Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Description and Demographic Features
2.2. Data Preparation and Analysis
3. Results
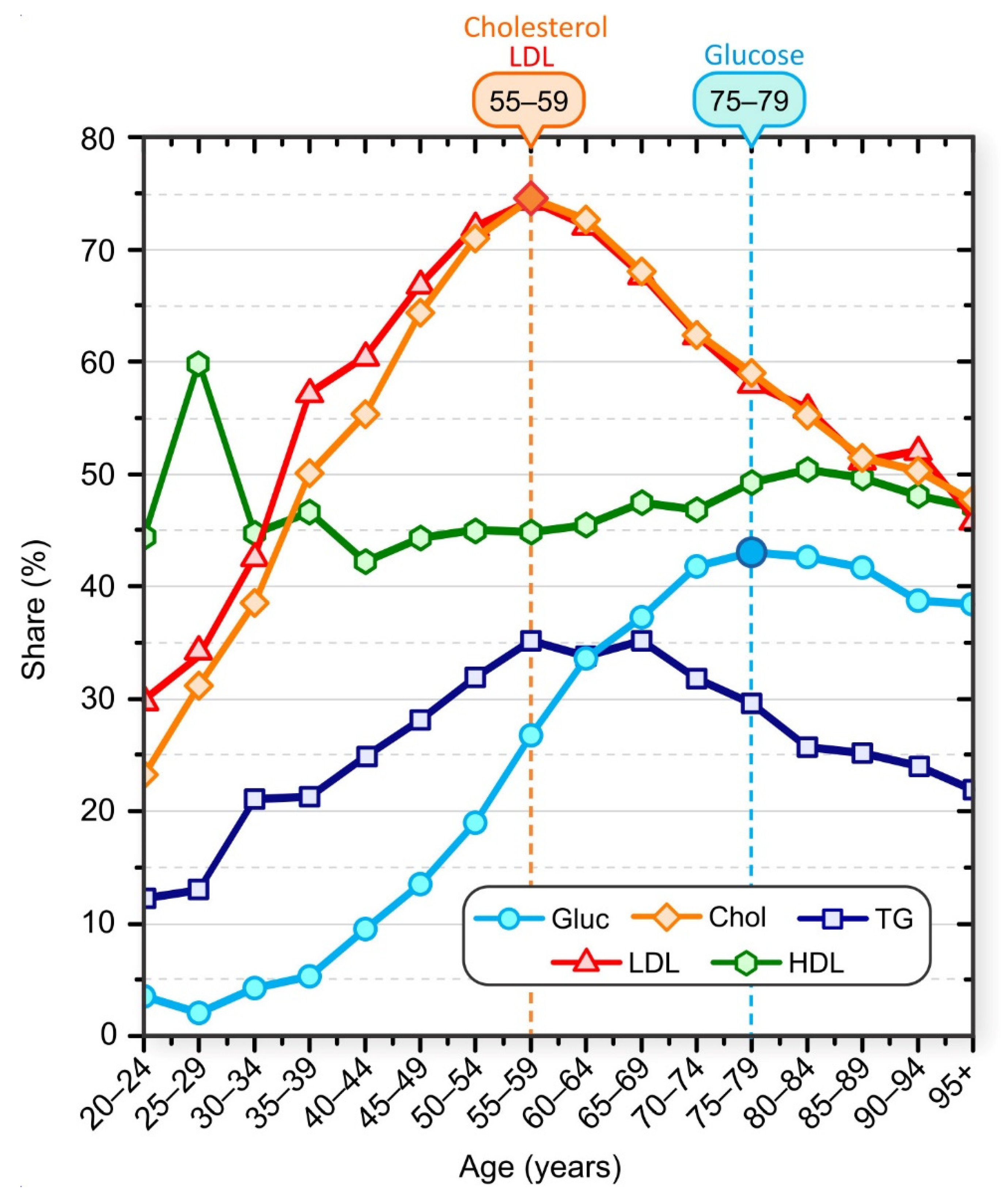
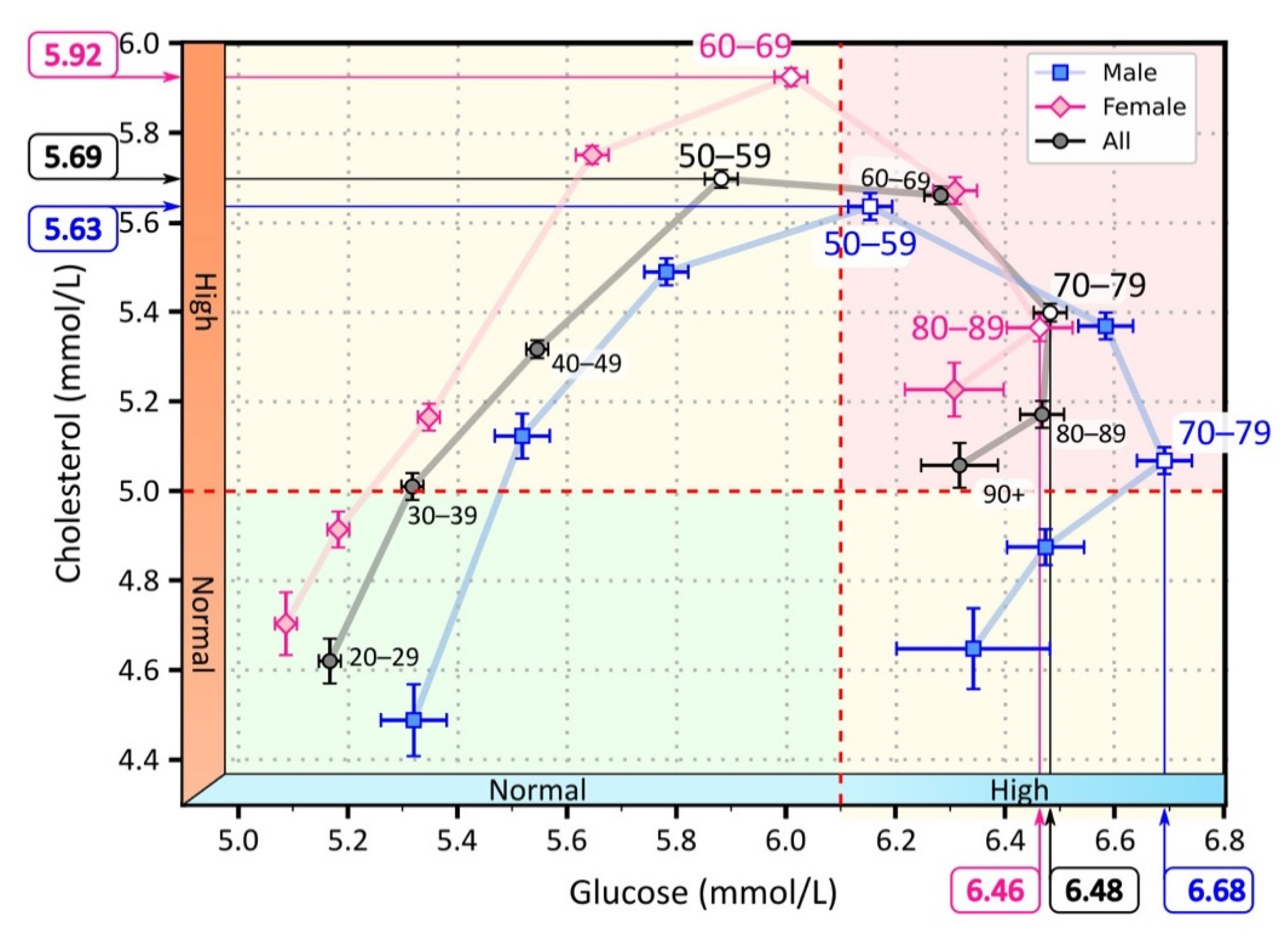
3.1. Lipid and Glucose Levels in Different Age Groups
3.2. Drug Use in Different Age Groups
3.3. Laboratory Results in the Mirror of Medication
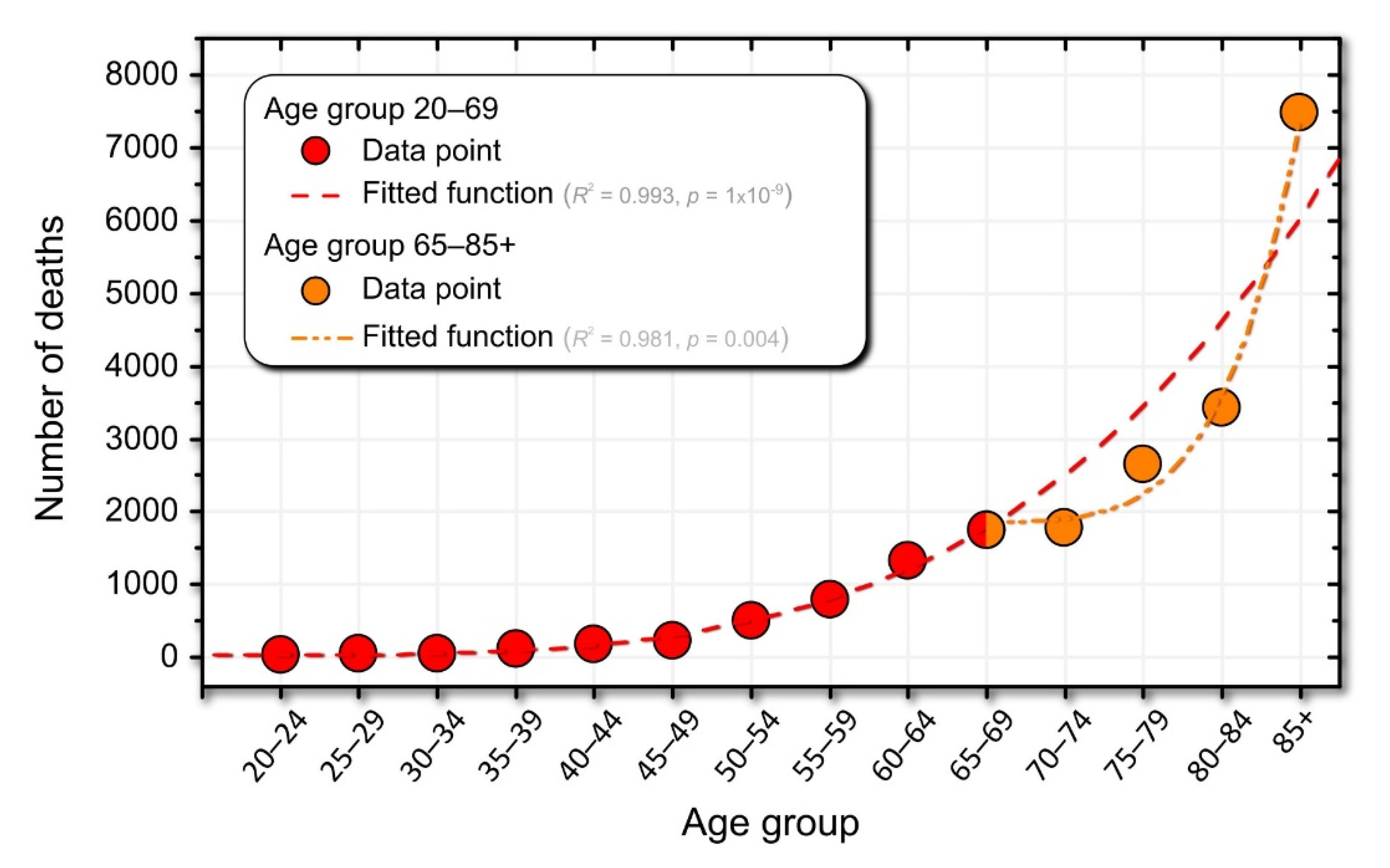
3.4. Mortality Dynamics in Association with Drug Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- International Diabetes Federation. IDF DIABETES ATLAS, 9th ed.; Karuranga, S., Malanda, B., Saeedi, P., Eds.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 978-2-930229-87-4. [Google Scholar]
- Elflein, J. Diabetes—Statistics & Facts | Statista. Available online: https://www.statista.com/topics/1723/diabetes/ (accessed on 22 April 2021).
- CDC. National Diabetes Statistics Report 2020. Estimates of Diabetes and Its Burden in the United States; CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 19 November 2021).
- Las, G.; Oliveira, M.F.; Shirihai, O.S. Emerging roles of β-cell mitochondria in type-2-diabetes. Mol. Aspects Med. 2020, 71, 100843. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Fructose: Metabolic, Hedonic, and Societal Parallels with Ethanol. J. Am. Diet. Assoc. 2010, 110, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.E.; Grootaert, C.; Van Camp, J. Rare mono- and disaccharides as healthy alternative for traditional sugars and sweeteners? Crit. Rev. Food Sci. Nutr. 2021, 61, 713–741. [Google Scholar] [CrossRef]
- Wali, J.A.; Milner, A.J.; Luk, A.W.S.; Pulpitel, T.J.; Dodgson, T.; Facey, H.J.W.; Wahl, D.; Kebede, M.A.; Senior, A.M.; Sullivan, M.A.; et al. Impact of dietary carbohydrate type and protein–carbohydrate interaction on metabolic health. Nat. Metab. 2021, 3, 810–828. [Google Scholar] [CrossRef]
- Meigs, J.B.; Rutter, M.K.; Sullivan, L.M.; Fox, C.S.; D’Agostino, R.B.; Wilson, P.W.F. Impact of Insulin Resistance on Risk of Type 2 Diabetes and Cardiovascular Disease in People With Metabolic Syndrome. Diabetes Care 2007, 30, 1219–1225. [Google Scholar] [CrossRef]
- Björntorp, P. Visceral Obesity: A “Civilization Syndrome”. Obes. Res. 1993, 1, 206–222. [Google Scholar] [CrossRef]
- Björntorp, P. Behavior and metabolic disease. Int. J. Behav. Med. 1996, 3, 285–302. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Taskinen, M.-R. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef]
- Taskinen, M.R. Type 2 Diabetes as a Lipid Disorder. Curr. Mol. Med. 2005, 5, 297–308. [Google Scholar] [CrossRef]
- Correa, R.; Wayar, F.; Reaven, P.; Corpas, E. Dyslipidemia in the Elderly. In Endocrinology of Aging; Corpas, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 607–650. ISBN 9780128196670. [Google Scholar]
- Lee, Y.; Hirose, H.; Ohneda, M.; Johnson, J.H.; McGarry, J.D.; Unger, R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: Impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA 1994, 91, 10878–10882. [Google Scholar] [CrossRef]
- McGarry, J.D. Banting Lecture 2001: Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef]
- Ebbert, J.; Jensen, M. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Investig. 1995, 96, 1261–1268. [Google Scholar] [CrossRef]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Rebrin, K.; Steil, G.M.; Getty, L.; Bergman, R.N. Free Fatty Acid as a Link in the Regulation of Hepatic Glucose Output by Peripheral Insulin. Diabetes 1995, 44, 1038–1045. [Google Scholar] [CrossRef]
- Saloranta, C.; Franssila-Kallunki, A.; Ekstrand, A.; Taskinen, M.-R.; Groop, L. Modulation of hepatic glucose production by non-esterified fatty acids in Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1991, 34, 409–415. [Google Scholar] [CrossRef]
- Shafrir, E.; Raz, I. Diabetes: Mellitus or lipidus? Diabetologia 2003, 46, 433–440. [Google Scholar] [CrossRef][Green Version]
- Yaney, G.C.; Corkey, B.E. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 2003, 46, 1297–1312. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Boden, G.; Jadali, F.; White, J.; Liang, Y.; Mozzoli, M.; Chen, X.; Coleman, E.; Smith, C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J. Clin. Investig. 1991, 88, 960–966. [Google Scholar] [CrossRef]
- Hennes, M.M.; Shrago, E.; Kissebah, A.H. Receptor and postreceptor effects of free fatty acids (FFA) on hepatocyte insulin dynamics. Int. J. Obes. 1990, 14, 831–841. [Google Scholar]
- Kissebah, A.H.; Alfarsi, S.; Adams, P.W.; Wynn, V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 1976, 12, 563–571. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Erion, K.; Corkey, B.E. β-Cell Failure or β-Cell Abuse? Front. Endocrinol. 2018, 9, 532. [Google Scholar] [CrossRef]
- Asghari, G.; Hasheminia, M.; Heidari, A.; Mirmiran, P.; Guity, K.; Shahrzad, M.K.; Azizi, F.; Hadaegh, F. Adolescent metabolic syndrome and its components associations with incidence of type 2 diabetes in early adulthood: Tehran lipid and glucose study. Diabetol. Metab. Syndr. 2021, 13, 1. [Google Scholar] [CrossRef]
- Adiels, M.; Olofsson, S.-O.; Taskinen, M.-R.; Borén, J. Diabetic dyslipidaemia. Curr. Opin. Lipidol. 2006, 17, 238–246. [Google Scholar] [CrossRef]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Nian, S.; Tong, Z.; Zhu, Y.; Li, Y.; Zhang, C.; Bai, X.; Luo, X.; Wu, M.; Yan, Z. Age-related trends in lipid levels: A large-scale cross-sectional study of the general Chinese population. BMJ Open 2020, 10, e034226. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.M.; Rogers, N.L.; Remsberg, K.E.; Sun, S.S.; Chumlea, W.C.; Demerath, E.W.; Czerwinski, S.A.; Towne, B.; Siervogel, R.M. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: The Fels Longitudinal Study. Int. J. Obes. 2006, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hou, X.; Hu, W.; Chen, L.; Chen, S. Serum lipid and lipoprotein levels of middle-aged and elderly Chinese men and women in Shandong Province. Lipids Health Dis. 2019, 18, 58. [Google Scholar] [CrossRef]
- Marhoum, T.A.; Abdrabo, A.A.; Marhoum, T.A.; Lutfi, M.F. Effects of age and gender on serum lipid profile in over 55 years-old apparently healthy Sudanese individuals. Asian J. Biomed. Pharm. Sci. 2013, 3, 10–14. [Google Scholar]
- Davison, G.W.; Irwin, R.E.; Walsh, C.P. The metabolic-epigenetic nexus in type 2 diabetes mellitus. Free Radic. Biol. Med. 2021, 170, 194–206. [Google Scholar] [CrossRef]
- Leao, S.C.; Carvalho, T.S.; Galvão, M.P.; Silva, R.V.; Rocha, M.F.; Queiroz, A.A.F.; Almeida, R.O.; Araujo, R.R.; Souto, M.J.S.; de Andrade Rodrigues, T.M. A Decade of Lipid Profiles: A Gender Focus. Heart Res.-Open J. 2016, 3, 9–15. [Google Scholar] [CrossRef]
- Natali, A.; Baldi, S.; Bonnet, F.; Petrie, J.; Trifirò, S.; Tricò, D.; Mari, A. Plasma HDL-cholesterol and triglycerides, but not LDL-cholesterol, are associated with insulin secretion in non-diabetic subjects. Metabolism 2017, 69, 33–42. [Google Scholar] [CrossRef]
- Huang, W.; Xu, W.; Zhu, P.; Yang, H.; Su, L.; Tang, H.; Liu, Y. Analysis of blood glucose distribution characteristics in a health examination population in Chengdu (2007–2015). Medicine 2017, 96, e8765. [Google Scholar] [CrossRef]
- Toth, P.P.; Zarotsky, V.; Sullivan, J.M.; Laitinen, D. Lipid therapy utilization rates in a managed-care mixed dyslipidemia population. J. Clin. Lipidol. 2008, 2, 365–374. [Google Scholar] [CrossRef]
- Pettersson, B.; Ambegaonkar, B.; Sazonov, V.; Martinell, M.; Stålhammar, J.; Wändell, P. Prevalence of lipid abnormalities before and after introduction of lipid modifying therapy among Swedish patients with dyslipidemia (PRIMULA). BMC Public Health 2010, 10, 737. [Google Scholar] [CrossRef]
- Hopstock, L.A.; Bønaa, K.H.; Eggen, A.E.; Grimsgaard, S.; Jacobsen, B.K.; Løchen, M.-L.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T. Longitudinal and secular trends in total cholesterol levels and impact of lipid-lowering drug use among Norwegian women and men born in 1905–1977 in the population-based Tromsø Study 1979–2016. BMJ Open 2017, 7, e015001. [Google Scholar] [CrossRef]
- Tuppin, P.; Ricci-Renaud, P.; de Peretti, C.; Fagot-Campagna, A.; Gastaldi-Menager, C.; Danchin, N.; Alla, F.; Allemand, H. Antihypertensive, antidiabetic and lipid-lowering treatment frequencies in France in 2010. Arch. Cardiovasc. Dis. 2013, 106, 274–286. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Huang, X.; Zhou, W.; Wang, T.; Zhu, L.; Ding, C.; Tao, Y.; Bao, H.; Cheng, X. A U-shaped association between the LDL-cholesterol to HDL-cholesterol ratio and all-cause mortality in elderly hypertensive patients: A prospective cohort study. Lipids Health Dis. 2020, 19, 238. [Google Scholar] [CrossRef]
- Yi, S.-W.; Yi, J.-J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- Yi, S.-W.; Park, S.; Lee, Y.-H.; Park, H.-J.; Balkau, B.; Yi, J.-J. Association between fasting glucose and all-cause mortality according to sex and age: A prospective cohort study. Sci. Rep. 2017, 7, 8194. [Google Scholar] [CrossRef]
- Ares, J.; Valdés, S.; Botas, P.; Sánchez-Ragnarsson, C.; Rodríguez-Rodero, S.; Morales-Sánchez, P.; Menéndez-Torre, E.; Delgado, E. Mortality risk in adults according to categories of impaired glucose metabolism after 18 years of follow-up in the North of Spain: The Asturias Study. PLoS ONE 2019, 14, e0211070. [Google Scholar] [CrossRef]
- Gardette, V.; Bongard, V.; Dallongeville, J.; Arveiler, D.; Bingham, A.; Ruidavets, J.-B.; Amouyel, P.; Haas, B.; Ducimetière, P.; Ferrières, J. Ten-Year All-Cause Mortality in Presumably Healthy Subjects on Lipid-Lowering Drugs (from the Prospective Epidemiological Study of Myocardial Infarction [PRIME] prospective cohort). Am. J. Cardiol. 2009, 103, 381–386. [Google Scholar] [CrossRef]
- Ko, D.T.; Mamdani, M.; Alter, D.A. Lipid-Lowering Therapy with Statins in High-Risk Elderly Patients. JAMA 2004, 291, 1864. [Google Scholar] [CrossRef]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of Blood Lipid Concentrations over the Adult Life Course and Risk of Cardiovascular Disease and All-Cause Mortality: Observations from the Framingham Study over 35 Years. J. Am. Heart Assoc. 2019, 8, e011433. [Google Scholar] [CrossRef]
- Laboratorijske Preiskave—UKC Maribor. Available online: https://www.ukc-mb.si/oddelki-službe-enote/skupni-medicinski-oddelki/oddelek-za-laboratorijsko-diagnostiko/laboratorijske-preiskave/ (accessed on 19 November 2021).
- SiStat Database. Available online: https://pxweb.stat.si/SiStat/en/Podrocja/Index/100/population (accessed on 28 April 2021).
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Fisher, R.A. The Conditions under Which χ2 Measures the Discrepancey between Observation and Hypothesis. J. R. Stat. Soc. 1924, 87, 442–450. [Google Scholar]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009; ISBN 1441412697. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar]
- 1 Life Expectancy at Birth. Available online: https://www.stat.si/Pages/en/goals/goal-3.-ensure-healthy-lives-and-promote-well-being-for-all-at-all-ages/3.1-life-expectancy-at-birth (accessed on 28 April 2021).
- Ko, G.T.C.; Wai, H.P.S.; Tang, J.S.F. Effects of age on plasma glucose levels in non-diabetic Hong Kong Chinese. Croat. Med. J. 2006, 47, 709–713. [Google Scholar] [PubMed]
- Elahi, D.; Muller, D. Carbohydrate metabolism in the elderly. Eur. J. Clin. Nutr. 2000, 54, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Gobal, F.A.; Mehta, J.L. Management of dyslipidemia in the elderly population. Ther. Adv. Cardiovasc. Dis. 2010, 4, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Tura, A.; Gastaldelli, A.; Ferrannini, E. Assessing Insulin Secretion by Modeling in Multiple-Meal Tests Role of Potentiation. Diabetes 2002, 51, S221–S226. [Google Scholar] [CrossRef]
- Kreisberg, R.A.; Kasim, S. Cholesterol metabolism and aging. Am. J. Med. 1987, 82, 54–60. [Google Scholar] [CrossRef]
- Ettinger, W.H.; Wahl, P.W.; Kuller, L.H.; Bush, T.L.; Tracy, R.P.; Manolio, T.A.; Borhani, N.O.; Wong, N.D.; O’Leary, D.H. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation 1992, 86, 858–869. [Google Scholar] [CrossRef]
- Rhee, E.-J.; Kim, H.C.; Kim, J.H.; Lee, E.Y.; Kim, B.J.; Kim, E.M.; Song, Y.; Lim, J.H.; Kim, H.J.; Choi, S.; et al. 2018 Guidelines for the management of dyslipidemia. Korean J. Intern. Med. 2019, 34, 723–771. [Google Scholar] [CrossRef]
- National Center for Health. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health; National Center for Health: Washington, DC, USA, 2011.
- Information Centre for Health and Social Care. Health Survey for England. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2006-cvd-and-risk-factors-for-adults-obesity-and-risk-factors-for-children (accessed on 3 November 2020).
- Hernáez, Á.; Soria-Florido, M.T.; Schröder, H.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; et al. Role of HDL function and LDL atherogenicity on cardiovascular risk: A comprehensive examination. PLoS ONE 2019, 14, e0218533. [Google Scholar] [CrossRef]
- Jensen, P.B.; Jensen, L.J.; Brunak, S. Mining electronic health records: Towards better research applications and clinical care. Nat. Rev. Genet. 2012, 13, 395–405. [Google Scholar] [CrossRef]
- Richens, J.G.; Lee, C.M.; Johri, S. Improving the accuracy of medical diagnosis with causal machine learning. Nat. Commun. 2020, 11, 3923. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Henri, C.; Leekitcharoenphon, P.; Mistou, M.; Hendriksen, R.S.; Hald, T. Machine Learning Methods as a Tool for Predicting Risk of Illness Applying Next-Generation Sequencing Data. Risk Anal. 2018, 39, 1397–1413. [Google Scholar] [CrossRef]
- Cho, G.; Yim, J.; Choi, Y.; Ko, J.; Lee, S.-H. Review of Machine Learning Algorithms for Diagnosing Mental Illness. Psychiatry Investig. 2019, 16, 262–269. [Google Scholar] [CrossRef]
- Haynos, A.F.; Wang, S.B.; Lipson, S.; Peterson, C.B.; Mitchell, J.E.; Halmi, K.A.; Agras, W.S.; Crow, S.J. Machine learning enhances prediction of illness course: A longitudinal study in eating disorders. Psychol. Med. 2020, 51, 1392–1402. [Google Scholar] [CrossRef]
- Mustafina, S.V.; Rymar, O.D.; Shcherbakova, L.V.; Verevkin, E.G.; Pikhart, H.; Sazonova, O.V.; Ragino, Y.I.; Simonova, G.I.; Bobak, M.; Malyutina, S.K.; et al. The Risk of Type 2 Diabetes Mellitus in a Russian Population Cohort According to Data from the HAPIEE Project. J. Pers. Med. 2021, 11, 119. [Google Scholar] [CrossRef]
- Koras, K.; Juraeva, D.; Kreis, J.; Mazur, J.; Staub, E.; Szczurek, E. Feature selection strategies for drug sensitivity prediction. Sci. Rep. 2020, 10, 9377. [Google Scholar] [CrossRef]
- Ali, M.; Aittokallio, T. Machine learning and feature selection for drug response prediction in precision oncology applications. Biophys. Rev. 2019, 11, 31–39. [Google Scholar] [CrossRef]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovič, R.; Grubelnik, V.; Vošner, H.B.; Kokol, P.; Završnik, M.; Janša, K.; Zupet, M.; Završnik, J.; Marhl, M. Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study. J. Pers. Med. 2022, 12, 280. https://doi.org/10.3390/jpm12020280
Markovič R, Grubelnik V, Vošner HB, Kokol P, Završnik M, Janša K, Zupet M, Završnik J, Marhl M. Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study. Journal of Personalized Medicine. 2022; 12(2):280. https://doi.org/10.3390/jpm12020280
Chicago/Turabian StyleMarkovič, Rene, Vladimir Grubelnik, Helena Blažun Vošner, Peter Kokol, Matej Završnik, Karmen Janša, Marjeta Zupet, Jernej Završnik, and Marko Marhl. 2022. "Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study" Journal of Personalized Medicine 12, no. 2: 280. https://doi.org/10.3390/jpm12020280
APA StyleMarkovič, R., Grubelnik, V., Vošner, H. B., Kokol, P., Završnik, M., Janša, K., Zupet, M., Završnik, J., & Marhl, M. (2022). Age-Related Changes in Lipid and Glucose Levels Associated with Drug Use and Mortality: An Observational Study. Journal of Personalized Medicine, 12(2), 280. https://doi.org/10.3390/jpm12020280








