Does Locally Advanced Thyroid Cancer Have Different Features? Results from a Single Academic Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thyroid FNAC Specimens
2.2. Molecular Analysis for BRAFV600E and TERT Mutation
2.3. Histopathology Specimens
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.Y.; Nixon, I.J.; Snehal, G.; Palmer, F.L.; Tuttle, R.M.; Shaha, A.; Shah, J.P.; Ganly, I. Operative management of locally advanced, differentiated thyroid cancer. Surgery 2016, 160, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, N.D.; Brierley, J.; Tuttle, M.R. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on cancer staging manual eight edition. CA Cancer J. Clin. 2018, 68, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Lee, Y.M.; Oh, H.S.; Jeon, M.J.; Song, D.E.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Sung, T.-Y.; Kim, W.G. A relook at the T stage of differentiated thyroid carcinoma with a focus on gross extrathyroidal extension. Thyroid 2019, 29, 202–208. [Google Scholar] [CrossRef]
- Tam, S.; Boonsripitayanon, M.; Amit, M.; Fellman, B.M.; Li, Y.; Busaidy, N.L.; Cabanillas, M.E.; Dadu, R.; Sherman, S.; Waguespack, S.G.; et al. Survival in differentiated thyroid cancer: Comparing the AJCC cancer staging seventh and eight editions. Thyroid 2018, 28, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.T.; Asa, S.L.; Baloch, Z.W.; Faquin, W.C.; Fellegara, G.; Ghossein, R.A.; Giordano, T.J.; LiVolsi, V.A.; Lloyd, R.; Mete, O.; et al. Interobserver variability in the histopathologica assessment of extrathyroidal extension of well differentiated thyroid carcinoma supports the new American joint committee on cancer eight edition criteria for tumor staging. Thyroid 2019, 29, 619–624. [Google Scholar] [CrossRef]
- Kim, B.Y.; Choi, J.E.; Lee, E.; Son, Y.-I.; Baek, C.-H.; Kim, S.W.; Chung, M.K. Prognostic factors for recurrence of locally advanced differentiated thyroid cancer. J. Surg. Oncol. 2017, 116, 877–883. [Google Scholar] [CrossRef]
- Amin, M.B.; Gress, D.M.; Meyer, L.R.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. AJCC Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asa, S.L. The current histologic classification of thyroid cancer. Endocrinol. Metab. Clin. 2019, 48, 1–22. [Google Scholar] [CrossRef]
- Shteinshnaider, M.; Kalmovich, L.M.; Koren, S.; Or, K.; Cantrell, D.; Benbassat, C. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: A comparative study. Thyroid 2018, 28, 201–209. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Limberg, J.; Ullmann, T.M.; Stefanova, D.; Buicko, J.L.; Finnerty, B.M.; Zarnegar, R.; Fahey, T.J.; Beninato, T. Does aggressive variants histology without invasive features predict overall survival in papillary thyroid cancer? A national cancer database analysis. Ann. Surg. 2019, 274, e276–e281. [Google Scholar] [CrossRef]
- Coca-Pelaza, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartlet, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary thyroid cancer-aggressive variants and impact on management: A narrative review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New therapies for advanced thyroid cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Ardito, G.; Revelli, L.; Giustozzi, E.; Salvatori, M.; Fadda, G.; Ardito, F.; Avenia, N.; Ferretti, A.; Rampin, L.; Chondrogiannis, S.; et al. Aggressive papillary thyroid microcarcinoma: Prognostic factors and therapeutic strategy. Clin. Nucl. Med. 2013, 38, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nature 2021, 17, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Holoubek, S.A.; Yan, H.; Khokar, A.H.; Roman, S.A. Aggressive variants of papillary thyoid microcarcinoma are associated with high-risk features but not decreased survival. Surgery 2019, 167, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Miftari, R.; Topciu, V.; Nura, A.; Haxhibeqiri, V. Management of the patient with aggressive and resistant papillary thyroid carcinoma. Med. Arch. 2016, 70, 314–317. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Shen, F.; Cai, W.; Gan, X.; Deng, X.; Xu, B. Survival of aggressive variants of papillary thyroid carcinoma in patients under 55 years old: A SEER population-based retrospective analysis. Endocrine 2018, 61, 499–505. [Google Scholar] [CrossRef]
- Kazaure, H.S.; Roman, S.A.; Sosa, J.A. Aggressive variants of papillary thyroid cancer: Incidence, characteristics and predictors of survival among 43,738 patients. Ann. Surg. Oncol. 2012, 19, 1874–1878. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.Z.; Edmund, S.C. The Bethesda System for Reporting Thyroid Cytopathology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Rossi, E.D.; Faquin, W.C.; Pantanowitz, L. Cytologic features of aggressive variants of follicular-derived thyroid carcinoma. Cancer Cytopathol. 2019, 127, 432–446. [Google Scholar] [CrossRef]
- Straccia, P.; Brunelli, C.; Rossi, E.D.; Lanza, P.; Martini, M.; Musarra, T.; Lombardi, C.P.; Pontecorvi, A.; Fadda, G. The immunocytochemical expression of VE-1 (BRAF V600E-related) antibody identifies the aggressive variants of papillary thyroid carcinoma on liquid-based cytology. Cytopathology 2019, 30, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Evranos, B.; Polat, S.B.; Baser, H.; Kilicarslan, A.; Yalcin, A.; Ersoy, R.; Cakir, B. Bethesda classification is a valuable guide for fine needle aspiration reports and highly predictive especially for diagnosing aggressive variants of papillary thyroid carcinoma. Cytopathology 2017, 28, 259–267. [Google Scholar] [CrossRef]
- Gharib, H.; Goellner, R.J.; Johnson, D.A. Fine needle aspiration of the thyroid: A 12 years experience with 11.000 biopsies. Clin. Lab. Med. 1993, 13, 699–709. [Google Scholar] [CrossRef]
- Perros, P.; Colley, S.; Boelaert, K.; Evans, C.; Evans, R.M.; Gerrard, G.E.; Gilbert, J.A.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the Management of Thyroid Cancer. Report of the Thyroid Cancer Guidelines Update Group, 3rd ed.; British Thyroid Association, Royal College of Physicians: London, UK, 2007. [Google Scholar]
- Fadda, G.; Basolo, F.; Bondi, A.; Bussolati, G.; Crescenzi, A.; Nappi, O.; Nardi, F.; Papotti, M.; Taddei, G.; Palombini, L. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian consensus working group. Pathologica 2010, 102, 40405–40829. [Google Scholar]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Fadda, G.; Rossi, E.D. Liquid based cytology in fine needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011, 55, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Fadda, G.; Rossi, E.D.; Raffaelli, M.; Pontecorvi, A.; Sioletic, S.; Morassi, F.; Lombardi, C.P.; Zannoni, F.; Rindi, G. Follicular thyroid neoplasms can be classified as low and high risk according to HBME-1 and Galectin 3 expression on liquid based fine needle cytology. Eur. J. Endocrinol. 2011, 165, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Cenci, T.; Bilotta, M.; Pierconti, F.; Pontecorvi, A.; Lombardi, C.P.; Fadda, G.; Larocca, L.M. Morphology combined with ancillary techniques: An algorithm approach for thyroid nodules. Cytopathology 2018, 29, 418–427. [Google Scholar] [CrossRef]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Cenci, T.; Straccia, P.; Angrisani, B.; Ricci, C.; Lanza, P.; Lombardi, C.P.; Pontecorvi, A.; et al. Analysis of immunocytochemical and molecular BRAF expression in thyroid carcinomas: A cyto-histological institutional experience. Cancer Cytopathol. 2014, 122, 527–535. [Google Scholar] [CrossRef]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Lombardi, C.P.; Pontecorvi, A.; Vellone, V.G.; Zannoni, G.F.; Larocca, L.M.; Fadda, G. BRAF (v600e) mutation analysis on LBC-processed aspiration biopsies predicts bilaterality and nodal involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013, 121, 291–297. [Google Scholar] [CrossRef]
- Dell’aquila, M.; Fiorentino, V.; Martini, M.; Capodimonti, S.; Cenci, T.; Lombardi, C.P.; Raffaelli, M.; Pontecorvi, A.; Fadda, G.; Pantanowitz, L.; et al. How limited molecular testing can also offer diagnostic and prognostic evaluation of thyroid nodules processed with liquid-based cytology: Role of TERT promoter and BRAF V600E mutation analysis. Cancer Cytopathol. 2021, 129, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. (Eds.) WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barlettaet, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyroid Cancer Genome. Available online: http//cancer.gov/about-nci/organization/ccg/research/sructural-genomics/tcga/studied-cancers/thyroid (accessed on 2 December 2021).
- Aschebrook-Kilfoy, B.; Ward, M.H.; Sabra, M.M.; Devesa, S.S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011, 21, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Marcadis, A.R.; Jennifer, C.; Ashok, K.S. Management of Locally Advanced Thyroid Cancer. In Evidence-Based Endocrine Surgery; Springer: Singapore, 2018; pp. 85–95. [Google Scholar]
- Kasperbauer, J.L. Locally advanced thyroid carcinoma. Ann. Otol. Rhinol. Laryngol. 2004, 113, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Electron, K.; Clark, O.H. Locally advanced differentiated thyroid cancer. Surg. Oncol. 2003, 122, 91–99. [Google Scholar]
- Radowsky, J.S.; Howard, R.S.; Burch, H.B.; Stojadinovic, A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014, 24, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Shaha, A.R. Locally advanced thyroid cancer. Curr. Opin. Otolar. Head Neck Surg. 2005, 13, 112–116. [Google Scholar] [CrossRef]
- Carling, T.; Udelsman, R. Thyroid cancer. Ann. Rev. Med. 2014, 65, 125–137. [Google Scholar] [CrossRef]
- Lombardi, C.P.; Bellantone, R.; De Crea, C.; Paladino, N.C.; Fadda, G.; Salvatori, M.; Raffaelli, M. Papillary thyroid microcarcinoma: Extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter area. World J. Surg. 2010, 34, 1214–1221. [Google Scholar] [CrossRef]
- Giannini, R.; Moretti, S.; Ugolini, C.; Macerola, E.; Menicali, E.; Nucci, N.; Morelli, S.; Colella, R.; Mandarano, M.; Sidoni, A.; et al. Immune profiling of thyroid carcinomas suggest the existence of two major phenotypes: An ATC-Like and a PDTC-like. J. Clin. Endocrinol. Metab. 2019, 104, 3557–3575. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.-Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007, 246, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, T.; Zhu, G.; Xing, M. Regulation of mutant TERT by BRAFV600E/MAP kinase pathway through FOS/GABP in human cancer. Nat. Commun. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.S.; Park, Y.J. Mechanisms of TERT reactivation and its interaction with brafv600e. Endocrinol. Metab. 2020, 35, 515–525. [Google Scholar] [CrossRef]
- Song, Y.S.; Yoo, S.K.; Kim, H.H.; Jung, G.; Oh, A.R.; Cha, J.Y.; Kim, S.; Cho, S.W.; Lee, K.E.; Seo, J.-S.; et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr. Relat. Cancer 2019, 26, 629–641. [Google Scholar] [CrossRef]
- Liu, X.; Bishop, J.; Shan, Y.; Pail, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, A. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Chen, E.; Dong, S.; Cai, Y.; Zhang, X.; Zhou, Y.; Zeng, R.; Yang, F.; Pan, C.; Liu, Y.; et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget 2016, 7, 18346–18355. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Nathani, R.; Venkat, J.; Bharda, A.; Vanere, V.; Bhatia, S.; Das, B.R. Molecular evaluation of BRAF gene mutation in thyroid tumors: Significant association with papillary tumors and extra thyroidal extension indicating its role as a biomarker of aggressive disease. Exp. Mol. Pathol. 2018, 105, 380–386. [Google Scholar] [CrossRef]
- Sugitani, I.; Kasai, N.; Fujimoto, Y.; Yanagisawa, A. A novel classifcation system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassifcation during the follow-up period. Surgery 2004, 135, 139–148. [Google Scholar] [CrossRef]

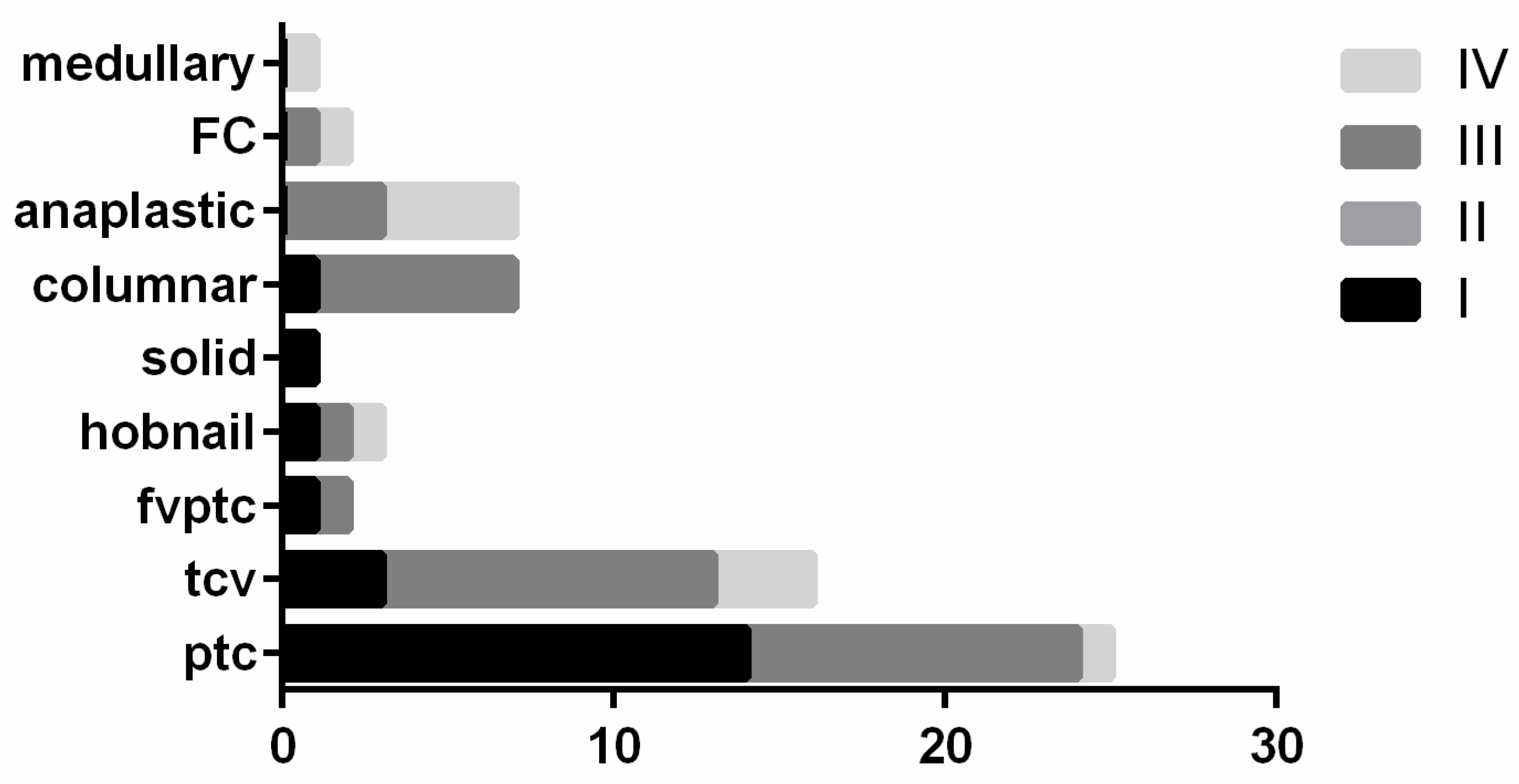




| Clinical-Pathological Features | Proportion (n = 65 Cases) |
|---|---|
| Age | |
| Mean | 60.15 years |
| Median | 60 years |
| Range | 22–87 years |
| Gender | |
| Male | 23 (35%) |
| Female | 42 (65%) |
| Cytology diagnosis (n = 42 cases) | |
| Non-diagnostic | 3 (7.1%) |
| Benign | 0 (0%) |
| AUS/FLUS | 2 (4.7%) |
| FN/SFN | 3 (7.1%) |
| SFM | 5 (11.9%) |
| Malignant | 29 (69%) |
| Histopathology diagnosis | |
| Benign | 0 (0%) |
| Malignant | 65 (100%) |
| Lymph node involvement | |
| Central VI level | 28 (42.6%) |
| Lateral cervical involvement | 37 (57.45) |
| Multifocality | 33 |
| Histological Diagnoses | Number of Cases | Cases with FNAC |
|---|---|---|
| Classical PTC | 25 (38.4%) | 15 (60%) |
| I-FVPTC | 2 (3%) | 2 (100%) |
| TCV | 17 (26.1%) | 8 (47%) |
| CC-PTC | 7 (10.7%) | 6 (85.7%) |
| Hobnail PTC | 3 (4.6%) | 3 (100%) |
| Solid variant PTC | 1 (1.5%) | 1 (100%) |
| FTC | 2 (3%) | 1 (50%) |
| MTC | 1 (1.5%) | 0 |
| ATC | 7 (10.7%) | 6 (85.7%) |
| Diagnoses | cPTC | I-FVPTC | FTC | TCV | SOLID PTC | HOBNAIL | CC-PTC | ATC |
|---|---|---|---|---|---|---|---|---|
| PM | 9 | 1 | 0 | 5 | 1 | 3 | 5 | 5 |
| SFM | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 |
| SFN/FN | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| AUS/FLUS | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BENIGN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ND | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Aquila, M.; Tralongo, P.; De Ruggieri, G.; Curatolo, M.; Revelli, L.; Lombardi, C.P.; Pontecorvi, A.; Fadda, G.; Larocca, L.M.; Raffaelli, M.; et al. Does Locally Advanced Thyroid Cancer Have Different Features? Results from a Single Academic Center. J. Pers. Med. 2022, 12, 221. https://doi.org/10.3390/jpm12020221
Dell’Aquila M, Tralongo P, De Ruggieri G, Curatolo M, Revelli L, Lombardi CP, Pontecorvi A, Fadda G, Larocca LM, Raffaelli M, et al. Does Locally Advanced Thyroid Cancer Have Different Features? Results from a Single Academic Center. Journal of Personalized Medicine. 2022; 12(2):221. https://doi.org/10.3390/jpm12020221
Chicago/Turabian StyleDell’Aquila, Marco, Pietro Tralongo, Giuseppe De Ruggieri, Mariangela Curatolo, Luca Revelli, Celestino Pio Lombardi, Alfredo Pontecorvi, Guido Fadda, Luigi Maria Larocca, Marco Raffaelli, and et al. 2022. "Does Locally Advanced Thyroid Cancer Have Different Features? Results from a Single Academic Center" Journal of Personalized Medicine 12, no. 2: 221. https://doi.org/10.3390/jpm12020221
APA StyleDell’Aquila, M., Tralongo, P., De Ruggieri, G., Curatolo, M., Revelli, L., Lombardi, C. P., Pontecorvi, A., Fadda, G., Larocca, L. M., Raffaelli, M., Pantanowitz, L., & Rossi, E. D. (2022). Does Locally Advanced Thyroid Cancer Have Different Features? Results from a Single Academic Center. Journal of Personalized Medicine, 12(2), 221. https://doi.org/10.3390/jpm12020221








