Liver Tumor Markers, HALP Score, and NLR: Simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow-Up and Data
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
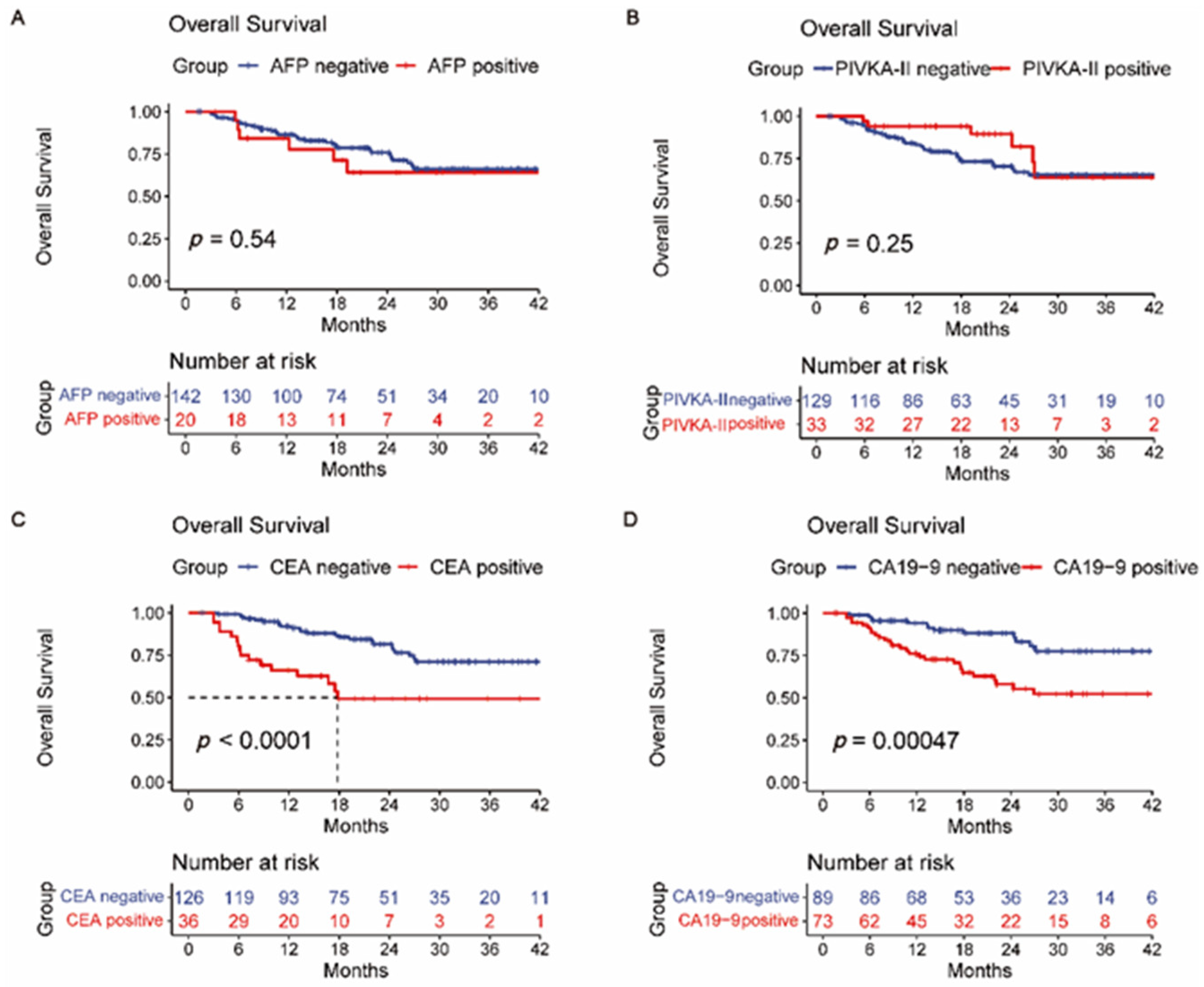
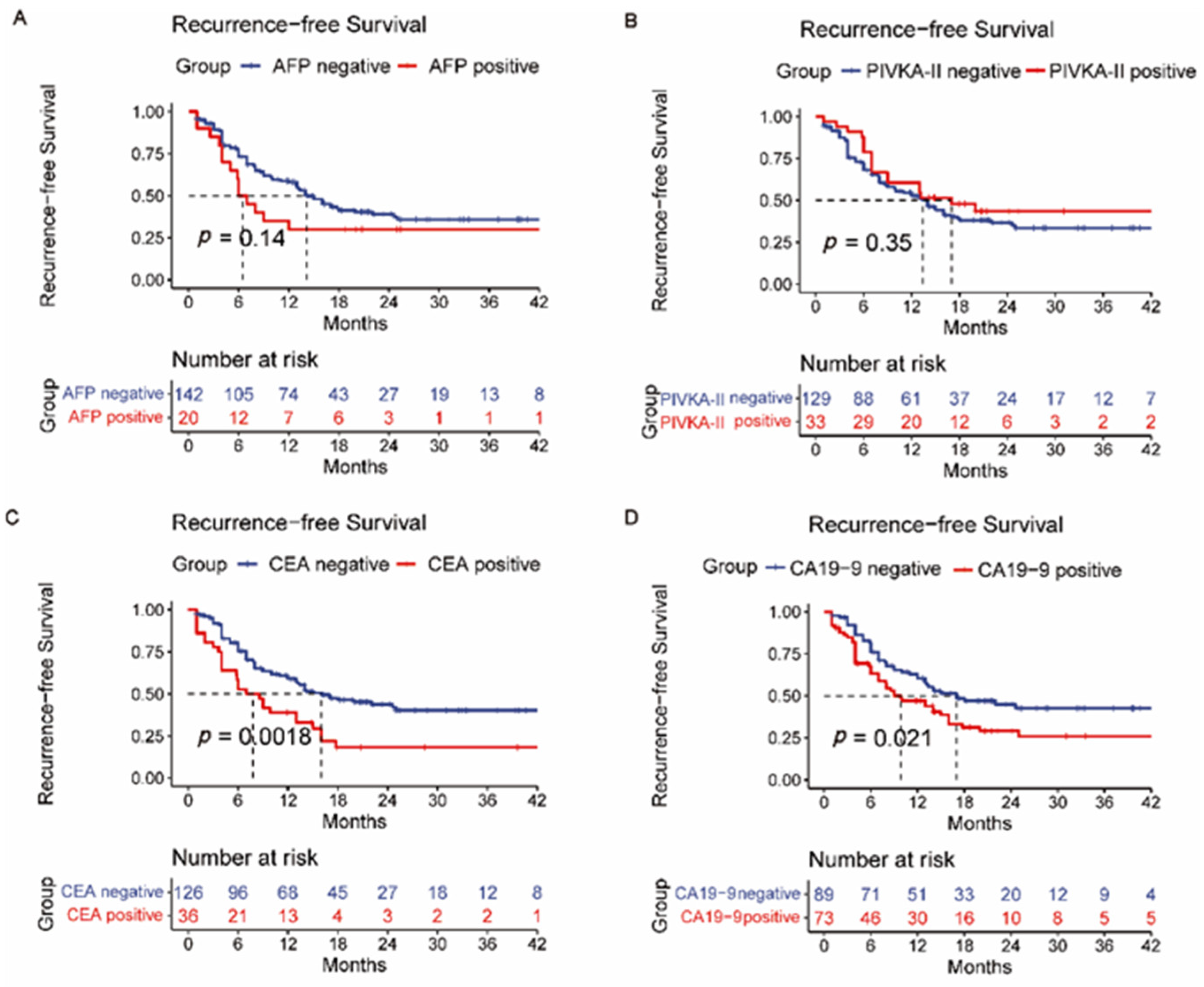
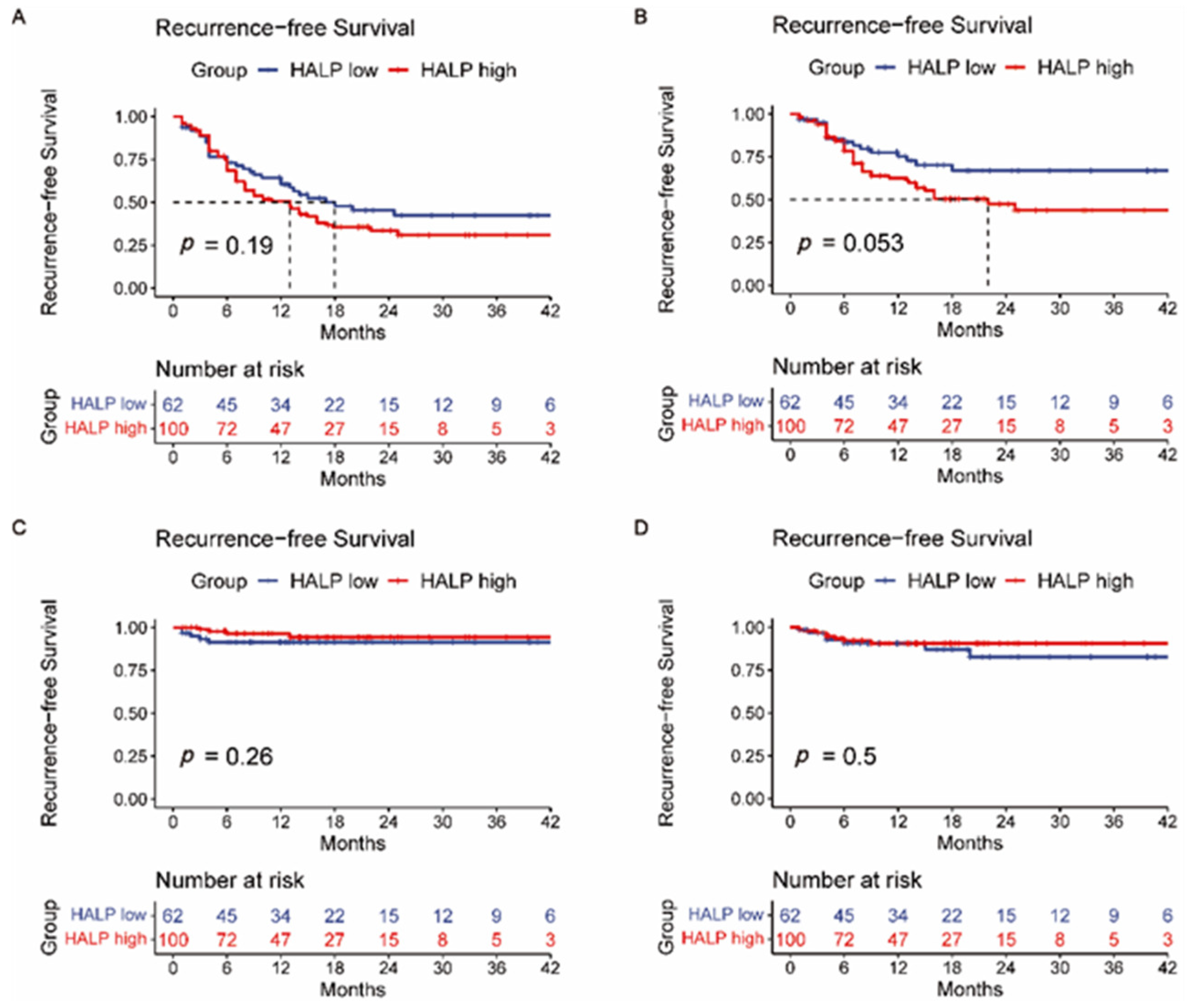
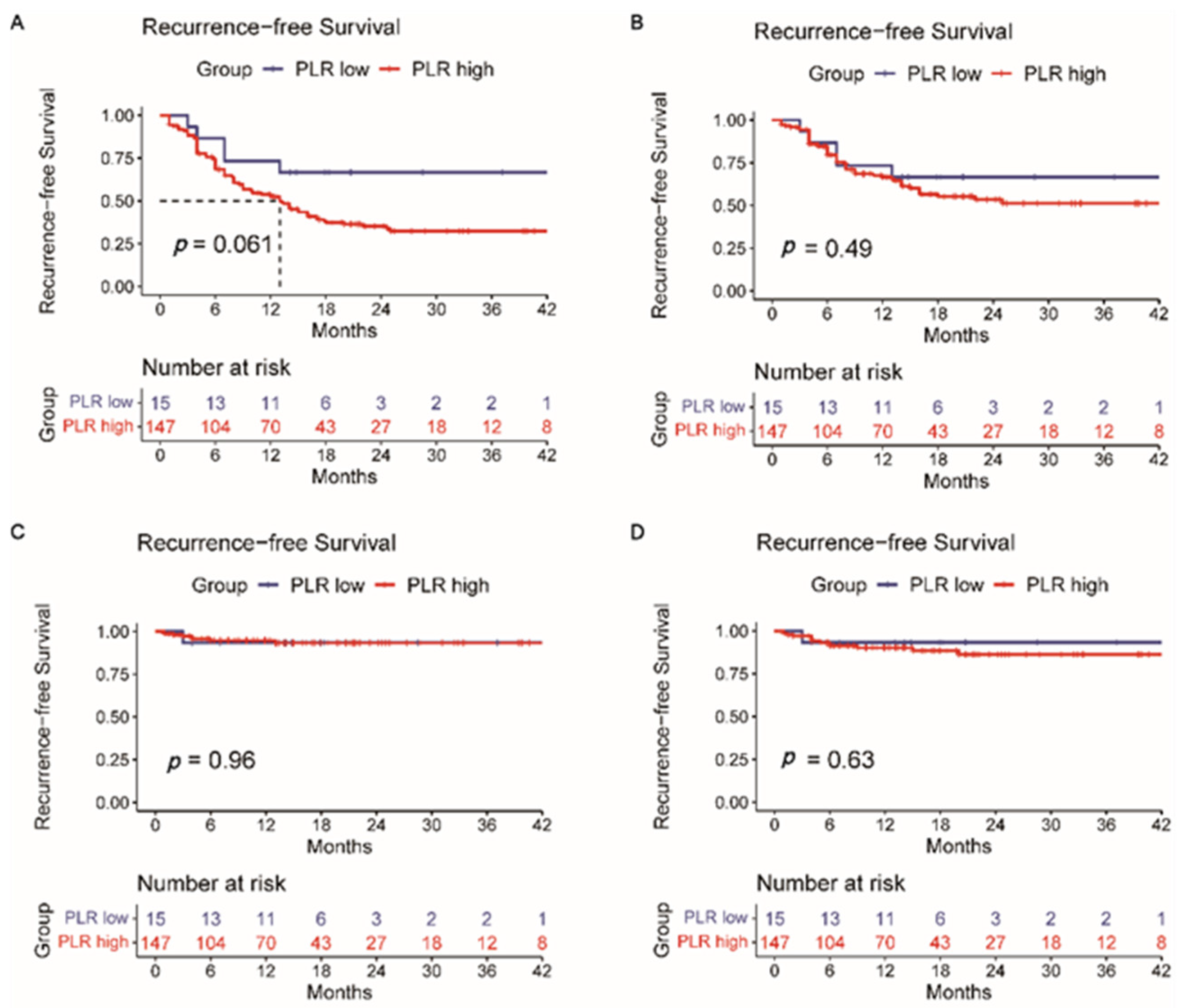
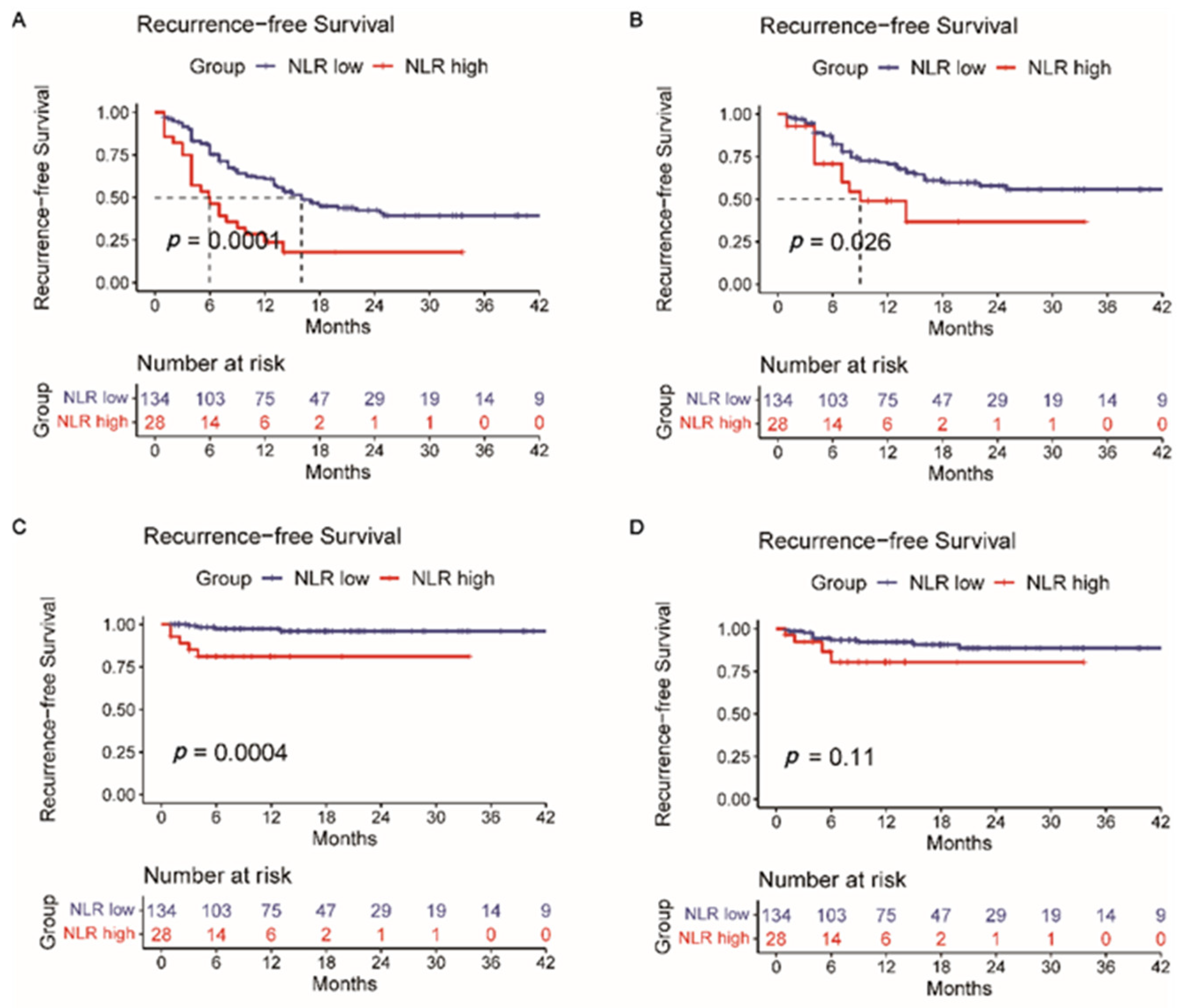
3.2. Overall Survival and Recurrence-Free Survival Analysis
3.3. Univariate and Multivariable Cox Regression Analyses in Cohorts
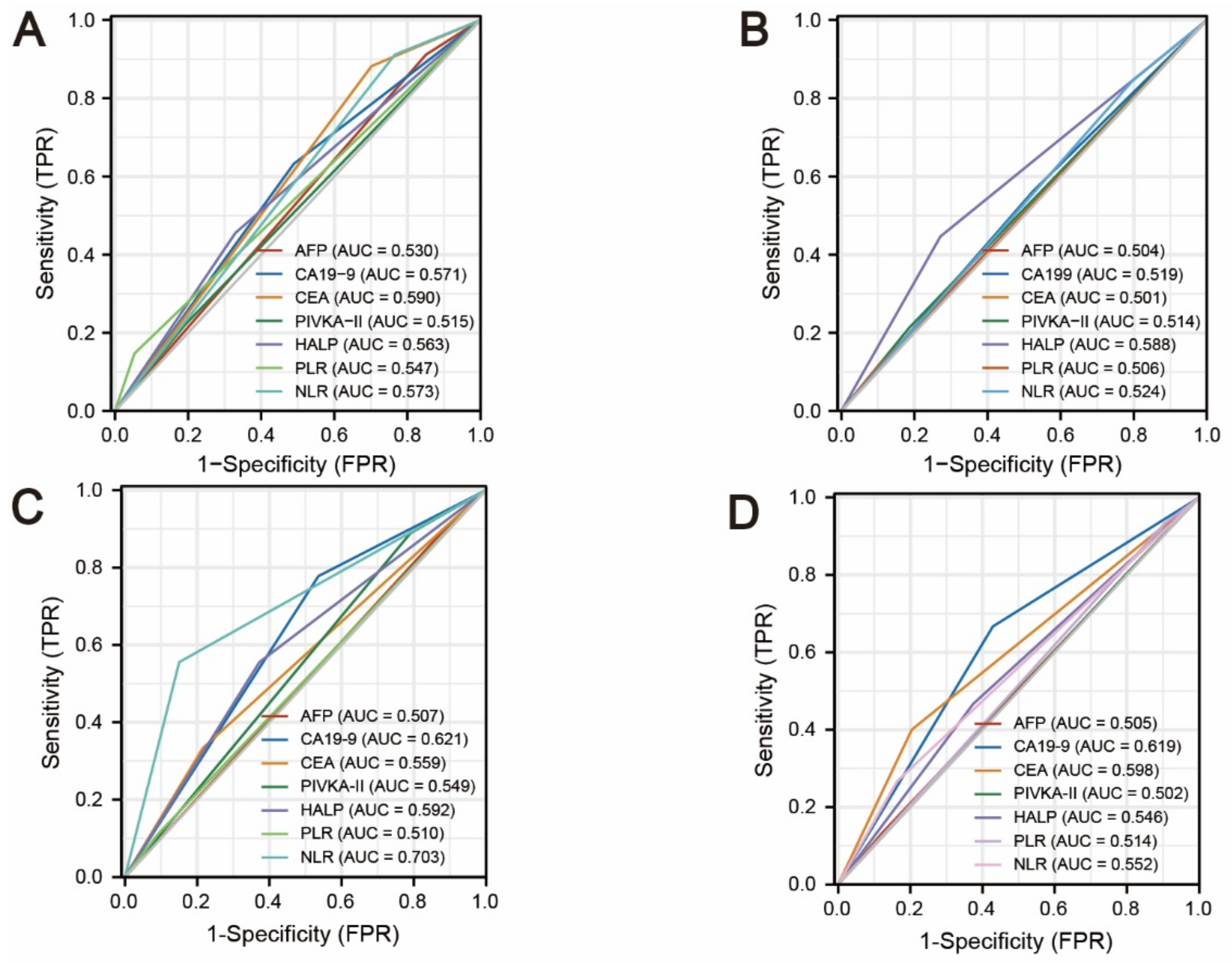
3.4. Comparison of AFP, PIVKA-II, CEA, CA19-9, HALP Score, PLR, and NLR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [Google Scholar]
- Mavros, M.; Economopoulos, K.; Alexiou, V.; Pawlik, T.J.J. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [PubMed]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [PubMed]
- Jiang, W.; Zeng, Z.C.; Tang, Z.Y.; Fan, J.; Sun, H.C.; Zhou, J.; Zeng, M.S.; Zhang, B.H.; Ji, Y.; Chen, Y.X. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: The Fudan score. Ann. Oncol. 2011, 22, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar]
- Shen, W.F.; Zhong, W.; Xu, F.; Kan, T.; Geng, L.; Xie, F.; Sui, C.J.; Yang, J.M. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2009, 15, 5976–5982. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, L.; He, Q.; Wang, S.; Pan, Z.; Wang, T.; Zhao, Y. Diagnostic accuracy of serum CA19-9 in patients with cholangiocarcinoma: A systematic review and meta-analysis. J. Pharmacol. Exp. Ther. 2015, 21, 3555–3563. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Paladina, I.; Giordano, M.; Malaguarnera, M.; Bertino, G.; Berretta, M. Serum markers of intrahepatic cholangiocarcinoma. Dis. Mark. 2013, 34, 219–228. [Google Scholar] [CrossRef]
- Nichols, J.C.; Gores, G.J.; LaRusso, N.F.; Wiesner, R.H.; Nagorney, D.M.; Ritts, R.E., Jr. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin. Proc. 1993, 68, 874–879. [Google Scholar] [CrossRef]
- Singh, S.; Tang, S.; Sreenarasimhaiah, J.; Lara, L.; Siddiqui, A. The clinical utility and limitations of serum carbohydrate antigen (CA19-9) as a diagnostic tool for pancreatic cancer and cholangiocarcinoma. Dig. Dis. Sci. 2011, 56, 2491–2496. [Google Scholar]
- Dolscheid-Pommerich, R.C.; Manekeller, S.; Walgenbach-Brünagel, G.; Kalff, J.C.; Hartmann, G.; Wagner, B.S.; Holdenrieder, S. Clinical performance of CEA, CA19-9, CA15-3, CA125 and AFP in gastrointestinal cancer using LOCI™-based assays. Anticancer Res. 2017, 37, 353–359. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Z.R.; Shi, J.S.; Lu, M.; Wang, L.; He, Q.R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004, 10, 427–432. [Google Scholar]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Koch, A.; Vucur, M.; Schneider, A.T.; Binnebösel, M.; Ulmer, T.F.; Lurje, G.; Schoening, W.; et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017, 7, 16975. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Song, Y.; Wang, J.; Xing, K.; Lin, X.; Li, S. Preoperative CEA levels are supplementary to CA19-9 levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J. Cancer 2018, 9, 3117–3128. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Miyagawa, Y.; Ishida, K.; Shimada, R.; Miyagawa, S.; Makuuchi, M.; Kawasaki, S. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br. J. Surg. 1999, 86, 1032–1038. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, X.; Hu, Z.; Xie, W.; Nie, K.; Fang, A.; Zhang, Y.; Fu, Y.; Chen, J.; Wang, J.; et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in China: An analysis of 4792 patients. J. Hepatocell. Carcinoma 2021, 8, 657–670. [Google Scholar] [CrossRef]
- Güç, Z.G.; Alacacıoğlu, A.; Kalender, M.E.; Oflazoğlu, U.; Ünal, S.; Yıldız, Y.; Salman, T.; Küçükzeybek, Y.; Tarhan, M.O. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front. Nutr. 2022, 9, 905292. [Google Scholar]
- Mouchli, M.; Reddy, S.; Gerrard, M.; Boardman, L.; Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article. Ann. Hepatol. 2021, 22, 100249. [Google Scholar] [CrossRef]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I.C. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Meyer, J.E.; Karachristos, A.; Maranki, J.L.; Camp, E.R.; Denlinger, C.S. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer 2016, 122, 1349–1369. [Google Scholar] [PubMed]
- Wang, Q.; Chen, Q.; Zhang, X.; Lu, X.L.; Du, Q.; Zhu, T.; Zhang, G.Y.; Wang, D.S.; Fan, Q.M. Diagnostic value of gamma-glutamyltransferase/aspartate aminotransferase ratio, protein induced by vitamin K absence or antagonist II, and alpha-fetoprotein in hepatitis B virus-related hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 5515–5529. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, X.; Du, Q.; Zhang, G.; Wang, D.; Wang, Q.; Guo, X. Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular carcinoma. Sci. Rep. 2020, 10, 13519. [Google Scholar] [PubMed]
- Inagaki, Y.; Tang, W.; Makuuchi, M.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int. Off. J. Int. Assoc. Study Liver 2011, 31, 22–35. [Google Scholar] [CrossRef]
- Suttie, J.W. Recent advances in hepatic vitamin K metabolism and function. Hepatology 1987, 7, 367–376. [Google Scholar] [CrossRef]
- Zakhary, N.I.; Khodeer, S.M.; Shafik, H.E.; Abdel Malak, C.A. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J. Adv. Res. 2013, 4, 539–546. [Google Scholar] [CrossRef]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Zhou, H.B.; Hu, J.Y.; Hu, H.P. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2014, 20, 5721–5729. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Lei, Z.; Wu, D.; Si, A.; Wang, K.; Wang, Y.; Wan, X.; Lau, W.Y.; Shen, F. Prognosis of intrahepatic cholangiocarcinomas with HBV infection is better than those with hepatolithiasis after R0 liver resection: A propensity score matching analysis. Ann. Surg. Oncol. 2017, 24, 1579–1587. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Pang, X.; Pu, H.; Lei, M.; Fan, B.; Lv, J.; You, D.; Li, Z.; Zhang, T. Trajectories of perioperative serum tumor markers and colorectal cancer outcomes: A retrospective, multicenter longitudinal cohort study. EBioMedicine 2021, 74, 103706. [Google Scholar] [CrossRef]
- Reitz, D.; Gerger, A.; Seidel, J.; Kornprat, P.; Samonigg, H.; Stotz, M.; Szkandera, J.; Pichler, M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J. Clin. Pathol. 2015, 68, 427–433. [Google Scholar] [CrossRef]
- Cai, H.; Kong, W.T.; Chen, C.B.; Shi, G.M.; Huang, C.; Shen, Y.H.; Sun, H.C. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: A meta-analysis of observational studies. BMC Cancer 2015, 15, 831. [Google Scholar]
| Patient Characteristics | Number | Percentage | |
|---|---|---|---|
| Age | ≥56 | 91 | 56.2% |
| <56 | 71 | 43.8% | |
| Gender | Male | 100 | 61.7% |
| Female | 62 | 37.4% | |
| Tumor numbers | Single | 108 | 66.7% |
| Multiple | 54 | 33.3% | |
| Tumor size | ≤2.0 cm | 5 | 3.1% |
| 2.1–5.0 cm | 70 | 43.2% | |
| >5.0 cm | 87 | 53.7% | |
| Hepatis B surface antigen | Positive | 85 | 52.5% |
| Negative | 77 | 47.5% | |
| Hepatitis C virus antibody | Positive | 1 | 0.6% |
| Negative | 161 | 99.4% | |
| Platelet count (×109/L) | 242.8 ± 87.1 | ||
| Albumin (mg/dL) | 43.6 ± 3.7 | ||
| Alanine aminotransferase (U/L) | 35.4 ± 33.6 | ||
| Aspartate aminotransferase (U/L) | 32.6 ± 27.2 | ||
| Total bilirubin (umol/L) | 13.9 ± 19.9 | ||
| Prothrombin time (s) | 12.0 ± 4.9 | ||
| T stage | 1 | 89 | 54.9% |
| 2 | 58 | 35.8% | |
| 3 | 1 | 0.6% | |
| 4 | 14 | 8.6% | |
| N stage | 0 | 135 | 83.3% |
| 1 | 27 | 16.7% | |
| M stage | 0 | 153 | 94.4% |
| 1 | 9 | 5.6% | |
| AJCC TNM staging system | I | 82 | 50.6% |
| II | 41 | 25.3% | |
| III | 27 | 16.7% | |
| IV | 12 | 7.4% | |
| The number of elevated pretreatment serum liver tumor markers | 0 | 57 | 35.2% |
| 1 | 55 | 34.0% | |
| ≥2 | 50 | 30.8% | |
| Vascular invasion | Yes | 33 | 20.4% |
| No | 129 | 79.6% | |
| Microvascular invasion | Yes | 43 | 26.5% |
| No | 117 | 72.2% | |
| Unknown | 2 | 1.2% | |
| Nerve tract invasion | Yes | 30 | 18.5% |
| No | 130 | 80.2% | |
| Unknown | 2 | 1.2% | |
| Tumor differentiations | Well | 1 | 0.6% |
| Well-moderate | 1 | 0.6% | |
| Moderate | 45 | 27.8% | |
| Moderate to poor | 83 | 51.2% | |
| Poor | 21 | 13.0% | |
| Unknown | 11 | 6.8% | |
| Alpha-fetoprotein (25 ng/mL) | 421.9 ± 3664.4 | ||
| Cancer antigen 199 (35 U/mL) | 715.4 ± 2306.8 | ||
| Carcinoembryonic antigen (5 ng/mL) | 6.76 ± 19.6 | ||
| Protein induced by vitamin K absence or Antagonist-II (40 mAU/mL) | 400.7 ± 4112.3 | ||
| Alpha-fetoprotein (25 ng/mL) | Elevated | 20 | 12.3% |
| Normal | 142 | 87.7% | |
| Cancer antigen 199 (35 U/mL) | Elevated | 73 | 45.1% |
| Normal | 89 | 54.9% | |
| Carcinoembryonic antigen (5 ng/mL) | Elevated | 36 | 22.2% |
| Normal | 126 | 77.8% | |
| Protein induced by vitamin K absence or Antagonist-II (40 mAU/mL) | Elevated | 33 | 20.4% |
| Normal | 129 | 79.6% | |
| Hemoglobin, albumin, lymphocyte, and platelet score | 54.3 ± 30.1 | ||
| Neutrophil-to-lymphocyte ratio | 2.9 ± 2.8 | ||
| Platelet-to-lymphocyte ratio | 140.0 ± 66.6 | ||
| History of cholelithiasis | Yes | 30 | 18.5% |
| No | 132 | 81.5% | |
| Child–Pugh classifications | A | 160 | 98.8% |
| B | 2 | 1.2% | |
| Barcelona Clinic Liver Cancer staging | A | 100 | 61.7% |
| B | 20 | 12.3% | |
| C | 42 | 25.9% | |
| Recurrence | Yes | 94 | 58.0 |
| NO | 68 | 42.0% | |
| Intrahepatic tumor recurrence | Yes | 59 | 36.4% |
| NO | 103 | 63.6% | |
| Lymph node metastasis | Yes | 9 | 5.6% |
| NO | 153 | 94.4% | |
| Distant metastasis | Yes | 15 | 9.3% |
| NO | 147 | 90.7% | |
| Variables | Overall Survival Univariate Analysis | Overall Survival Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≥56 vs. <56) | 1.725 | 0.916–3.248 | 0.091 | |||
| Gender (male vs. female) | 0.977 | 0.718–1.330 | 0.883 | |||
| Cirrhosis (yes vs. no) | 1.146 | 0.614–2.138 | 0.669 | |||
| Tumor number (multiple vs. single) | 1.757 | 0.953–3.239 | 0.071 | |||
| Tumor size (>5 cm vs. ≤5 cm) | 1.800 | 0.963–3.364 | 0.065 | |||
| Vascular invasion | 2.992 | 1.615–5.543 | <0.001 * | 2.347 | 1.216–4.530 | 0.011 * |
| Pathology microvascular invasion | 1.311 | 0.681–2.523 | 0.418 | |||
| Pathology nerve tract invasion | 2.846 | 1.490–5.437 | 0.002 * | 3.018 | 1.536–5.927 | 0.001 * |
| Pathology differentiation degree (Moderate to poor, poor vs. well, well to moderate, moderate) | 1.483 | 0.719–3.056 | 0.286 | |||
| AFP (≥25 vs. <25) | 1.236 | 0.521–2.935 | 0.631 | |||
| CA19-9 (≥35 vs. <35) | 2.653 | 1.410–4.989 | 0.002 * | 2.071 | 1.030–4.167 | 0.041 * |
| CEA (≥5 vs. <5) | 3.384 | 1.818–6.300 | <0.001 * | 1.965 | 0.880–3.746 | 0.107 |
| PIVKA-II (≥40 vs. <40) | 0.463 | 0.182–1.180 | 0.107 | |||
| History of cholelithiasis | 1.660 | 0.909–3.032 | 0.099 | |||
| Hepatitis B | 0.920 | 0.502–1.687 | 0.788 | |||
| HALP (≥43.63 vs. <43.63) | 1.258 | 0.662–2.391 | 0.483 | |||
| NLR (≥3.73 vs. <3.73) | 0.475 | 0.238–0.946 | 0.034 * | 1.463 | 0.691–3.098 | 0.320 |
| PLR (≥76.51 vs. <76.51) | 0.217 | 0.030–1.580 | 0.132 | |||
| Variables | Recurrence-Free Survival Univariate Analysis | Recurrence-Free Survival Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≥56 vs. <56) | 1.088 | 0.723–1.636 | 0.686 | |||
| Gender (male vs. female) | 1.172 | 0.948–1.449 | 0.142 | |||
| Cirrhosis (yes vs.no) | 1.277 | 0.844–1.933 | 0.247 | |||
| Tumor number (multiple vs. single) | 2.491 | 1.651–3.759 | <0.001 * | 2.060 | 1.250–3.397 | 0.005 * |
| Tumor size (>5 cm vs. ≤5 cm) | 1.925 | 1.265–2.928 | 0.002 * | 1.315 | 0.813–2.127 | 0.265 |
| Vascular invasion | 2.539 | 1.619–3.982 | <0.001 * | 1.561 | 0.931–2.617 | 0.091 |
| Pathology microvascular invasion | 1.980 | 1.290–3.040 | 0.002 * | 1.800 | 1.121–2.892 | 0.015 * |
| Pathology nerve tract invasion | 1.204 | 0.719–2.015 | 0.481 | |||
| Pathology differentiation degree (moderate to poor, poor vs. well, well to moderate, moderate) | 1.976 | 1.187–3.290 | 0.009* | 1.826 | 1.054–3.163 | 0.032 * |
| AFP (≥25 vs. <25) | 1.514 | 0.858–2.675 | 0.153 | |||
| CA19-9 (≥35 vs. <35) | 1.587 | 1.058–2.380 | 0.025 * | 1.794 | 1.117–2.882 | 0.016 * |
| CEA (≥5 vs. <5) | 1.972 | 1.264–3.074 | 0.003 * | 1.037 | 0.609–1.766 | 0.893 |
| PIVKA-II (≥40 vs. <40) | 0.789 | 0.472–1.320 | 0.789 | |||
| History of cholelithiasis | 1.326 | 0.809–2.173 | 0.263 | |||
| Hepatitis B | 1.056 | 0.704–1.585 | 0.792 | |||
| HALP (≥43.63 vs. <43.63) | 1.319 | 0.857–2.030 | 0.208 | |||
| NLR (≥3.73 vs. <3.73) | 0.400 | 0.246–0.652 | 0.000 * | 2.424 | 1.386–4.239 | 0.002 * |
| PLR (≥76.51 vs. <76.51) | 0.439 | 0.178–1.082 | 0.074 | |||
| Intrahepatic Tumor Recurrence | Lymph Node Metastasis | Distant Metastasis | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Univariate Analysis | ||||
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age (≥56 vs. <56) | 1.320 (0.782–2.229) | 0.298 | 1.635 (0.409–6.538) | 0.487 | 0.549 (0.195–1.542) | 0.255 |
| Gender (male vs. female) | 1.202 (0.918–1.574) | 0.182 | 0.924 (0.478–1.783) | 0.813 | 1.036 (0.617–1.738) | 0.895 |
| Cirrhosis (yes vs. no) | 1.196 (0.711–2.012) | 0.500 | 0.634 (0.170–2.361) | 0.497 | 0.923 (0.335–2.548) | 0.878 |
| Tumor number (multiple vs. single) | 2.461 (1.465–4.135) | 0.001 * | 1.852 (0.494–6.946) | 0.361 | 1.667 (0.588–4.727) | 0.336 |
| Tumor size (>5 cm vs. ≤5 cm) | 1.977 (1.162–3.364) | 0.012 * | 7.836 (0.977–62.857) | 0.053 | 2.971 (0.938–9.408) | 0.064 |
| Vascular invasion | 1.983 (1.094–3.591) | 0.024 * | 0.584 (0.073–4.682) | 0.613 | 3.496 (1.227–9.966) | 0.019 * |
| Pathology microvascular invasion | 1.832 (1.063–3.158) | 0.029 * | 0.438 (0.054–3.562) | 0.440 | 1.301 (0.406–4.170) | 0.658 |
| Pathology nerve tract invasion | 0.794 (0.377–1.676) | 0.546 | 0.600 (0.075–4.799) | 0.630 | 0.354 (0.046–2.690) | 0.315 |
| Pathology differentiation degree (moderate to poor, poor vs. well, well to moderate, moderate) | 1.971 (1.037–3.743) | 0.038 * | 3.919 (0.490–31.359) | 0.198 | 2.911 (0.643–13.173) | 0.165 |
| AFP (≥25 vs. <25) | 1.171 (0.531–2.579) | 0.696 | 0.983 (0.123–7.875) | 0.987 | 1.233 (0.278–5.472) | 0.783 |
| CA19-9 (≥35 vs. <35) | 1.365 (0.818–2.277) | 0.234 | 0.393 (0.082–1.894) | 0.244 | 2.891 (0.986–8.473) | 0.053 |
| CEA (≥5 vs. <5) | 1.302 (0.702–2.414) | 0.403 | 2.012 (0.502–8.059) | 0.323 | 2.920 (1.033–8.255) | 0.043 * |
| PIVKA-II (≥40 vs. <40) | 0.763 (0.962–1.141) | 0.419 | 0.435 (0.054–3.480) | 0.433 | 0.858 (0.242–3.042) | 0.813 |
| History of cholelithiasis | 1.259 (0.668–2.347) | 0.476 | 2.324 (0.581–9.296) | 0.233 | 3.106 (1.105–8.729) | 0.032 * |
| Hepatitis B | 0.976 (0.585–1.627) | 0.925 | 1.068 (0.287–3.980) | 0.922 | 1.741 (0.595–5.099) | 0.312 |
| HALP (≥43.63 vs. <43.63) | 1.732 (0.975–3.078) | 0.061 | 0.478 (0.128–1.782) | 0.272 | 0.703 (0.255–1.941) | 0.703 |
| NLR (≥3.73 vs. <3.73) | 2.020 (1.061–3.847) | 0.032 * | 7.757 (2.031–29.619) | 0.003 * | 2.468 (0.769–7.921) | 0.129 |
| PLR (≥76.51 vs. <76.51) | 1.375 (0.549–3.438) | 0.497 | 0.944 (0.118–7.566) | 0.957 | 1.639 (0.215–12.467) | 0.633 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zeng, H.; Pan, Y.; Zhao, Y.; Wang, X.; Chen, J.; Wang, J.; Zhang, Y.; Zhou, Z.; Xu, L.; et al. Liver Tumor Markers, HALP Score, and NLR: Simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery. J. Pers. Med. 2022, 12, 2041. https://doi.org/10.3390/jpm12122041
Zhang D, Zeng H, Pan Y, Zhao Y, Wang X, Chen J, Wang J, Zhang Y, Zhou Z, Xu L, et al. Liver Tumor Markers, HALP Score, and NLR: Simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery. Journal of Personalized Medicine. 2022; 12(12):2041. https://doi.org/10.3390/jpm12122041
Chicago/Turabian StyleZhang, Deyao, Huilan Zeng, Yangxun Pan, Yumo Zhao, Xin Wang, Jinbin Chen, Juncheng Wang, Yaojun Zhang, Zhongguo Zhou, Li Xu, and et al. 2022. "Liver Tumor Markers, HALP Score, and NLR: Simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery" Journal of Personalized Medicine 12, no. 12: 2041. https://doi.org/10.3390/jpm12122041
APA StyleZhang, D., Zeng, H., Pan, Y., Zhao, Y., Wang, X., Chen, J., Wang, J., Zhang, Y., Zhou, Z., Xu, L., Chen, M., & Hu, D. (2022). Liver Tumor Markers, HALP Score, and NLR: Simple, Cost-Effective, Easily Accessible Indexes for Predicting Prognosis in ICC Patients after Surgery. Journal of Personalized Medicine, 12(12), 2041. https://doi.org/10.3390/jpm12122041






