Long-Term Follow-Up in IgG4-Related Ophthalmic Disease: Serum IgG4 Levels and Their Clinical Relevance
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Eishi, Y.; Koike, M.; Tsuruta, K.; Okamoto, A.; Egawa, N.; Nakajima, H. A new clinicopathological entity of IgG4-related autoimmune disease. J. Gastroenterol. 2003, 38, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Okazaki, K.; Masaki, Y.; Kawano, M.; Yamamoto, M.; Saeki, T.; Matsui, S.; Yoshino, T.; Nakamura, S.; Kawa, S.; et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod. Rheumatol. 2012, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Takahira, M.; Azumi, A. Japanese Study Group for IgG4-Related Ophthalmic Disease. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn. J. Ophthalmol. 2015, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Deshpande, V.; Stone, J.H. Ophthalmic manifestations of IgG4-related disease: Single-center experience and literature review. Semin. Arthritis Rheum. 2014, 43, 806–817. [Google Scholar] [CrossRef]
- Khosroshahi, A.; Wallace, Z.S.; Crowe, J.L.; Akamizu, T.; Azumi, A.; Carruthers, M.N.; Chari, S.T.; Della-Torre, E.; Frulloni, L.; Goto, H.; et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015, 67, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Detiger, S.E.; Karim, A.F.; Verdijk, R.M.; Van Hagen, P.M.; Van Laar, J.A.M.; Paridaens, D. The treatment outcomes in IgG4-related orbital disease: A systematic review of the literature. Acta Ophthalmol. 2019, 97, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Kang, S.; Song, M.K.; Ahn, C.J.; Sa, H. Clinicoserological factors associated with response to steroid treatment and recurrence in patients with IgG4-related ophthalmic disease. Br. J. Ophthalmol. 2018, 102, 1591–1595. [Google Scholar] [CrossRef]
- Kubota, T.; Katayama, M.; Nishimura, R.; Moritani, S. Long- term outcomes of ocular adnexal lesions in IgG4- related ophthalmic disease. Br. J. Ophthalmol. 2020, 104, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Luo, X.; Fei, Y.; Peng, L.; Zhou, J.; Li, J.; Lu, H.; Liu, Z.; Zhang, P.; Liu, X.; et al. Long-Term Outcomes of IgG4-Related Ophthalmic Disease in a Chinese IgG4-Related Disease Cohort. Front. Med. 2021, 8, 784520. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yen, S.H.; Tsai, C.C.; Kao, S.C.; Lee, F.L. Adjunctive Orbital Radiotherapy for Ocular Adnexal IgG4-related Disease: Preliminary Experience in Patients Refractory or Intolerant to Corticosteroid Therapy. Ocul. Immunol. Inflamm. 2015, 23, 162–167. [Google Scholar] [CrossRef]
- Xu, W.L.; Ling, Y.C.; Wang, Z.K.; Deng, F. Diagnostic performance of serum IgG4 level for IgG4-related disease: A meta-analysis. Sci. Rep. 2016, 6, 32035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.S.; Fan, C.H.; Liu, Y.Y. Diagnostic performances of serum IgG4 concentration and IgG4/IgG ratio in IgG4-related disease. Clin. Rheumatol. 2017, 36, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.K.; Kao, S.C.; Yang, C.F.; Lee, F.L.; Tsai, C.C. Ocular adnexal IgG4-related disease: Clinical features, outcome, and factors associated with response to systemic steroids. Jpn. J. Ophthalmol. 2015, 59, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, C.; Tsuboi, H.; Yokosawa, M.; Hagiwara, S.; Hirota, T.; Takai, C.; Asashima, H.; Miki, H.; Umeda, N.; Horikoshi, M.; et al. Clinicopathological features of IgG4-related disease complicated with orbital involvement. Mod. Rheumatol. 2014, 24, 471–476. [Google Scholar] [CrossRef]
- Woo, Y.J.; Kim, J.W.; Yoon, J.S. Clinical implications of serum IgG4 levels in patients with IgG4-related ophthalmic disease. Br. J. Ophthalmol. 2017, 101, 256–260. [Google Scholar]
- Son, K.Y.; Woo, K.I.; Kim, Y.D. Clinical Outcomes of IgG4-Related Ophthalmic Disease and Idiopathic Sclerosing Orbital Inflammation. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 34–39. [Google Scholar] [CrossRef]
- Zen, Y.; Nakanuma, Y. IgG4-related disease: A cross-sectional study of 114 cases. Am. J. Surg. Pathol. 2010, 34, 1812–1819. [Google Scholar] [CrossRef]
- Hamano, H.; Arakura, N.; Muraki, T.; Ozaki, Y.; Kiyosawa, K.; Kawa, S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J. Gastroenterol. 2006, 41, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Kamisawa, T.; Shimosegawa, T.; Okazaki, K.; Nishino, T.; Watanabe, H.; Kanno, A.; Okumura, F.; Nishikawa, T.; Kobayashi, K.; Ichiya, T.; et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009, 58, 1504–1507. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- Davies, A.M.; Sutton, B.J. Human IgG4: A structural perspective. Immunol. Rev. 2015, 268, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zee, J.S.; Van Swieten, P.; Aalberse, R.C. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J. Immunol. 1986, 137, 3566–3571. [Google Scholar] [PubMed]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-related disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Dodev, T.S.; Bowen, H.; Shamji, M.H.; Bax, H.J.; Beavil, A.J.; McDonnell, J.M.; Durham, S.R.; Sutton, B.J.; Gould, H.J.; James, L.K. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy 2015, 70, 720–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.F.; James, L.K.; Bahnson, H.T.; Shamji, M.H.; Couto-Francisco, N.C.; Islam, S.; Houghton, S.; Clark, A.T.; Stephens, A.; Turcanu, V.; et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J. Allergy Clin. Immunol. 2015, 135, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.K.; Tsai, C.C.; Kao, S.C.; Liu, C.J.L. Immunoglobulin G4-related ophthalmic disease. Taiwan J. Ophthalmol. 2018, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, P.; Ye, H.; Xiao, W.; Chen, R.; Mao, Y.; Ai, S.; Liu, Z.; Tang, L.; Yang, H. Clinical features and outcomes of IgG4-related idiopathic orbital inflammatory disease: From a large southern China-based cohort. Eye 2021, 35, 1248–1255. [Google Scholar] [CrossRef]
- Goto, H.; Ueda, S.I.; Nemoto, R.; Ohshima, K.I.; Sogabe, Y.; Kitagawa, K.; Ogawa, Y.; Oyama, T.; Furuta, M.; Azumi, A.; et al. Clinical features and symptoms of IgG4-related ophthalmic disease: A multicenter study. Jpn. J. Ophthalmol. 2021, 65, 651–656. [Google Scholar] [CrossRef]
- Andrew, N.; Kearney, D.; Selva, D. IgG4-related orbital disease: A meta-analysis and review. Acta Ophthalmol. 2013, 91, 694–700. [Google Scholar] [CrossRef]
- Zhao, Z.; Mou, D.; Wang, Z.; Zeng, Q.; Wang, Z.; Xue, J.; Ren, L.; Liu, Y.; Su, Y. Clinical features and relapse risks of IgG4-related ophthalmic disease: A single-center experience in China. Arthritis Res. Ther. 2021, 23, 98. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age (mean, year) | 60.68 |
| Male gender, n (%) | 17 (68%) |
| Bilateral, n (%) | 14 (56%) |
| Systemic involvement, n (%) | 8 (32%) |
| Ocular involvement | |
| Lacrimal gland, n (%) | 19 (76%) |
| Orbital soft tissue, n (%) | 9 (36%) |
| Extraocular muscle, n (%) | 5 (20%) |
| Infraorbital nerve, n (%) | 5 (20%) |
| Recurrence, n (%) | 11 (44%) |
| Duration of steroid treatment (mean, month) | 14.53 |
| Follow-up time, month (mean, month) | 57.72 |
| Last follow-up status of IgG4 level * | |
| Normalized, n (%) | 9 (36%) |
| Elevated, n (%) | 16 (64%) |
| Variable | IgG4 Normalized * (n = 9) | IgG4 Elevated * (n = 16) | p Value |
|---|---|---|---|
| Age (mean, year) | 61.22 | 60.38 | 0.718 |
| Male gender | 77.8% | 62.5% | 0.661 |
| Systemic involvement | 44.4% | 25% | 0.394 |
| Ocular involvement | |||
| Lacrimal gland | 55.6% | 87.5% | 0.142 |
| Orbital soft tissue | 44.4% | 31.3% | 0.671 |
| Extraocular muscle | 11.1% | 25% | 0.621 |
| Infraorbital nerve | 33.3% | 12.5% | 0.312 |
| Recurrence | 22.2% | 56.3% | 0.208 |
| IgG level at baseline (mean, mg/dL) | 1875.38 | 2227.13 | 0.636 |
| IgG4 level at baseline (mean, mg/dL) | 580.78 | 916.19 | 0.276 |
| Lowest IgG4 level after initial steroid treatment (mean, mg/dL) | 82.19 | 229.33 | 0.002 |
| IgG4 level at last follow-up (mean, mg/dL) | 78.50 | 605.60 | 0 |
| Duration of steroid treatment (mean, month) | 17.36 | 12.94 | 0.890 |
| Variable | Value |
|---|---|
| IgG level at baseline (mean, mg/dL) (n = 24, 1 missing datum) | 2104.78 |
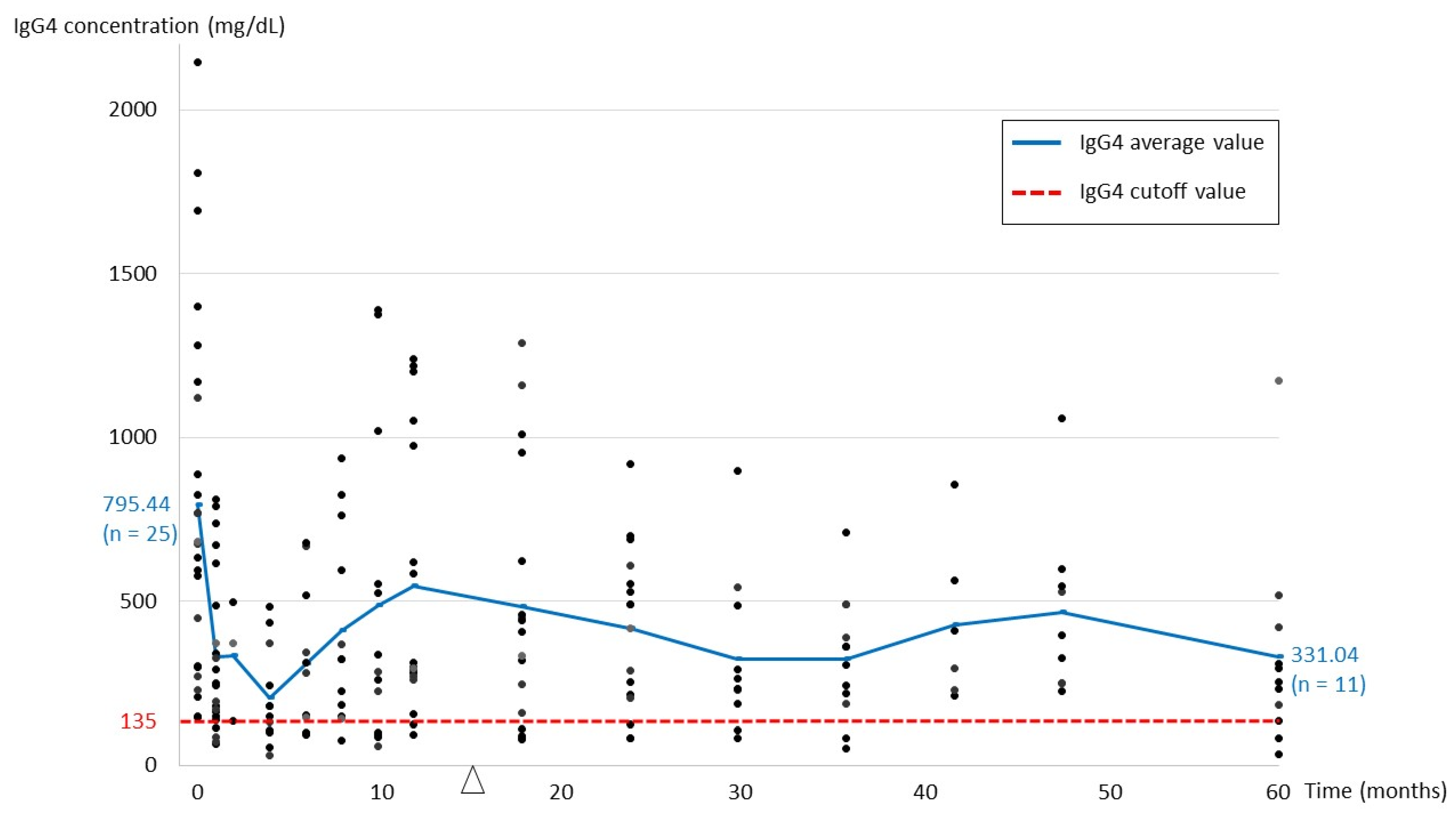
| IgG4 level at baseline (mean, mg/dL) (n = 25) | 795.44 |
| IgG4/IgG ratio at baseline (n = 24, 1 missing datum) | 0.3586 |
| Lowest IgG4 level after initial steroid treatment (mean, mg/dL) (n = 25) | 176.36 |
| IgG4 level during recurrence (mean, mg/dL) (n = 11) | 780.14 |
| IgG4 level at baseline in patients with recurrence (mean, mg/dL) (n = 11) | 789.44 |
| IgG4 level at last follow-up (mean, mg/dL) (n = 25) | 415.84 |
| In patients with remission (mean, mg/dL) (n = 19) | 326.25 |
| In patients with stable disease (mean, mg/dL) (n = 6) | 699.55 |
| Variable | Group 1 (Remission with Normalized IgG4) 1 (n = 9) | Group 2 (Remission with Elevated IgG4) 1 (n = 10) | p Value |
|---|---|---|---|
| Last follow-up IgG4 (mean, mg/dL) | 78.50 | 549.23 | NA 2 |
| Age (mean, year) | 61.22 | 63.10 | 0.905 |
| Male gender | 77.80% | 60% | 0.628 |
| Bilateral | 33.30% | 70% | 0.179 |
| Systemic involvement | 44.40% | 20% | 0.35 |
| Ocular involvement | |||
| Lacrimal gland | 55.56% | 90% | 0.141 |
| Orbital soft tissue | 44.44% | 20% | 0.35 |
| Extraocular muscle | 11.11% | 30% | 0.582 |
| Infraorbital nerve | 33.33% | 20% | 0.628 |
| Recurrence | 22.22% | 60% | 0.17 |
| IgG level at baseline (mean, mg/dL) | 1875.38 | 1857.67 | 0.888 |
| IgG4 level at baseline (mean, mg/dL) | 553.95 | 701.37 | 0.696 |
| Lowest IgG4 level after initial steroid treatment (mean, mg/dL) | 82.19 | 152.80 | 0.043 |
| Reduction in IgG4 level (%) 3 | 75.58% | 75.68% | 0.847 |
| Duration of steroid treatment (mean, month) | 17.36 | 5.84 | 0.968 |
| Variable | Unilateral (n = 11) | Bilateral (n = 14) | p Value |
|---|---|---|---|
| Age (mean, year) | 63.00 | 58.86 | 0.291 |
| Male gender | 63.6% | 71.4% | 1 |
| Systemic involvement | 36.4% | 28.6% | 1 |
| Ocular involvement | |||
| Lacrimal gland | 45.5% | 100.0% | 0.003 |
| Orbital soft tissue | 45.5% | 28.6% | 0.434 |
| Extraocular muscle | 18.2% | 21.4% | 1 |
| Infraorbital nerve | 9.1% | 28.6% | 0.341 |
| Recurrence | 44.4% | 40.0% | 1 |
| IgG level at baseline (mean, mg/dL) | 1649.55 | 2522.08 | 0.007 |
| IgG4 level at baseline (mean, mg/dL) | 455.09 | 1083.43 | 0.004 |
| Lowest IgG4 level after initial steroid treatment (mean, mg/dL) | 159.49 | 189.61 | 0.572 |
| IgG4 during recurrence (mean, mg/dL) | 205.80 | 1186.00 | 0.114 |
| IgG4 level at last follow-up (mean, mg/dL) | 281.98 | 521.02 | 0.183 |
| Reduction in IgG4 level (%) 1 | 64.72 | 81.50 | 0.005 |
| Change in IgG4 level at recurrence (%) 2 | −8.21 | 21.93 | 0.667 |
| Duration of steroid treatment (mean, month) | 10.83 | 17.44 | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, W.-Y.; Tsai, C.-Y.; Tsai, C.-C. Long-Term Follow-Up in IgG4-Related Ophthalmic Disease: Serum IgG4 Levels and Their Clinical Relevance. J. Pers. Med. 2022, 12, 1963. https://doi.org/10.3390/jpm12121963
Chou W-Y, Tsai C-Y, Tsai C-C. Long-Term Follow-Up in IgG4-Related Ophthalmic Disease: Serum IgG4 Levels and Their Clinical Relevance. Journal of Personalized Medicine. 2022; 12(12):1963. https://doi.org/10.3390/jpm12121963
Chicago/Turabian StyleChou, Wei-Yi, Ching-Yao Tsai, and Chieh-Chih Tsai. 2022. "Long-Term Follow-Up in IgG4-Related Ophthalmic Disease: Serum IgG4 Levels and Their Clinical Relevance" Journal of Personalized Medicine 12, no. 12: 1963. https://doi.org/10.3390/jpm12121963
APA StyleChou, W.-Y., Tsai, C.-Y., & Tsai, C.-C. (2022). Long-Term Follow-Up in IgG4-Related Ophthalmic Disease: Serum IgG4 Levels and Their Clinical Relevance. Journal of Personalized Medicine, 12(12), 1963. https://doi.org/10.3390/jpm12121963







