Factors Likely to Affect the Uptake of Genomic Approaches to Cancer Screening in Primary Care: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
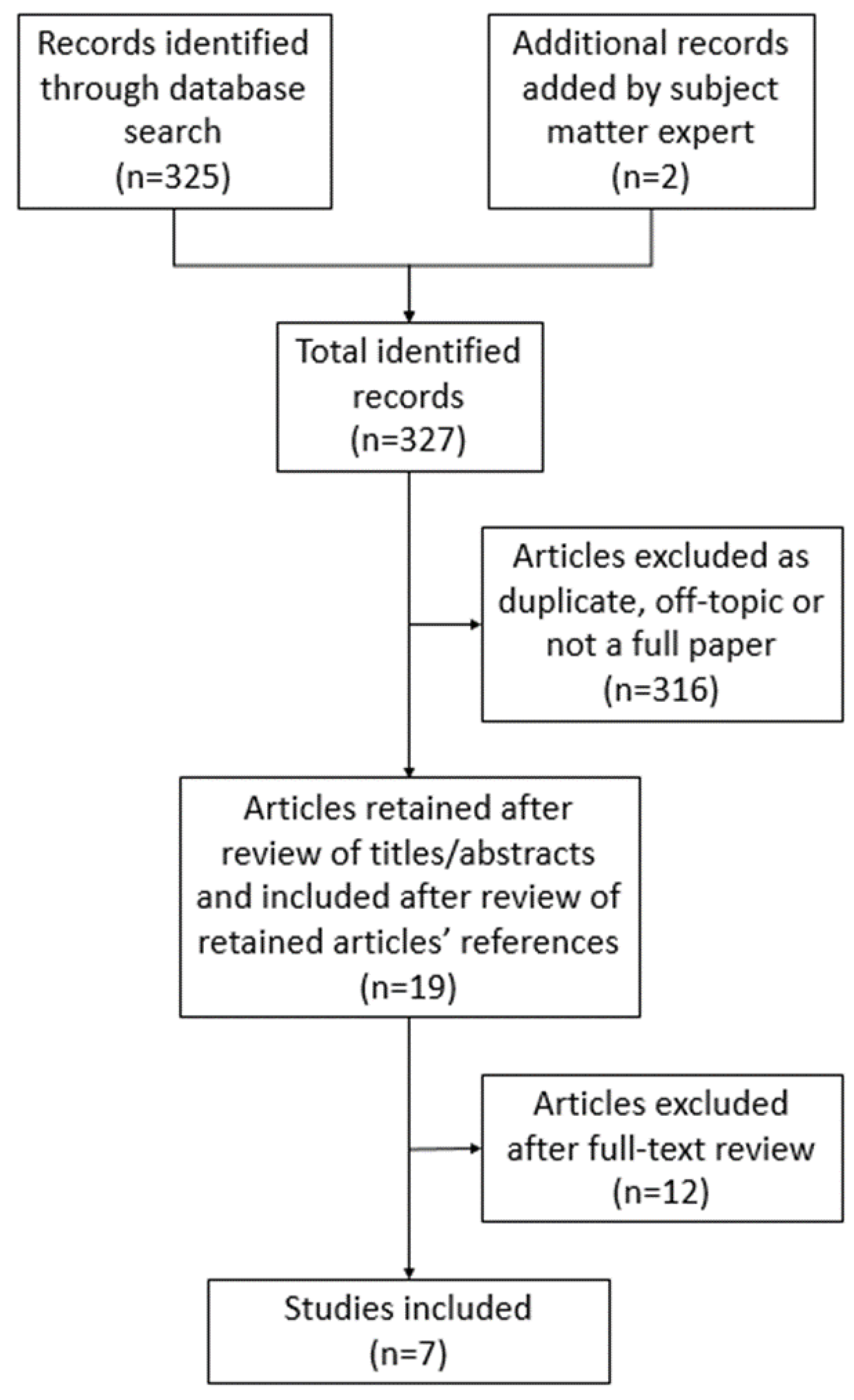
- The research team performed an initial search on the databases PubMed, Ovid, and Scopus, followed by the manual removal of duplicates across the results. Additionally, a subject matter expert on the research team (REM) suggested the inclusion of 2 additional articles. Search terms included: “Multi-cancer screening” or “Multi-cancer early detection” or “MCED” or “multi-analyte blood testing” or “multi-analyte blood test” or “multi-analyte assay”.
- Members of the team (KVD and MHH) reviewed the search results based on title and abstract. Articles that were not related in any way to the perception of liquid biopsy or MCED were excluded.
- Members of the team (KVD and MHH) reviewed the full text of each of the remaining articles. Any article that did not discuss patient or provider views related to the use of liquid biopsy or MCED testing were excluded.
- Members of the team (KVD and MHH) reviewed references of the included articles for any other relevant articles.
- Members of the team (KVD, MHH, and REM) analyzed the final articles, recorded details of each study, and summarized key themes (see Table 1).
3. Results
3.1. Provider Perspective
3.2. Patient Perspective
3.3. Perceptions of Liquid Biopsy and MCED Testing in Diverse Populations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- United States Preventive Services Taskforce. Recommendation: Lung Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/index.php/recommendation/lung-cancer-screening#bootstrap-panel--13 (accessed on 29 December 2021).
- United States Preventive Services Taskforce. Recommendation: Cervical Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening (accessed on 11 January 2022).
- United States Preventive Services Taskforce. Recommendation: Colorectal Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening (accessed on 11 January 2022).
- United States Preventive Services Taskforce. Recommendation: Breast Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening (accessed on 11 January 2022).
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.; Jorge, J.; Gonçalves, A.; Sarmento-Ribeiro, A.; Melo, J.; Carreira, I. Liquid biopsies: Applications for cancer diagnosis and monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Lokshin, A.; Bast, R.C.; Rodland, K. Circulating Cancer Biomarkers. Cancers 2021, 13, 802. [Google Scholar] [CrossRef]
- Mattox, A.K.; Bettegowda, C.; Zhou, S.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Applications of liquid biopsies for cancer. Sci. Transl. Med. 2019, 11, eaay1984. [Google Scholar] [CrossRef]
- Klein, E.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Hackshaw, A.; Cohen, S.S.; Reichert, H.; Kansal, A.R.; Chung, K.C.; Ofman, J.J. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br. J. Cancer 2021, 125, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D.; Ofman, J.J. Criteria for Evaluating Multi-cancer Early Detection Tests. Oncol. Haematol 2021, 17, 3–6. [Google Scholar] [CrossRef]
- Rollet, Q.; Tron, L.; De Mil, R.; Launoy, G.; Guillaume, É. Contextual factors associated with cancer screening uptake: A systematic review of observational studies. Prev. Med. 2021, 150, 106692. [Google Scholar] [CrossRef]
- Smith, J.; Dodd, R.H.; Gainey, K.M.; Naganathan, V.; Cvejic, E.; Jansen, J.; McCaffery, K.J. Patient-Reported Factors Associated With Older Adults’ Cancer Screening Decision-making: A Systematic Review. JAMA Netw. Open 2021, 4, e2133406. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Abola, M.V.; Fennimore, T.F.; Chen, M.M.; Chen, Z.; Sheth, A.K.; Cooper, G.; Li, L. Stool DNA-based versus colonoscopy-based colorectal cancer screening: Patient perceptions and preferences. Fam. Med. Community Health 2015, 3, 2–8. [Google Scholar] [CrossRef]
- Adler, A.; Geiger, S.; Keil, A.; Bias, H.; Schatz, P.; Devos, T.; Dhein, J.; Zimmermann, M.; Tauber, R.; Wiedenmann, B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014, 14, 183. [Google Scholar] [CrossRef]
- Benning, T.M.; Dellaert, B.G.C.; Dirksen, C.D.; Severens, J.L. Preferences for potential innovations in non-invasive colorectal cancer screening: A labeled discrete choice experiment for a Dutch screening campaign. Acta Oncol. 2014, 53, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.M.; Schroy, P.C.; Rosenberg, J.L.; Lai-Goldman, M.; Eisenberg, M.; Brown, T.; Rochelle, R.B.; Billings, P.R. Colorectal cancer screening using stool DNA analysis in clinical practice: Early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal tests. Clin. Color. Cancer 2006, 5, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Schroy, P.C.; Heeren, T.C. Patient perceptions of stool-based DNA testing for colorectal cancer screening. Am. J. Prev. Med. 2005, 28, 208–214. [Google Scholar] [CrossRef]
- Schroy, P.C.; Lal, S.; Glick, J.T.; Robinson, P.A.; Zamor, P.; Heeren, T.C. Patient preferences for colorectal cancer screening: How does stool DNA testing fare? Am. J. Manag. Care 2007, 13, 393–400. [Google Scholar]
- Yang, D.; Hillman, S.L.; Harris, A.M.; Sinicrope, P.S.; Devens, M.E.; Ahlquist, D.A. Patient perceptions of stool DNA testing for pan-digestive cancer screening: A survey questionnaire. World J. Gastroenterol. 2014, 20, 4972–4979. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.N. Transforming the landscape of early detection using blood tests—Commentary on current methodologies and future prospects. Br. J. Cancer 2021, 124, 1475–1477. [Google Scholar] [CrossRef]
- Lemke, A.A.; Amendola, L.M.; Kuchta, K.; Dunnenberger, H.M.; Thompson, J.; Johnson, C.; Ilbawi, N.; Oshman, L.; Hulick, P.J. Primary care physician experiences with integrated population-scale genetic testing: A mixed-methods assessment. J. Pers. Med. 2020, 10, 1065. [Google Scholar] [CrossRef]
- Harding, B.; Webber, C.; Ruhland, L.; Dalgarno, N.; Armour, C.M.; Birtwhistle, R.; Brown, G.; Carroll, J.C.; Flavin, M.; Phillips, S.; et al. Primary care providers’ lived experiences of genetics in practice. J. Community Genet. 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Mikat-Stevens, M.A.; Larson, L.A.; Tarini, B.A. Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genet. Med. 2015, 17, 169–176. [Google Scholar] [CrossRef]
- Rutten, L.J.F.; Parks, P.D.; Weiser, E.; Fan, C.; Jacobson, D.J.; Jenkins, G.D.; Zhu, X.; Griffin, J.M.; Limburg, P.J. Health care provider characteristics associated with colorectal cancer screening preferences and use. Mayo Clin. Proc. 2022, 97, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gelhorn, H.; Ross, M.M.; Kansal, A.R.; Fung, E.T.; Seiden, M.V.; Krucien, N.; Chung, K.C. Patient Preferences for Multi-Cancer Early Detection (MCED) Screening Tests. Patient 2022. online ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic-implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Etzioni, R.; Gulati, R.; Weiss, N.S. Multi-Cancer Early Detection: Learning from the past to Meet the Future. J. Natl. Cancer Inst. 2021, 114, 349–352. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Population and Sample Size | Study Design | Study Description | Outcomes |
|---|---|---|---|---|
| Abola et al., (2015) [17] | Patients 30–80 years of age at average risk for colorectal cancer (CRC) (n = 423) | Cross-sectional survey | Patients underwent both colonoscopy and stool DNA (sDNA) test, then completed a survey about their experience and preferences |
|
| Adler et al., (2014) [18] | Patients 50–75 years of age at average risk for CRC (n = 172) | Observational study | Patients who refused colonoscopy were offered either stool test or epi proColon blood test |
|
| Benning et al., (2014) [19] | Dutch adults 55–75 years of age (n = 815) | Case study using a discrete choice experiment (DCE) | Participants took an online survey where they were presented with attributes for blood, stool, or combination tests for colorectal cancer screening and asked to pick their preferred test |
|
| Berger et al., (2006) [20] | Patients whose doctors ordered an sDNA kit between August 2003 and July 2005 (n = 1211) | Cross-sectional survey | Patients answered a survey about their experience with the sDNA test |
|
| Schroy & Heeren (2005) [21] | Asymptomatic patients 50 years of age and older at mostly average risk for CRC (n = 4042) | Prospective survey | Patients completed a survey after undergoing sDNA, fecal occult blood test (FOBT), and colonoscopy |
|
| Schroy et al., (2007) [22] | Asymptomatic patients between the ages of 50 and 75 who had no prior screening for CRC other than FOBT (n = 263) | Cross-sectional survey | Patients reviewed a decision aid with a research assistant and were then asked to complete a survey to indicate their preferred CRC screening test and reasons for their choice |
|
| Yang et al., (2014) [23] | 1200 randomly selected patients stratified equally by gender and by the following age groups: 50–59, 60–69, and 70–79 received surveys. Only those returned were included. (n = 434) | Cross-sectional survey | Patients completed a survey regarding knowledge of, personal and family history of, and personal concern for cancer as well as past CRC screening behavior and interest in multi-organ stool DNA test (MUST) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, K.V.; Hallman, M.H.; DiCarlo, M.; Wambua, S.M.; Jaffe, R.L.; Welsh, A.W.; Kerber, C.; Yang, H.; Chambers, C.V.; Myers, R.E. Factors Likely to Affect the Uptake of Genomic Approaches to Cancer Screening in Primary Care: A Scoping Review. J. Pers. Med. 2022, 12, 2044. https://doi.org/10.3390/jpm12122044
Davis KV, Hallman MH, DiCarlo M, Wambua SM, Jaffe RL, Welsh AW, Kerber C, Yang H, Chambers CV, Myers RE. Factors Likely to Affect the Uptake of Genomic Approaches to Cancer Screening in Primary Care: A Scoping Review. Journal of Personalized Medicine. 2022; 12(12):2044. https://doi.org/10.3390/jpm12122044
Chicago/Turabian StyleDavis, Kaitlyn V., Mie H. Hallman, Melissa DiCarlo, Sophie M. Wambua, Rachel L. Jaffe, Allison W. Welsh, Cameron Kerber, Hushan Yang, Christopher V. Chambers, and Ronald E. Myers. 2022. "Factors Likely to Affect the Uptake of Genomic Approaches to Cancer Screening in Primary Care: A Scoping Review" Journal of Personalized Medicine 12, no. 12: 2044. https://doi.org/10.3390/jpm12122044
APA StyleDavis, K. V., Hallman, M. H., DiCarlo, M., Wambua, S. M., Jaffe, R. L., Welsh, A. W., Kerber, C., Yang, H., Chambers, C. V., & Myers, R. E. (2022). Factors Likely to Affect the Uptake of Genomic Approaches to Cancer Screening in Primary Care: A Scoping Review. Journal of Personalized Medicine, 12(12), 2044. https://doi.org/10.3390/jpm12122044






