Design of a Functional Eye Dressing for Treatment of the Vitreous Floater
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxygen Therapy
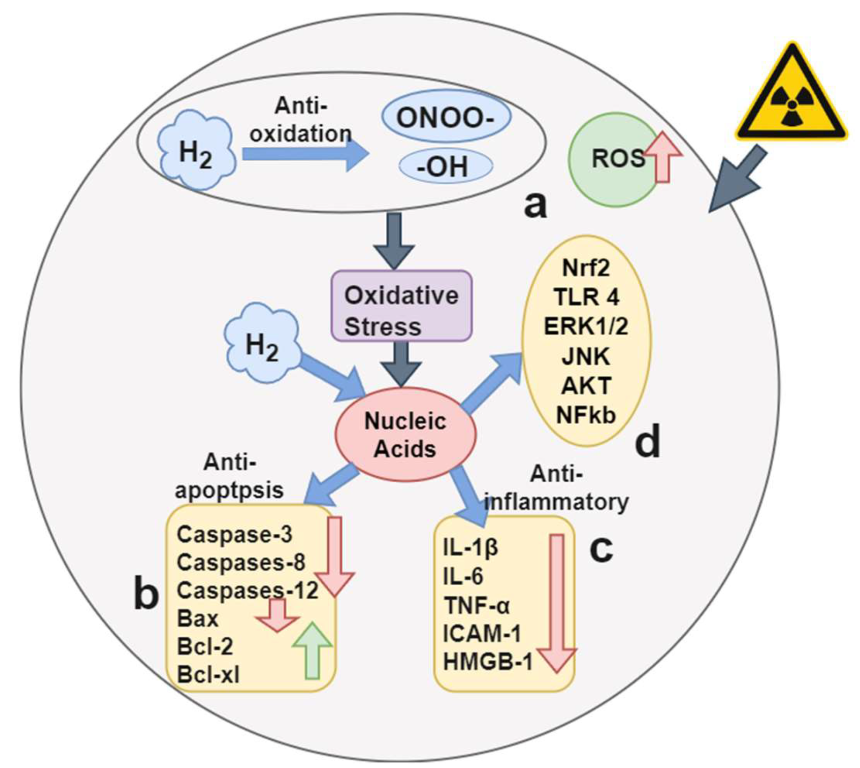
2.2. Hydrogen Therapy
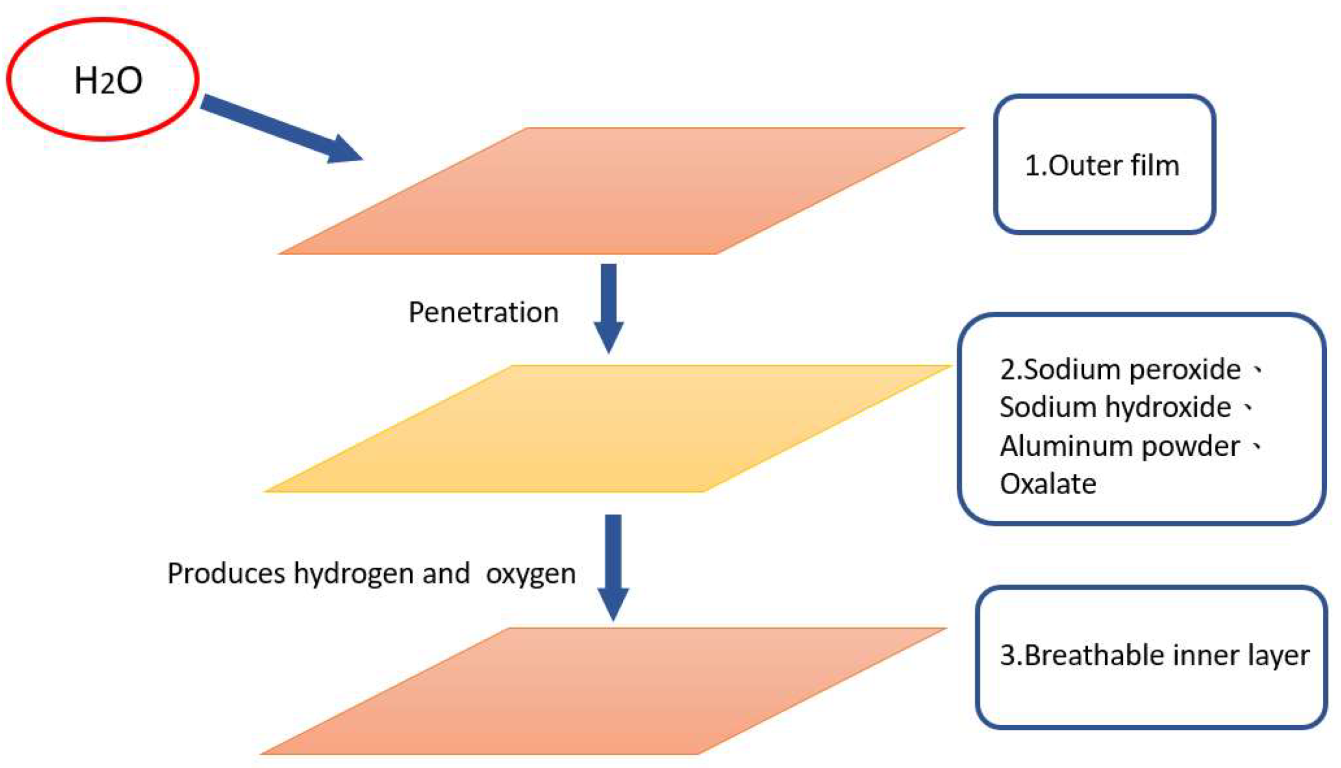
2.3. Functional Dressing
2.4. Type B Ultrasonic Scanner (Nidek RS-3000)
2.5. Symptoms of Vitreous Floaters
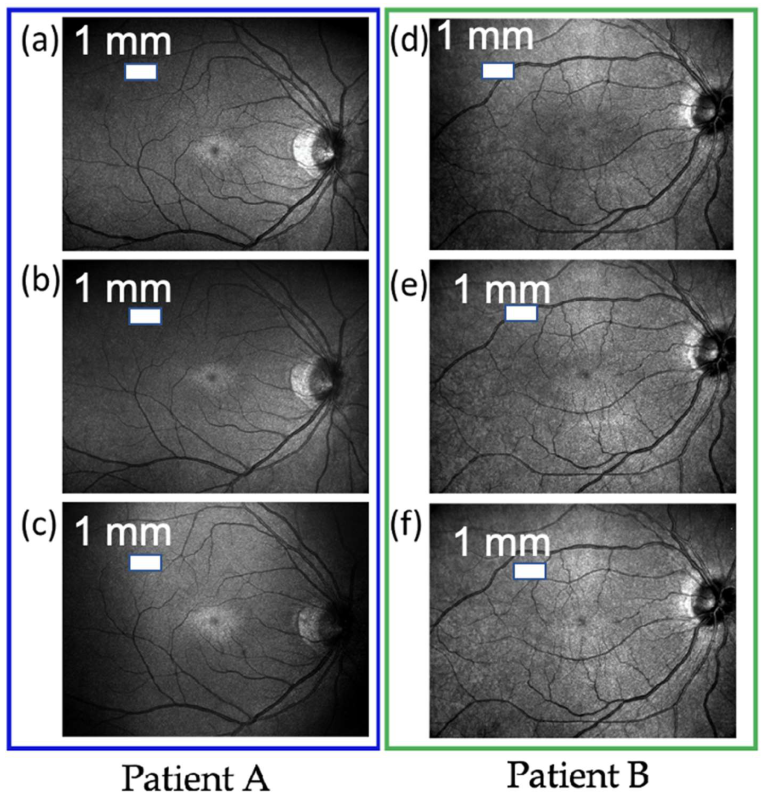
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lumi, X.; Hawlina, M.; Glavač, D.; Facskó, A.; Moe, M.C.; Kaarniranta, K.; Petrovski, G. Ageing of the vitreous: From acute onset floaters and flashes to retinal detachment. Ageing Res. Rev. 2015, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Habermann, T.M.; Steensma, D.P. Lymphadenopathy. Mayo Clin. Proc. 2000, 75, 723–732. [Google Scholar] [CrossRef]
- Nunes, G.; Ludwig, G.; Gemelli, H.; Serracarbassa, P.D.; Zanotele, M. Long Term Evaluation of the Efficacy And And Safety of Nd: Yag Laser Vitreolysis for Symptomatic Vitreous Floaters. Arq. Bras. Oftalmol. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Gopali, R.; Reddy, A.; Patel, K.K. The Readability of Ophthalmological Patient Education Materials Provided by Major Academic Hospitals. Semin. Ophthalmol. 2021, 37, 71–76. [Google Scholar] [CrossRef]
- Katsanos, A.; Tsaldari, N.; Gorgoli, K.; Lalos, F.; Stefaniotou, M.; Asproudis, I. Safety and efficacy of YAG laser vitreolysis for the treatment of vitreous floaters: An overview. Adv. Ther. 2020, 37, 1319–1327. [Google Scholar] [CrossRef]
- Ludwig, G.D.; Gemelli, H.; Nunes, G.M.; Serracarbassa, P.D.; Zanotele, M. Efficacy and safety of Nd:YAG laser vitreolysis for symptomatic vitreous floaters: A randomized controlled trial. Eur. J. Ophthalmol. 2021, 31, 909–914. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhao, J. Acute retinal detachment after Nd:YAG treatment for vitreous floaters and postertior capsule opacification: A case report. BMC Ophthalmol. 2020, 20, 157. [Google Scholar] [CrossRef]
- Wagle, A.M.; Lim, W.-Y.; Yap, T.-P.; Neelam, K.; Eong, K.-G.A. Utility values associated with vitreous floaters. Am. J. Ophthalmol. 2011, 152, 60–65.e1. [Google Scholar] [CrossRef]
- Sebag, J. Floaters and the quality of life. Am. J. Ophthalmol. 2011, 152, 3–4.e1. [Google Scholar] [CrossRef]
- Schulz-Key, S.; Carlsson, J.O.; Crafoord, S. Longterm follow-up of pars plana vitrectomy for vitreous floaters: Complications, outcomes and patient satisfaction. Acta Ophthalmol. 2011, 89, 159–165. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Brasse, K.; Hoerauf, H. Vitreous floaters. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2020, 117, 485–496. [Google Scholar]
- Son, G.; Sohn, J.; Kong, M. Acute symptomatic vitreous floaters assessed with ultra-wide field scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Sci. Rep. 2021, 11, 8930. [Google Scholar] [CrossRef]
- Zeydanli, E.O.; Parolini, B.; Ozdek, S.; Bopp, S.; Adelman, R.A.; Kuhn, F.; Gini, G.; Sallam, A.B.; Aksakal, N. Management of vitreous floaters: An international survey the European VitreoRetinal Society Floaters study report. Eye 2020, 34, 825–834. [Google Scholar] [CrossRef]
- Pinilla, G.; Kumar, A.; Floaters, M.K.; Pardo, C.A.; Rothstein, J.; Ilieva, H. Increased synthesis of pro-inflammatory cytokines in C9ORF72 patients. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 517–527. [Google Scholar] [CrossRef]
- Sebag, J. Age-related changes in human vitreous structure. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 89–93. [Google Scholar] [CrossRef]
- Sebag, J. Vitreous: In Health and Disease; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Gale, J.; Ikuno, Y. Myopic Vitreopathy II. In Vitreous: In Health and Disease; Springer: Berlin/Heidelberg, Germany, 2014; p. 113. [Google Scholar]
- Karitans, V.; Fomins, S.; Ozolinsh, M. Phase retrieval for studying the structure of vitreous floaters simulated in a model eye. J. Mod. Opt. 2021, 68, 792–797. [Google Scholar] [CrossRef]
- Menteş, J.; Yıldırım, Ş. Optical coherence tomography characteristics of quiescent type 1 neovascularization in eyes with nonexudative age-related macular degeneration. Turk. J. Ophthalmol. 2019, 49, 84. [Google Scholar] [CrossRef]
- Coupland, S. The pathologist’s perspective on vitreous opacities. Eye 2008, 22, 1318–1329. [Google Scholar] [CrossRef][Green Version]
- Spraul, C.W.; Grossniklaus, H.E. Vitreous hemorrhage. Surv. Ophthalmol. 1997, 42, 3–39. [Google Scholar] [CrossRef]
- Sun, I.-T.; Lee, T.-H.; Chen, C.-H. Rapid cataract progression after Nd:YAG vitreolysis for vitreous floaters: A case report and literature review. Case Rep. Ophthalmol. 2017, 8, 321–325. [Google Scholar] [CrossRef]
- Delaney, Y.; Oyinloye, A.; Benjamin, L. Nd:YAG vitreolysis and pars plana vitrectomy: Surgical treatment for vitreous floaters. Eye 2002, 16, 21–26. [Google Scholar] [CrossRef]
- Milston, R.; Madigan, M.C.; Sebag, J. Vitreous floaters: Etiology, diagnostics, and management. Surv. Ophthalmol. 2016, 61, 211–227. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Nguyen-Cuu, J.; Yu, F.; Yee, K.M.; Mamou, J.; Silverman, R.H.; Ketterling, J.; Sebag, J. Assessment of Vitreous Structure and Visual Function after Neodymium: Yttrium–Aluminum–Garnet Laser Vitreolysis. Ophthalmology 2019, 126, 1517–1526. [Google Scholar] [CrossRef]
- Souza, C.E.; Lima, L.H.; Nascimento, H.; Zett, C.; Belfort, R. Objective assessment of YAG laser vitreolysis in patients with symptomatic vitreous floaters. Int. J. Retin. Vitr. 2020, 6, 1. [Google Scholar] [CrossRef]
- Mandura, R.A. Acquired Senile Retinoschisis of the Peripheral Retina Imaged by Spectral Domain Optical Coherent Tomography. Cureus 2021, 13, e14540. [Google Scholar] [CrossRef]
- Lam, P.Y.; Shih, K.C.; Fong, P.Y.; Chan, T.C.Y.; Ng, A.L.-K.; Jhanji, V.; Tong, L. A review on evidence-based treatments for meibomian gland dysfunction. Eye Contact Lens 2020, 46, 3–16. [Google Scholar] [CrossRef]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for wound healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Pate, K.M.; Goswami, D.G.; Lake, M.; Lake, S.; Kant, R.; Ammar, D.; Tewari-Singh, N. A Supersaturated Oxygen Emulsion for the Topical Treatment of Ocular Trauma. Mil. Med. 2020, 185, e466–e472. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.-N.; Yin, X.-X.; Song, W.-G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.-C.; Kim, M.-S.; Jeong, J.; Kim, B.-S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Rosenband, V.; Gany, A. Application of activated aluminum powder for generation of hydrogen from water. Int. J. Hydrog. Energy 2010, 35, 10898–10904. [Google Scholar] [CrossRef]
- Oksala, A. Ultrasonic findings in the vitreous body at various ages. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1978, 207, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Mamou, J.; Wa, C.A.; Yee, K.M.; Silverman, R.H.; Ketterling, J.A.; Sadun, A.A.; Sebag, J. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R. Experimental Analysis of the Advanced Clinical System for the Treatment of Patients with Vitreous Floaters. In Proceedings of the 2020 IEEE 2nd Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS), Tainan, Taiwan, 29–31 May 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 12–15. [Google Scholar]

| Levels of Floaters | Mild | Moderate | Severe |
|---|---|---|---|
| Number of patients | 10 | 12 | 6 |
| Clinical symptoms |
|
|
|
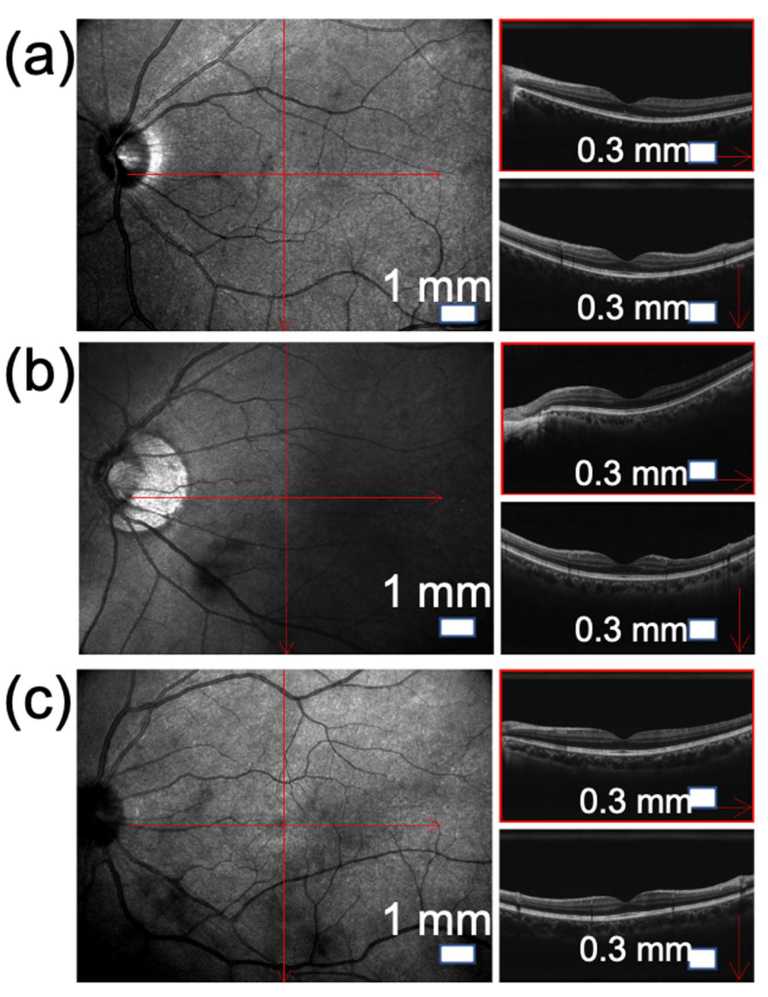
| Pathological signs shown in OCT | Slight shadow and blackness appear on the vitreous | Local shadows appear inside the vitreous | The shadows and blackness appear wider and darker |
| Levels of Floaters | Mild | Moderate | Severe |
|---|---|---|---|
| Number of patients | 10 | 12 | 6 |
| Number of recovery patients | 7 | 8 | 6 |
| Avarage recovery rate | 70.0% | 66.7% | 83.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.-S.; Huang, S.-Y.; Nguyen, H.-T.; Ho, W.-T.; Chao, W.-H.; Lin, F.-C.; Wang, H.-C. Design of a Functional Eye Dressing for Treatment of the Vitreous Floater. J. Pers. Med. 2022, 12, 1659. https://doi.org/10.3390/jpm12101659
Fan W-S, Huang S-Y, Nguyen H-T, Ho W-T, Chao W-H, Lin F-C, Wang H-C. Design of a Functional Eye Dressing for Treatment of the Vitreous Floater. Journal of Personalized Medicine. 2022; 12(10):1659. https://doi.org/10.3390/jpm12101659
Chicago/Turabian StyleFan, Wen-Shuang, Shuan-Yu Huang, Hong-Thai Nguyen, Wen-Tsung Ho, Wen-Hung Chao, Fen-Chi Lin, and Hsiang-Chen Wang. 2022. "Design of a Functional Eye Dressing for Treatment of the Vitreous Floater" Journal of Personalized Medicine 12, no. 10: 1659. https://doi.org/10.3390/jpm12101659
APA StyleFan, W.-S., Huang, S.-Y., Nguyen, H.-T., Ho, W.-T., Chao, W.-H., Lin, F.-C., & Wang, H.-C. (2022). Design of a Functional Eye Dressing for Treatment of the Vitreous Floater. Journal of Personalized Medicine, 12(10), 1659. https://doi.org/10.3390/jpm12101659








