Impact of Scaling and Periodontal Treatment during Pregnancy on the Risk of Adverse Birth Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Data Sources
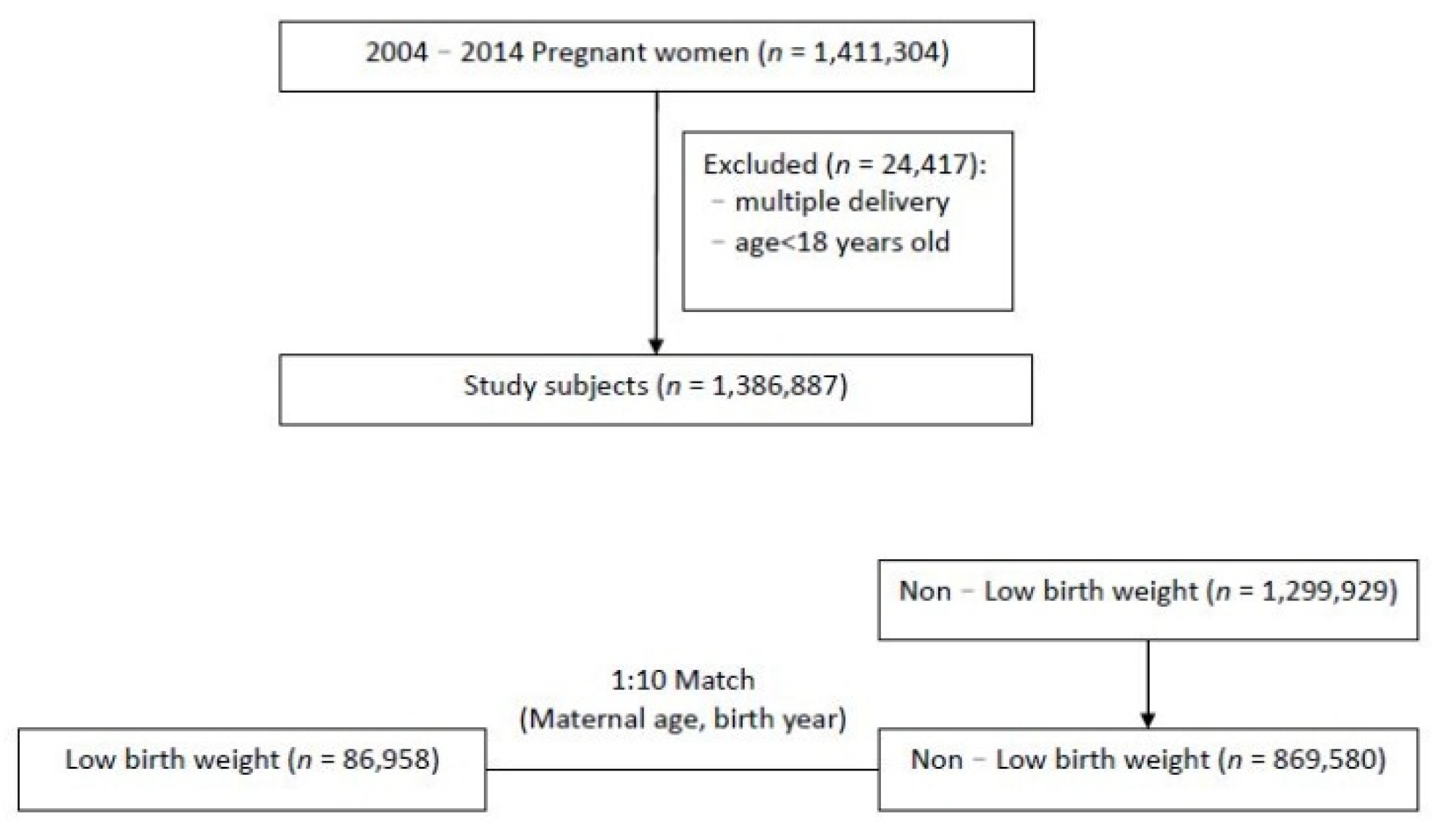
2.3. Study Design
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebersole, J.L.; Cappelli, D. Acute-phase reactants in infections and inflammatory diseases. Periodontology 2000 2000, 23, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Buekens, P.; Fraser, W.D.; Beck, J.; Offenbacher, S. Periodontal disease and adverse pregnancy outcomes: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.K.; Sammel, M.D.; Stamilio, D.M.; Clothier, B.; Jeffcoat, M.K.; Parry, S.; Macones, G.A.; Elovitz, M.A.; Metlay, J. Periodontal disease and adverse pregnancy outcomes: Is there an association? Am. J. Obstet. Gynecol. 2009, 200, 497.e1–497.e8. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Pileri, P.; Villa, A.; Calabrese, S.; Ottolenghi, L.; Abati, S. Pathogenic mechanisms linking periodontal diseases with adverse pregnancy outcomes. Reprod. Sci. 2012, 19, 633–641. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F. Preterm birth and periodontal disease. N. Engl. J. Med. 2006, 355, 1925–1927. [Google Scholar] [CrossRef]
- Jarjoura, K.; Devine, P.C.; Perez-Delboy, A.; Herrera-Abreu, M.; D’Alton, M.; Papapanou, P.N. Markers of periodontal infection and preterm birth. Am. J. Obstet. Gynecol. 2005, 192, 513–519. [Google Scholar] [CrossRef]
- Shetty, M.; Shetty, P.K.; Ramesh, A.; Thomas, B.; Prabhu, S.; Rao, A. Periodontal disease in pregnancy is a risk factor for preeclampsia. Acta Obstet. Gynecol. Scand. 2010, 89, 718–721. [Google Scholar] [CrossRef]
- Paquette, D.W. The periodontal infection-systemic disease link: A review of the truth or myth. J. Int. Acad. Periodontol. 2002, 4, 101–109. [Google Scholar]
- Lieff, S.; Boggess, K.A.; Murtha, A.P.; Jared, H.; Madianos, P.N.; Moss, K.; Beck, J.; Offenbacher, S. The oral conditions and pregnancy study: Periodontal status of a cohort of pregnant women. J. Periodontol. 2004, 75, 116–126. [Google Scholar] [CrossRef]
- Haerian-Ardakani, A.; Eslami, Z.; Rashidi-Meibodi, F.; Haerian, A.; Dallalnejad, P.; Shekari, M.; Taghavi, A.M.; Akbari, S. Relationship between maternal periodontal disease and low birth weight babies. Iran. J. Reprod. Med. 2013, 11, 625–630. [Google Scholar]
- Muwazi, L.; Rwenyonyi, C.M.; Nkamba, M.; Kutesa, A.; Kagawa, M.; Mugyenyi, G.; Kwizera, G.; Okullo, I. Periodontal conditions, low birth weight and preterm birth among postpartum mothers in two tertiary health facilities in Uganda. BMC Oral Health 2014, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.R.; Hamissi, J.H.; Naeini, S.R.; Karimi, M. The Relationship Between Maternal Periodontal Status of and Preterm and Low Birth Weight Infants in Iran: A Case Control Study. Glob. J. Health Sci. 2016, 8, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Dasanayake, A.P.; Boyd, D.; Madianos, P.N.; Offenbacher, S.; Hills, E. The association between Porphyromonasgingivalis-specific maternal serum IgG and low birth weight. J. Periodontol. 2001, 72, 1491–1497. [Google Scholar] [CrossRef]
- McGaw, T. Periodontal disease and preterm delivery of low-birth-weight infants. J.-Can. Dent. Assoc. 2002, 68, 165–169. [Google Scholar] [PubMed]
- Hughes, M.M.; Black, R.E.; Katz, J. 2500-g Low Birth Weight Cutoff: History and Implications for Future Research and Policy. Matern. Child Health J. 2017, 21, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanthi, V.; Vanka, A.; Bhambal, A.; Saxena, V.; Saxena, S.; Kumar, S.S. Association of pregnant women periodontal status to preterm and low-birth weight babies: A systematic and evidence-based review. Dent. Res. J. 2012, 9, 368–380. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, T.A.; Cheng, D.; Strobino, D. Dental cleaning before and during pregnancy among Maryland mothers. Matern. Child Health J. 2013, 17, 110–118. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Middleton, P.; Esposito, M.; Glenny, A.M. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst. Rev. 2017, 6, CD005297. [Google Scholar] [CrossRef]
- Lopez, N.J.; Smith, P.C.; Gutierrez, J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: A randomized controlled trial. J. Periodontol. 2002, 73, 911–924. [Google Scholar] [CrossRef]
- Alves, R.T.; Ribeiro, R.A. Relationship between maternal periodontal disease and birth of preterm low weight babies. Braz. Oral Res. 2006, 20, 318–323. [Google Scholar] [CrossRef]
- Jeffcoat, M.K.; Geurs, N.C.; Reddy, M.S.; Cliver, S.P.; Goldenberg, R.L.; Hauth, J.C. Periodontal infection and preterm birth: Results of a prospective study. J. Am. Dent. Assoc. 2001, 132, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, S.A.; Giraldo, P.C.; Saba-Chujfi, E.; Amaral, R.L.G.; Morais, S.S.; Fachini, A.M.; Gonçalves, A.K.S. Chronic periodontitis and pre-term labour in Brazilian pregnant women: An association to be analysed. J. Clin. Periodontol. 2007, 34, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Diagnosis of endotoxemia with gram-negative bacteremia is bacterial species dependent: A meta-analysis of clinical studies. J. Clin. Microbiol. 2009, 47, 3826–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Buekens, P.; Vastardis, S.; Stella, M.Y. Periodontal disease and pregnancy outcomes: State-of-the-science. Obstet. Gynecol. Surv. 2007, 62, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood J. Am. Soc. Hematol. 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K.; Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef]
- Bergman, D.; Halje, M.; Nordin, M.; Engstrom, W. Insulin-like growth factor 2 in development and disease: A mini-review. Gerontology 2013, 59, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Mahumud, R.A.; Sultana, M.; Sarker, A.R. Distribution and Determinants of Low Birth Weight in Developing Countries. J. Prev. Med. Public Health 2017, 50, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NHI Taiwan. National Health Insurance Administration. Available online: https://www.nhi.gov.tw/English/Content_List.aspx?n=8FC0974BBFEFA56D&topn=ED4A30E51A609E49 (accessed on 19 January 2022).

| Characteristic | Non-LBW | LBW | p Value |
|---|---|---|---|
| (n = 869,580) (%) a | (n = 86,958) (%) a | ||
| Maternal age (years) (%) | |||
| <25 | 160,880 (18.5) | 16,088 (18.5) | |
| 25–34 | 564,270 (64.9) | 56,427 (64.9) | |
| ≥35 | 144,430 (16.6) | 14,443 (16.6) | |
| Income (TWD) (%) | <0.001 | ||
| ≤15,840 | 148,445 (17.1) | 17,616 (20.3) | |
| 15,841–28,800 | 435,297 (50.1) | 42,982 (49.4) | |
| 28,801–45,800 | 201,303 (23.1) | 18,986 (21.8) | |
| >45,800 | 84,535 (9.7) | 7374 (8.5) | |
| Urbanization of residence (%) | <0.001 | ||
| Urban | 529,899 (61) | 51,571 (59.3) | |
| Suburban | 111,103 (12.8) | 11,537 (13.3) | |
| Rural | 228,062 (26.2) | 23,805 (27.4) | |
| Mode of delivery (%) | <0.001 | ||
| Vaginal delivery | 574,754 (66.1) | 51,866 (59.6) | |
| Cesarean section | 294,826 (33.9) | 35,092 (40.4) | |
| Maternal comorbidity (%) | <0.001 | ||
| Diabetes mellitus | 5741 (0.7) | 895 (1) | |
| Hypertension | 5595 (0.6) | 1972 (2.3) | |
| Hyperlipidemia | 10,698 (1.2) | 1577 (1.8) | |
| Pregnancy-related complication (%) | |||
| Gestational diabetes mellitus | 17,366 (2) | 2049 (2.4) | <0.001 |
| Gestational hypertension | 4557 (0.5) | 1532 (1.8) | <0.001 |
| Pre-eclampsia or eclampsia | 8881 (1) | 7315 (8.4) | <0.001 |
| Placenta previa and abruptio placentae | 27,030 (3.1) | 7867 (9) | <0.001 |
| Variables | OR | 95% CI | p Value a | OR | 95% CI | p Value a | ||
|---|---|---|---|---|---|---|---|---|
| Procedures (during–within 2 years before pregnancy) | ||||||||
| Periodontal emergency treatment | - | - | - | - | 1.00 | 0.98 | 1.03 | 0.673 |
| Scaling localized/full mouth | 0.93 | 0.91 | 0.94 | <0.001 | 0.93 | 0.91 | 0.94 | <0.001 |
| Maternal age | ||||||||
| <25 | 1.00 (reference) | 1.00 (reference) | ||||||
| 25–34 | 1.00 | 0.98 | 1.02 | 0.977 | 1.00 | 0.98 | 1.02 | 0.980 |
| ≥35 | 0.89 | 0.87 | 0.91 | <0.001 | 0.89 | 0.87 | 0.91 | <0.001 |
| Income | ||||||||
| ≤15,840 | 1.00 (reference) | 1.00 (reference) | ||||||
| 15,841–28,800 | 0.84 | 0.82 | 0.85 | <0.001 | 0.84 | 0.82 | 0.85 | <0.001 |
| 28,801–45,800 | 0.81 | 0.79 | 0.82 | <0.001 | 0.81 | 0.79 | 0.82 | <0.001 |
| >45,800 | 0.75 | 0.73 | 0.77 | <0.001 | 0.75 | 0.73 | 0.77 | <0.001 |
| Urbanization of residence | ||||||||
| Urban | 1.00 (reference) | 1.00 (reference) | ||||||
| Suburban | 1.04 | 1.02 | 1.07 | <0.001 | 1.04 | 1.02 | 1.07 | <0.001 |
| Rural | 1.04 | 1.03 | 1.06 | <0.001 | 1.04 | 1.03 | 1.06 | <0.001 |
| Mode of delivery | ||||||||
| Vaginal delivery | 1.00 (reference) | 1.00 (reference) | ||||||
| Cesarean section | 1.06 | 1.04 | 1.07 | <0.001 | 1.06 | 1.04 | 1.07 | <0.001 |
| Maternal comorbidity | ||||||||
| Diabetes mellitus | 0.94 | 0.86 | 1.01 | 0.106 | 0.94 | 0.86 | 1.01 | 0.105 |
| Hypertension | 2.07 | 1.95 | 2.20 | <0.001 | 2.07 | 1.95 | 2.20 | <0.001 |
| Hyperlipidemia | 1.09 | 1.02 | 1.15 | 0.008 | 1.09 | 1.02 | 1.15 | 0.008 |
| Pregnancy-related complication | ||||||||
| Gestational diabetes mellitus | 0.99 | 0.95 | 1.04 | 0.757 | 0.99 | 0.95 | 1.04 | 0.756 |
| Gestational hypertension | 2.67 | 2.51 | 2.84 | <0.001 | 2.67 | 2.51 | 2.84 | <0.001 |
| Pre-eclampsia or eclampsia | 7.91 | 7.65 | 8.18 | <0.001 | 7.91 | 7.65 | 8.18 | <0.001 |
| Placenta previa and abruptio placentae | 3.07 | 2.98 | 3.15 | <0.001 | 3.07 | 2.98 | 3.15 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Wu, D.-R.; Lin, W.-S.; Chen, I.-C.; Liu, J.-F.; Chen, H.-L.; Lin, C.-H. Impact of Scaling and Periodontal Treatment during Pregnancy on the Risk of Adverse Birth Outcomes. J. Pers. Med. 2022, 12, 137. https://doi.org/10.3390/jpm12020137
Chen J-J, Wu D-R, Lin W-S, Chen I-C, Liu J-F, Chen H-L, Lin C-H. Impact of Scaling and Periodontal Treatment during Pregnancy on the Risk of Adverse Birth Outcomes. Journal of Personalized Medicine. 2022; 12(2):137. https://doi.org/10.3390/jpm12020137
Chicago/Turabian StyleChen, Jhih-Jhen, Dai-Rong Wu, Wei-Szu Lin, I-Chieh Chen, Jeng-Fen Liu, Hui-Ling Chen, and Ching-Heng Lin. 2022. "Impact of Scaling and Periodontal Treatment during Pregnancy on the Risk of Adverse Birth Outcomes" Journal of Personalized Medicine 12, no. 2: 137. https://doi.org/10.3390/jpm12020137
APA StyleChen, J.-J., Wu, D.-R., Lin, W.-S., Chen, I.-C., Liu, J.-F., Chen, H.-L., & Lin, C.-H. (2022). Impact of Scaling and Periodontal Treatment during Pregnancy on the Risk of Adverse Birth Outcomes. Journal of Personalized Medicine, 12(2), 137. https://doi.org/10.3390/jpm12020137






