Correlation between Androgen Receptor Expression in Luminal B (HER–2 Negative) Breast Cancer and Disease Outcomes
Abstract
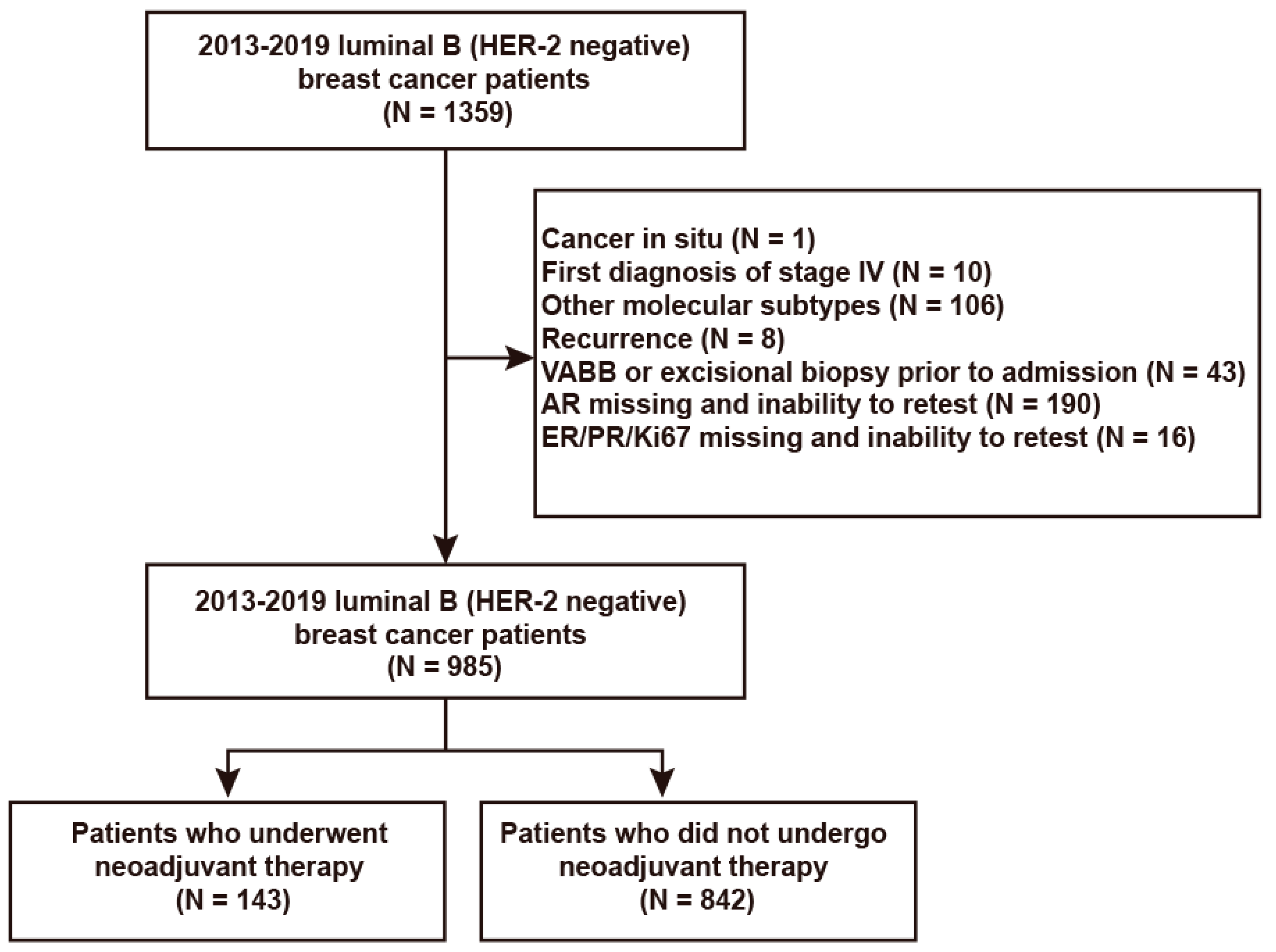
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Specimen Preparation and Staining
2.3. Immunohistochemistry (IHC) and Fluorescent In Situ Hybridization (FISH)
2.4. Neoadjuvant Treatment Regimen and Evaluation Criteria
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prognosis–Related Risk Factors in the Overall Luminal B (HER–2 Negative) Breast Cancer Population
3.3. Prognosis–Related AR Cutoff Values in the Overall Luminal B (HER–2 Negative) Breast Cancer Population
3.4. Prognosis–Related Risk Factors in the Non–pCR Population of Luminal B (HER–2 Negative) Breast Cancer Who Underwent Neoadjuvant Therapy
3.5. Prognosis–Related AR/ER and Residual Tumor Ki67 Cutoff Values in the Non–pCR Population of Luminal B (HER–2 Negative) Breast Cancer Who Underwent Neoadjuvant Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Tang, L.; Xiao, Z. Analysis of clinical features and molecular subtype distribution of 2324 breast cancer patients in Hunan province. Chin. J. Gen. Surg. (Nat. Sci. Ed.) 2013, 22, 1403–1409. [Google Scholar]
- Linghu, R.; Si, W.; Li, Y.; Yang, J. Epidemiological and clinicopathological characteristics of patients with breast cancer: A retrospective analysis of 3846 case. Acad. J. Chin. PLA Med. (Nat. Sci. Ed.) 2015, 36, 1017–1038. [Google Scholar]
- Liu, Q.; Zhang, Y.; Zhou, X.; Lv, L.; Luo, C.; Wang, X. A single center, retrospective analysis on clinical epidemiology and pathologic characteristics of breast cancer in Beijing from 2009 to 2018. Tumor (Nat. Sci. Ed.) 2020, 40, 431–439. [Google Scholar]
- Park, S.; Koo, J.; Kim, M.; Park, H.; Lee, J.; Kim, S.; Park, B.-W.; Lee, K. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor–positive breast cancers. Ann. Oncol. 2011, 22, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Venema, C.M.; Bense, R.D.; Steenbruggen, T.G.; Nienhuis, H.H.; Qiu, S.-Q.; van Kruchten, M.; Brown, M.; Tamimi, R.M.; Hospers, G.A.; Schröder, C.P.; et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol. Ther. 2019, 200, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven–Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay–Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor–positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [Green Version]
- Cejalvo, J.M.; de Dueñas, E.M.; Galván, P.; García–Recio, S.; Gasión, O.B.; Paré, L.; Antolín, S.; Martinello, R.; Blancas, I.; Adamo, B.; et al. Intrinsic Subtypes and Gene Expression Profiles in Primary and Metastatic Breast Cancer. Cancer Res. 2017, 77, 2213–2221. [Google Scholar] [CrossRef] [Green Version]
- Daemen, A.; Manning, G. HER2 is not a cancer subtype but rather a pan–cancer event and is highly enriched in AR–driven breast tumors. Breast Cancer Res. 2018, 20, 8. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumours of the Breast, 4th ed.; IARC, Press: Lyon, France, 2012. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Arch. Pathol. Lab. Med. 2013, 138, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J.; Panel Members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long–term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, G.; Bravaccini, S.; Ravaioli, S.; Puccetti, M.; Scarpi, E.; Andreis, D.; Tumedei, M.M.; Sarti, S.; Cecconetto, L.; Pietri, E.; et al. Androgen Receptor Expression in Breast Cancer: What Differences Between Primary Tumor and Metastases? Transl. Oncol. 2018, 11, 950–956. [Google Scholar] [CrossRef]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrøm, M.J.; Bofin, A.M. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar] [CrossRef]
- Kono, M.; Fujii, T.; Lim, B.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Androgen Receptor Function and Androgen Receptor–Targeted Therapies in Breast Cancer. JAMA Oncol. 2017, 3, 1266–1273. [Google Scholar] [CrossRef]
- Kensler, K.H.; Poole, E.M.; Heng, Y.J.; Collins, L.C.; Glass, B.; Beck, A.H.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. JNCI J. Natl. Cancer Inst. 2018, 111, 700–708. [Google Scholar] [CrossRef]
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J.; et al. Adjuvant Ovarian Suppression in Premenopausal Breast Cancer. N. Engl. J. Med. 2015, 372, 436–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccart, M.; Veer, L.J.V.; Poncet, C.; Cardozo, J.M.N.L.; Delaloge, S.; Pierga, J.-Y.; Vuylsteke, P.; Brain, E.; Vrijaldenhoven, S.; A Neijenhuis, P.; et al. 70–gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021, 22, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin–Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Bianco–Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.A.; Sakko, A.J.; et al. The Magnitude of Androgen Receptor Positivity in Breast Cancer Is Critical for Reliable Prediction of Disease Outcome. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Min, A.; Lee, K.-H.; Ryu, H.S.; Kim, T.-Y.; Woo, G.-U.; Suh, K.J.; Lee, D.-W.; Lee, H.-B.; Moon, H.-G.; et al. Prognostic Role of Androgen Receptor Expression in Surgically Resected Early Breast Cancer Patients. J. Breast Cancer 2020, 23, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Witzel, I.; Loibl, S.; Wirtz, R.; Fasching, P.A.; Denkert, C.; Weber, K.; Lück, H.-J.; Huober, J.; Karn, T.; Von Mackelenbergh, M.; et al. Androgen receptor expression and response to chemotherapy in breast cancer patients treated in the neoadjuvant TECHNO and PREPARE trial. Br. J. Cancer 2019, 121, 1009–1015. [Google Scholar] [CrossRef]
- Rangel, N.; Lagos, M.R.; Annaratone, L.; Osella–Abate, S.; Metovic, J.; Mano, M.P.; Bertero, L.; Cassoni, P.; Sapino, A.; Castellano, I. The role of the AR/ER ratio in ER–positive breast cancer patients. Endocr. Relat. Cancer 2018, 25, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Rangel, N.; Rondon–Lagos, M.; Annaratone, L.; Aristizábal–Pachon, A.F.; Cassoni, P.; Sapino, A.; Castellano, I. AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors. Cells 2020, 9, 1064. [Google Scholar] [CrossRef]
- Bronte, G.; Rocca, A.; Ravaioli, S.; Scarpi, E.; Bonafè, M.; Puccetti, M.; Maltoni, R.; Andreis, D.; Martinelli, G.; Bravaccini, S. Evaluation of Androgen Receptor in Relation to Estrogen Receptor (AR/ER) and Progesterone Receptor (AR/PgR): A New Must in Breast Cancer? J. Oncol. 2019, 2019, 1393505. [Google Scholar] [CrossRef]
- Chia, K.M.; Milioli, H.; Portman, N.; Laven–Law, G.; Coulson, R.; Yong, A.; Segara, D.; Parker, A.; E Caldon, C.; Deng, N.; et al. Non–canonical AR activity facilitates endocrine resistance in breast cancer. Endocr. Relat. Cancer 2019, 26, 251–264. [Google Scholar] [CrossRef]
- Rajarajan, S.; Korlimarla, A.; Alexander, A.; Anupama, C.E.; Ramesh, R.; Srinath, B.S.; Sridhar, T.S.; Prabhu, J.S. Pre–Menopausal Women With Breast Cancers Having High AR/ER Ratios in the Context of Higher Circulating Testosterone Tend to Have Poorer Outcomes. Front. Endocrinol. 2021, 12, 679756. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Cinausero, M.; Iacono, D.; Pelizzari, G.; Bonotto, M.; Vitale, M.G.; Gerratana, L.; Puglisi, F. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat. Rev. 2017, 61, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Karamouzis, M.V.; Papavassiliou, K.A.; Adamopoulos, C.; Papavassiliou, A.G. Targeting Androgen/Estrogen Receptors Crosstalk in Cancer. Trends Cancer 2015, 2, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. AR pathway activity correlates with AR expression in a HER2–dependent manner and serves as a better prognostic factor in breast cancer. Cell Oncol. 2020, 43, 321–333. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schmitt, W.D.; Loibl, S.; Müller, B.M.; Blohmer, J.U.; Sinn, B.V.; Eidtmann, H.; Eiermann, W.; Gerber, B.; Tesch, H.; et al. Ki67 Measured after Neoadjuvant Chemotherapy for Primary Breast Cancer. Clin. Cancer Res. 2013, 19, 4521–4531. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Ross, H.A.S.; Von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome Prediction for Estrogen Receptor–Positive Breast Cancer Based on Postneoadjuvant Endocrine Therapy Tumor Characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Goncalves, R.; Sanati, S.; Creighton, C.J.; DeSchryver, K.; Crouch, E.; Brink, A.; Watson, M.; et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J. Clin. Oncol. 2017, 35, 1061–1069. [Google Scholar] [CrossRef]
- Khan, Q.J.; O’Dea, A.; Bardia, A.; Kalinsky, K.; Wisinski, K.B.; O’Regan, R.; Yuan, Y.; Ma, C.X.; Jahanzeb, M.; Trivedi, M.S.; et al. Letrozole + ribociclib versus letrozole + placebo as neoadjuvant therapy for ER+ breast cancer (FELINE trial). J. Clin. Oncol. 2020, 38, 505. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez–Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.-S.; Barriga, S.; et al. Potent Cell–Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2− Breast Cancer. Clin. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef]



| Baseline Characteristics | Total | Neoadjuvant Chemotherapy | Non–Neoadjuvant Chemotherapy | p Value | |
|---|---|---|---|---|---|
| 985 (100%) | 143 (14.5%) | 842 (85.5%) | |||
| Sex | Female | 978 (99.3%) | 143 (100%) | 835 (99.2%) | 0.602 |
| Male | 7 (0.7%) | 0 (0%) | 7 (0.8%) | ||
| Age | ≤40 | 93 (9.5%) | 28 (19.6%) | 65 (7.7%) | <0.001 |
| >40 | 891 (90.5%) | 115 (80.4%) | 776 (92.3%) | ||
| BMI | <24.0 | 439 (45.8%) | 56 (39.7%) | 383 (46.8%) | 0.118 |
| ≥24.0 | 520 (54.2%) | 85 (60.3%) | 435 (53.2%) | ||
| Menopause | No | 352 (38.1%) | 73 (52.5%) | 279 (35.5%) | <0.001 |
| Yes | 573 (61.9%) | 66 (47.5%) | 507 (64.5%) | ||
| T stage | T0–2 | 940 (85.8%) | 118 (84.9%) | 822 (97.6%) | <0.001 |
| T3–4 | 31 (14.2%) | 11 (15.1%) | 20 (2.4%) | ||
| N stage | N0 | 556 (57.1%) | 31 (22.3%) | 525 (62.9%) | <0.001 |
| N+ | 418 (42.9%) | 108 (77.7%) | 310 (37.1%) | ||
| Grade | G1–2 | 750 (77.2%) | 83 (59.7%) | 667 (80.2%) | <0.001 |
| G3 | 221 (22.8%) | 56 (40.3%) | 165 (19.8%) | ||
| CNB ER | 1–10% | 21 (2.1%) | 9 (6.3%) | 12 (1.4%) | <0.001 |
| 11–30% | 17 (1.7%) | 7 (4.9%) | 10 (1.2%) | ||
| ≥30% | 947 (96.1%) | 127 (88.8%) | 820 (97.4%) | ||
| CNB PR | 0–10% | 286 (29%) | 53 (37.1%) | 233 (27.7%) | 0.032 |
| 11–30% | 90 (9.1%) | 12 (8.4%) | 78 (9.3%) | ||
| ≥30% | 609 (61.8%) | 78 (54.4%) | 531 (63.1%) | ||
| CNB HER–2 | IHC − | 347 (35.3%) | 41 (28.9%) | 306 (36.3%) | 0.085 |
| IHC + ~ ++ | 637 (64.7%) | 101 (71.1%) | 536 (63.7%) | ||
| CNB AR | 0 | 22 (2.2%) | 11 (7.7%) | 11 (1.3%) | <0.001 |
| 1–64% | 140 (14.2%) | 39 (27.3%) | 101 (12.0%) | ||
| 65–89% | 217 (22.0%) | 21 (14.7%) | 196 (23.3%) | ||
| ≥90% | 606 (61.5%) | 72 (50.3%) | 534 (63.4%) | ||
| AR/ER | ≤1.00 | 840 (85.3%) | 121 (84.6%) | 719 (85.4%) | 0.809 |
| >1.00 | 145 (14.7%) | 22 (15.4%) | 123 (14.6%) | ||
| AR/PR | ≤1.00 | 442 (44.9%) | 59 (41.3%) | 383 (45.5%) | 0.347 |
| >1.00 | 543 (55.1%) | 84 (58.7%) | 459 (54.5%) | ||
| CNB Ki67 | 0–10% | 54 (5.5%) | 1 (0.7%) | 53 (6.3%) | <0.001 |
| 11–30% | 629 (63.9%) | 47 (32.9%) | 582 (69.1%) | ||
| ≥30% | 302 (30.7%) | 95 (66.4%) | 207 (24.6%) | ||
| Median follow–up duration (month) | 42(4–101) | 46 (4–101) | 42 (8–79) | ||
| Occurrence of DFS | No | 830 (90.9%) | 101 (73.2%) | 729 (94.1%) | <0.001 |
| Yes | 83 (9.1%) | 37 (26.8%) | 46 (5.9%) | ||
| Occurrence of OS | No | 880 (96.8%) | 124 (92.5%) | 756 (97.5%) | 0.002 |
| Yes | 29 (3.2%) | 10 (7.5%) | 19 (2.5%) | ||
| Clinicopathologic Feature | No. of Patients (%) | |
|---|---|---|
| Neoadjuvant therapy | Chemotherapy | 134 (93.7%) |
| Anthracycline plus taxane | 126 (88.1%) | |
| Other chemotherapy | 8 (5.6%) | |
| Endocrine therapy | 9 (6.3%) | |
| Miller–Payne stage | G1 | 31 (21.7%) |
| G2 | 10 (7%) | |
| G3 | 50 (35%) | |
| G4 | 32 (22.4%) | |
| G5 | 7 (4.9%) | |
| Unknown | 13 (9.1%) | |
| pCR | No | 136 (95.1%) |
| Yes | 7 (4.9%) | |
| ypT stage | ypT0 | 7 (4.9%) |
| ypT1–2 | 125 (87.4%) | |
| ypT3–4 | 11 (7.7%) | |
| ypN stage | ypN0 | 46 (32.2%) |
| ypN+ | 96 (67.1%) | |
| Unknown | 1 (0.7%) | |
| ER of residual tumor | 1–10% | 16 (11.2%) |
| 11–30% | 6 (1.2%) | |
| ≥30% | 111 (77.6%) | |
| pCR/unknown | 10 (7.0%) | |
| PR of residual tumor | 1–10% | 68 (47.6%) |
| 11–30% | 18 (12.6%) | |
| ≥30% | 47 (32.9%) | |
| pCR/unknown | 10 (7.0%) | |
| Ki67 of residual tumor | Ki67 ≤ 20% | 84 (58.7%) |
| Ki67 > 20% | 48 (33.6%) | |
| pCR/ unknown | 11 (7.7%) | |
| ΔKi67 | Large decrease | 31 (21.7%) |
| Medium decrease | 36 (25.2%) | |
| Slight decrease/no change | 48 (33.6%) | |
| Increase | 15 (10.5%) | |
| pCR/unknown | 13 (9.1%) | |
| Step | Factors Included | B | SE | Wald | HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|
| Step 1 | Age | ≤40 | reference | ||||
| >40 | −0.696 | 0.3 | 5.374 | 0.499 (0.277–0.898) | 0.02 | ||
| Baseline T stage | T0–2 | reference | |||||
| T3–4 | 0.923 | 0.347 | 7.05 | 2.516 (1.273–4.971) | 0.008 | ||
| Baseline N stage | N0 | reference | |||||
| N+ | 0.748 | 0.272 | 7.587 | 2.114 (1.241–3.6) | 0.006 | ||
| Grade | G1–2 | reference | |||||
| G3 | 0.726 | 0.262 | 7.694 | 2.067 (1.237–3.454) | 0.006 | ||
| CNB ER | 1–10% | 1.283 | reference | 0.526 | |||
| 11–30% | 0.629 | 0.88 | 0.51 | 1.875 (0.334–10.523) | 0.475 | ||
| ≥30% | 0.026 | 0.795 | 0.001 | 1.027 (0.216–4.875) | 0.973 | ||
| CNB PR | 0–10% | 1.647 | reference | 0.439 | |||
| 11–30% | −0.549 | 0.44 | 1.558 | 0.578 (0.244–1.367) | 0.212 | ||
| ≥30% | −0.041 | 0.327 | 0.015 | 0.96 (0.506–1.823) | 0.901 | ||
| CNB HER–2 | IHC − | reference | |||||
| IHC + ~ ++ | −0.236 | 0.254 | 0.864 | 0.79 (0.481–1.299) | 0.353 | ||
| CNB AR | 0–89% | reference | |||||
| ≥90% | −0.555 | 0.254 | 4.767 | 0.574 (0.349–0.945) | 0.029 | ||
| AR/ER | ≤1.00 | reference | |||||
| >1.00 | 0.326 | 0.321 | 1.037 | 1.386 (0.739–2.598) | 0.309 | ||
| AR/PR | ≤1.00 | reference | |||||
| >1.00 | 0.356 | 0.306 | 1.355 | 1.427 (0.784–2.598) | 0.244 | ||
| CNB Ki67 | 0–10% | 0.8 | reference | 0.67 | |||
| 11–30% | −0.379 | 0.631 | 0.36 | 0.685 (0.199–2.36) | 0.549 | ||
| ≥30% | −0.182 | 0.653 | 0.078 | 0.833 (0.232–2.994) | 0.78 | ||
| Neoadjuvant therapy | No | reference | |||||
| Yes | 0.56 | 0.282 | 3.932 | 1.75 (1.007–3.042) | 0.047 | ||
| Step 7 | Age | ≤40 | reference | ||||
| >40 | −0.665 | 0.29 | 5.268 | 0.514 (0.292–0.907) | 0.022 | ||
| Baseline T stage | T0–2 | reference | |||||
| T3–4 | 0.873 | 0.329 | 7.036 | 2.395 (1.256–4.565) | 0.008 | ||
| Baseline N stage | N0 | reference | |||||
| N+ | 0.679 | 0.266 | 6.52 | 1.972 (1.171–3.32) | 0.011 | ||
| Grade | G1–2 | reference | |||||
| G3 | 0.722 | 0.243 | 8.848 | 2.059 (1.279–3.313) | 0.003 | ||
| CNB AR | 0–89% | reference | |||||
| ≥90% | −0.555 | 0.235 | 5.575 | 0.574 (0.362–0.91) | 0.018 | ||
| Neoadjuvant therapy | No | reference | |||||
| Yes | 0.675 | 0.266 | 6.422 | 1.964 (1.165–3.31) | 0.011 | ||
| Step | Factors Included | B | SE | Wald | HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|
| Step 1 | Age | ≤40 | |||||
| >40 | 0.921 | 0.796 | 1.341 | 2.513 (0.528–11.947) | 0.247 | ||
| Baseline T stage | T0–2 | ||||||
| T3–4 | 1.077 | 0.599 | 3.231 | 2.937 (0.907–9.505) | 0.072 | ||
| Baseline N stage | N0 | ||||||
| N+ | 0.97 | 0.45 | 4.638 | 2.637 (1.091–6.375) | 0.031 | ||
| Grade | G1–2 | ||||||
| G3 | 0.885 | 0.431 | 4.221 | 2.424 (1.042–5.64) | 0.04 | ||
| CNB ER | 1–10% | 0.857 | 0.652 | ||||
| 11–30% | 0.619 | 1.375 | 0.203 | 1.857 (0.126–27.486) | 0.652 | ||
| ≥30% | −0.229 | 1.215 | 0.035 | 0.796 (0.074–8.602) | 0.851 | ||
| CNB PR | 0–10% | 0.343 | 0.842 | ||||
| 11–30% | −0.283 | 0.645 | 0.193 | 0.753 (0.213–2.665) | 0.66 | ||
| ≥30% | −0.311 | 0.61 | 0.26 | 0.733 (0.222–2.423) | 0.61 | ||
| CNB HER–2 | IHC − | ||||||
| IHC + ~ ++ | −0.854 | 0.405 | 4.443 | 0.426 (0.193–0.942) | 0.035 | ||
| CNB AR | 0–89% | ||||||
| ≥90% | −1.034 | 0.431 | 5.755 | 0.356 (0.153–0.828) | 0.016 | ||
| AR/ER | ≤1.00 | ||||||
| >1.00 | −0.638 | 0.693 | 0.845 | 0.529 (0.136–2.058) | 0.358 | ||
| AR/PR | ≤1.00 | ||||||
| >1.00 | −0.114 | 0.546 | 0.043 | 0.893 (0.306–2.602) | 0.835 | ||
| CNB Ki67 | 0–10% | 0.053 | 0.974 | ||||
| 11–30% | −0.059 | 1.078 | 0.003 | 0.943 (0.114–7.804) | 0.956 | ||
| ≥30% | 0.044 | 1.12 | 0.002 | 1.045 (0.116–9.383) | 0.969 | ||
| Neoadjuvant therapy | No | ||||||
| Yes | 0.013 | 0.489 | 0.001 | 1.013 (0.389–2.639) | 0.979 | ||
| Step 8 | Baseline T stage | T0–2 | |||||
| T3–4 | 1.045 | 0.518 | 4.065 | 2.842 (1.03–7.847) | 0.044 | ||
| Baseline N stage | N0 | ||||||
| N+ | 0.921 | 0.43 | 4.588 | 2.511 (1.081–5.831) | 0.032 | ||
| Grade | G1–2 | ||||||
| G3 | 0.872 | 0.39 | 4.982 | 2.391 (1.112–5.139) | 0.026 | ||
| CNB HER–2 | IHC − | ||||||
| IHC + ~ ++ | −0.825 | 0.389 | 4.488 | 0.438 (0.204–0.94) | 0.034 | ||
| CNB AR | 0–89% | ||||||
| ≥90% | −1.163 | 0.401 | 8.424 | 0.312 (0.142–0.685) | 0.004 | ||
| Group | DFS | OS |
|---|---|---|
| pCR | 85.71% | 100.00% |
| non–pCR | 73.53% | 92.65% |
| p value | 0.741 | >0.999 |
| Factors Included | B | SE | Wald | HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Age | ≤40 | reference | ||||
| >40 | −0.411 | 0.481 | 0.731 | 0.663 (0.258–1.701) | 0.393 | |
| Baseline T stage | T0–2 | reference | ||||
| T3–4 | 1.266 | 0.571 | 4.923 | 3.547 (1.159–10.854) | 0.027 | |
| Baseline N stage | N0 | reference | ||||
| N+ | −0.009 | 0.89 | 0 | 0.991 (0.173–5.671) | 0.992 | |
| Grade | G1–2 | reference | ||||
| G3 | 0.414 | 0.626 | 0.436 | 1.512 (0.443–5.157) | 0.509 | |
| CNB ER | 1–10% | 1.273 | reference | 0.529 | ||
| 11–30% | 1.686 | 1.498 | 1.266 | 5.396 (0.286–101.71) | 0.261 | |
| ≥30% | 1.49 | 1.674 | 0.793 | 4.439 (0.167–118.05) | 0.373 | |
| CNB PR | 0–10% | 1.481 | reference | 0.477 | ||
| 11–30% | −1.007 | 0.926 | 1.183 | 0.365 (0.06–2.242) | 0.277 | |
| ≥30% | −0.806 | 0.801 | 1.011 | 0.447 (0.093–2.148) | 0.315 | |
| CNB HER–2 | IHC − | reference | ||||
| IHC + ~ ++ | −0.86 | 0.571 | 2.269 | 0.423 (0.138–1.295) | 0.132 | |
| CNB AR | <65% | reference | ||||
| ≥65% | −0.041 | 0.572 | 0.005 | 0.96 (0.313–2.946) | 0.943 | |
| AR/ER | ≤1.00 | reference | ||||
| >1.00 | 1.683 | 0.739 | 5.183 | 5.381 (1.264–22.91) | 0.023 | |
| AR/PR | ≤1.00 | reference | ||||
| >1.00 | −0.82 | 0.611 | 1.8 | 0.441 (0.133–1.459) | 0.18 | |
| CNB Ki67 | 0–10% | 2.013 | reference | 0.365 | ||
| 11–30% | 5.187 | 85.207 | 0.004 | 178.85 (0–6.035 × 1074) | 0.951 | |
| ≥30% | 4.31 | 85.21 | 0.003 | 74.467 (0–2.528 × 1074) | 0.96 | |
| ypT stage | T0–2 | reference | ||||
| T3–4 | 0.969 | 0.884 | 1.201 | 2.637 (0.466–14.925) | 0.273 | |
| ypN stage | ypN0 | reference | ||||
| ypN+ | −0.046 | 0.761 | 0.004 | 0.955 (0.215–4.241) | 0.951 | |
| ER of residual tumor | 1–10% | 2.709 | reference | 0.258 | ||
| 11–30% | −1.385 | 1.556 | 0.792 | 0.25 (0.012–5.283) | 0.373 | |
| ≥30% | 0.695 | 1.181 | 0.346 | 2.004 (0.198–20.302) | 0.556 | |
| PR of residual tumor | 1–10% | 0.614 | reference | 0.736 | ||
| 11–30% | −0.08 | 0.812 | 0.01 | 0.923 (0.188–4.532) | 0.921 | |
| ≥30% | 0.395 | 0.625 | 0.4 | 1.485 (0.436–5.052) | 0.527 | |
| HER–2 of residual tumor | − | reference | ||||
| + ~ ++ | 0.241 | 0.369 | 0.427 | 1.273 (0.617–2.626) | 0.513 | |
| Ki67 of residual tumor | Ki67 ≤ 20% | reference | ||||
| Ki67 > 20% | 1.607 | 0.676 | 5.658 | 4.988 (1.327–18.748) | 0.017 | |
| ΔKi67 | Large decrease | 5.641 | reference | 0.13 | ||
| Medium decrease | 0.577 | 0.834 | 0.478 | 1.78 (0.347–9.132) | 0.489 | |
| Slight decrease/no change | −0.079 | 0.89 | 0.008 | 0.924 (0.162–5.286) | 0.929 | |
| Increase | −2.241 | 1.439 | 2.425 | 0.106 (0.006–1.785) | 0.119 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Li, J.; Zhang, H.; Zhang, S.; Ye, J.; Cheng, Y.; Liu, Q.; Xin, L.; Xiang, H.; Liu, Y.; et al. Correlation between Androgen Receptor Expression in Luminal B (HER–2 Negative) Breast Cancer and Disease Outcomes. J. Pers. Med. 2022, 12, 1988. https://doi.org/10.3390/jpm12121988
Yang F, Li J, Zhang H, Zhang S, Ye J, Cheng Y, Liu Q, Xin L, Xiang H, Liu Y, et al. Correlation between Androgen Receptor Expression in Luminal B (HER–2 Negative) Breast Cancer and Disease Outcomes. Journal of Personalized Medicine. 2022; 12(12):1988. https://doi.org/10.3390/jpm12121988
Chicago/Turabian StyleYang, Fan, Jiayi Li, Hong Zhang, Shuang Zhang, Jingming Ye, Yuanjia Cheng, Qian Liu, Ling Xin, Hongyu Xiang, Yinhua Liu, and et al. 2022. "Correlation between Androgen Receptor Expression in Luminal B (HER–2 Negative) Breast Cancer and Disease Outcomes" Journal of Personalized Medicine 12, no. 12: 1988. https://doi.org/10.3390/jpm12121988
APA StyleYang, F., Li, J., Zhang, H., Zhang, S., Ye, J., Cheng, Y., Liu, Q., Xin, L., Xiang, H., Liu, Y., Duan, X., & Xu, L. (2022). Correlation between Androgen Receptor Expression in Luminal B (HER–2 Negative) Breast Cancer and Disease Outcomes. Journal of Personalized Medicine, 12(12), 1988. https://doi.org/10.3390/jpm12121988






