong, J. Usefulness of oral mucosal epithelial cell apoptosis rate in nutritional assessment. Nutrition 2006, 22, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011, 142, 113–123. [Google Scholar]
- Agha, R.A.; Borrelli, M.R.; Vella-Baldacchino, M.; Thavayogan, R.; Orgill, D.P.; STROCSS Group. The STROCSS statement: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2017, 46, 198–202. [Google Scholar] [CrossRef]
- Gao, C.; Hasan, O.; Wei, X.; Zou, Y.; Yin, X.; Tao, D.; Gong, J. Assessment of nutritional status of clinical patients by determining normal range of oral mucosal apoptosis and proliferation rate. J. Huazhong Univ. Sci. Technol. 2012, 32, 680–685. [Google Scholar] [CrossRef]
- Chinese Society for Parenteral and Enteral Nutrition; Enhanced Recovery After Surgery Committee of China Medicine Education Association. Chinese expert consensus on perioperative nutritional support in enhanced recovery after surgery (2019 edition). Chin. J. Dig. Surg. 2019, 18, 897–902. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuta, A.; Saito, T.; Murata, S.; Makiura, D.; Inoue, J.; Okumura, M.; Sakai, Y.; Ono, R. Impact of preoperative cachexia on postoperative length of stay in elderly patients with gastrointestinal cancer. Nutrition 2019, 58, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Gharagozlian, S.; Mala, T.; Brekke, H.K.; Kolbjørnsen, L.C.; Ullerud, Å.A.; Johnson, E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer—A cross-sectional pilot study. Clin. Nutr. ESPEN 2020, 37, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Looijaard, W.; Dekker, I.M.; Beishuizen, A.; Girbes, A.R.J.; Straaten, H.M.O.-V.; Weijs, P.J.M. Early high protein intake and mortality in critically ill ICU patients with low skeletal muscle area and -density. Clin. Nutr. 2020, 39, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Constansia, R.D.N.; Hentzen, J.E.K.R.; Hogenbirk, R.N.M.; Plas, W.Y.; Rd, M.J.E.C.; Buis, C.I.; Kruijff, S.; Klaase, J.M. Actual postoperative protein and calorie intake in patients undergoing major open abdominal cancer surgery: A prospective, observational cohort study. Nutr. Clin. Pract. 2021, 37, 183–191. [Google Scholar] [CrossRef]
- Yeung, S.E.; Hilkewich, L.; Gillis, C.; Heine, J.A.; Fenton, T.R. Protein intakes are associated with reduced length of stay: A comparison between Enhanced RecoveryAfter Surgery (ERAS) and conventional care after elective colorectal surgery. Am. J. Clin. Nutr. 2017, 106, 44–51. [Google Scholar]
- Cronk, D.R.; Ferguson, D.C.; Thompson, J.S. Malnutrition impairs postresection intestinal adaptation. JPEN J. Parenter. Enter. Nutr. 2000, 24, 76–80. [Google Scholar] [CrossRef]
- Ortiz, R.; Cortés, L.; Cortés, E.; Medina, H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin. Exp. Immunol. 2009, 155, 96–106. [Google Scholar] [CrossRef]
| Variables | All Patients (N = 53) | No inflection Phenomenon (n = 23) | Inflection Phenomenon (n = 30) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age, mean (SD), y | 56.2 ± 10.7 | 53.7 ± 11.6 | 58.2 ± 9.7 | 0.130 |
| Gender, Male/Female | 28/25 | 12/11 | 16/14 | 0.933 |
| Body mass index mean (SD), kg/m2 | 22.3 ± 2.4 | 22.9 ± 2.5 | 21.9 ± 2.2 | 0.141 |
| Smoking | ||||
| No | 58.5% (31/53) | 56.5% (13/23) | 60.0% (18/30) | 0.799 |
| Yes | 41.5% (22/53) | 43.5% (10/23) | 40.0% (12/30) | |
| Alcohol | ||||
| No | 71.7% (38/53) | 73.9% (17/23) | 70.0% (21/30) | 0.754 |
| Yes | 28.3% (15/53) | 26.1% (6/23) | 30.0% (9/30) | |
| Comorbidity | ||||
| No | 88.7% (47/53) | 91.3% (21/23) | 86.7% (26/30) | 0.597 |
| Yes | 11.3% (6/53) | 8.7% (15/23) | 13.3% (4/30) | |
| Laboratory | ||||
| Albumin (g/L), mean (SD) | 38.4 ± 3.9 | 39.3 ± 3.6 | 37.7 ± 4.1 | 0.144 |
| Prealbumin (mg/L), mean (SD) | 225.6 ± 44.2 | 214.1 ± 41.9 | 234.5 ± 44.7 | 0.095 |
| Absolute neutrophil count (* 109/L), mean (SD) | 3.2 ± 1.1 | 3.2 ± 1.1 | 3.1 ± 1.2 | 0.780 |
| Absolute lymphocyte count (* 109/L), mean (SD) | 1.9 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.5 | 0.721 |
| Hemoglobin (g/L), mean (SD) | 122.4 ± 26.5 | 123.0 ± 24.5 | 121.7 ± 28.3 | 0.870 |
| Preoperative serum CEA | ||||
| Normal (<5 ng/mL) | 88.7% (47/53) | 82.6% (19/23) | 93.3% (28/30) | 0.222 |
| Elevated (≥5 ng/mL) | 11.3% (6/53) | 17.4% (4/23) | 6.7% (2/30) | |
| Preoperative serum CA19-9 | ||||
| Normal (<37 IU/mL) | 96.2% (51/53) | 95.7% (22/23) | 96.7% (29/30) | 0.848 |
| Elevated (≥37 IU/mL) | 3.8% (2/53) | 4.3% (1/23) | 3.3% (1/30) |
| Variables | All Patients (N = 53) | No inflection Phenomenon (n = 23) | Inflection Phenomenon (n = 30) | p-Value |
|---|---|---|---|---|
| Blood loss, mL, mean (SD) | 235 ± 114 | 235 ± 114 | 222 ± 133 | 0.76 |
| Operation time, min, mean (SD) | 324 ± 50 | 330 ± 45 | 318 ± 54 | 0.39 |
| Operation | 0.70 | |||
| Distal gastrectomy | 62.3% (33/53) | 65.2% (15/23) | 60.0% (18/30) | |
| Total gastrectomy | 37.7% (20/53) | 34.8% (8/23) | 40.0% (12/30) | |
| Average energy intake, Kcal/Kg/day | 27.4 ± 3.9 | 25.7 ± 3.1 | 28.8 ± 4.1 | <0.01 * |
| TNM stage | ||||
| I/II | 41.5% (21/53) | 39.1% (9/23) | 43.3% (13/30) | 0.76 |
| III/IV | 58.5% (31/53) | 60.9% (14/23) | 56.7% (17/30) | |
| Median length of stay, day | 11 (10–12) | 11 (10–17) | 10 (9–11) | 0.04 * |
| Complications | ||||
| No | 86.8% (46/53) | 90.0% (27/340) | 83.3% (19/23) | 0.431 |
| Grade 1 & 2 | 13.2% (7/53) | 10.0% (3/30) | 17.4% (4/23) | |
| Mortality | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 1.00 |
| Inflection Phenomenon | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Variable | HR (95%CI) | p | HR (95%CI) | p |
| Age | ||||
| ≤55 y | 1 (Ref) | 1 (Ref) | ||
| >55 y | 3.75 (1.19–11.79) | 0.024 * | 6.80 (1.45–31.84) | 0.015 * |
| Gender | ||||
| Male | 1 (Ref) | N/A | ||
| Female | 1.05 (0.35–3.11) | 0.93 | ||
| Body mass index, kg/m2 | ||||
| >24 | 1 (Ref) | 1 (Ref) | ||
| ≤24 | 4.18 (1.09–16.04) | 0.037 * | 5.83 (0.89–38.01) | 0.065 * |
| Smoking | ||||
| No | 1 (Ref) | N/A | ||
| Yes | 0.87 (0.29–2.61) | 0.80 | ||
| Alcohol | ||||
| No | 1 (Ref) | N/A | ||
| Yes | 1.21 (0.38–4.09) | 0.75 | ||
| Comorbidity | N/A | |||
| No | 1 (Ref) | |||
| Yes | 1.62 (0.27–9.70) | 0.60 | ||
| Albumin | ||||
| ≥35 g/L | 1 (Ref) | NS (p = 0.902) | ||
| <35 g/L | 4.44 (1.08–18.32) | 0.04 * | ||
| Prealbumin | 1.01 (1.00–1.03) | 0.105 | N/A | |
| Hemoglobin | 1.00 (0.98–1.02) | 0.87 | N/A | |
| TNM stage | ||||
| I/II | 1 (Ref) | N/A | ||
| III/IV | 0.84 (0.28–2.54) | 0.76 | ||
| Operation time | ||||
| >300 min | 1 (Ref) | 1 (Ref) | ||
| ≤300 min | 4.55 (1.30–16.67) | 0.018 * | 7.69 (1.59–33.33) | 0.012 * |
| Blood loss | 1.00 (1.00–1.01) | 0.76 | N/A | |
| Operation | ||||
| Distal gastrectomy | 1 (Ref) | N/A | ||
| Total gastrectomy | 1.25 (0.41–3.86) | 0.70 | ||
| Average energy intake | ||||
| <25 Kcal/kg/day | 1 (Ref) | 1 (Ref) | ||
| ≥25 Kcal/kg/day | 5.96 (1.57–22.60) | <0.01 * | 5.32 (1.10–25.79) | 0.038 * |
| Complication | ||||
| No | 1 (Ref) | N/A | ||
| Yes | 0.53 (0.11–2.64) | 0.44 | ||
| Variables | Inflection-Point Nutrition Group (n = 27) | Formular Nutrition Group (n = 26) | p-Value |
|---|---|---|---|
| First flatus, h, median (IQR) | 60 (48, 72) | 68 (48, 80) | 0.56 |
| Overall complications | 3 (11.1%) | 4 (15.4%) | 0.65 |
| Fever | 0 | 1 | |
| Pulmonary infection | 0 | 1 | |
| Small amount of pleural effusion | 2 | 0 | |
| Gastroparesis | 1 | 0 | |
| Wound problem | 0 | 1 | |
| Intraluminal bleeding | 0 | 1 | |
| Total hospital stay, days, median (IQR) | 18 (16, 21) | 17 (15, 24) | 0.71 |
| Postoperative hospital stay, days, median (IQR) | 11 (10, 12) | 11 (9, 13) | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Li, Z.; Zhang, S.; Cao, D.; Yu, Y.; Zhang, Y.; Chen, H.; Fu, D.; Gong, J. Inflection-Point Nutrition Support Determined by Oral Mucosal Apoptosis Rate Is a Novel Assessment Strategy for Personalized Nutrition: A Prospective Cohort Study. J. Pers. Med. 2022, 12, 358. https://doi.org/10.3390/jpm12030358
Gao C, Li Z, Zhang S, Cao D, Yu Y, Zhang Y, Chen H, Fu D, Gong J. Inflection-Point Nutrition Support Determined by Oral Mucosal Apoptosis Rate Is a Novel Assessment Strategy for Personalized Nutrition: A Prospective Cohort Study. Journal of Personalized Medicine. 2022; 12(3):358. https://doi.org/10.3390/jpm12030358
Chicago/Turabian StyleGao, Chun, Zike Li, Sheng Zhang, Dengyi Cao, Yang Yu, Yujie Zhang, Hao Chen, Dehua Fu, and Jianping Gong. 2022. "Inflection-Point Nutrition Support Determined by Oral Mucosal Apoptosis Rate Is a Novel Assessment Strategy for Personalized Nutrition: A Prospective Cohort Study" Journal of Personalized Medicine 12, no. 3: 358. https://doi.org/10.3390/jpm12030358
APA StyleGao, C., Li, Z., Zhang, S., Cao, D., Yu, Y., Zhang, Y., Chen, H., Fu, D., & Gong, J. (2022). Inflection-Point Nutrition Support Determined by Oral Mucosal Apoptosis Rate Is a Novel Assessment Strategy for Personalized Nutrition: A Prospective Cohort Study. Journal of Personalized Medicine, 12(3), 358. https://doi.org/10.3390/jpm12030358





