Kidney Function Trajectory within Six Months after Acute Kidney Injury Inpatient Care and Subsequent Adverse Kidney Outcomes: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
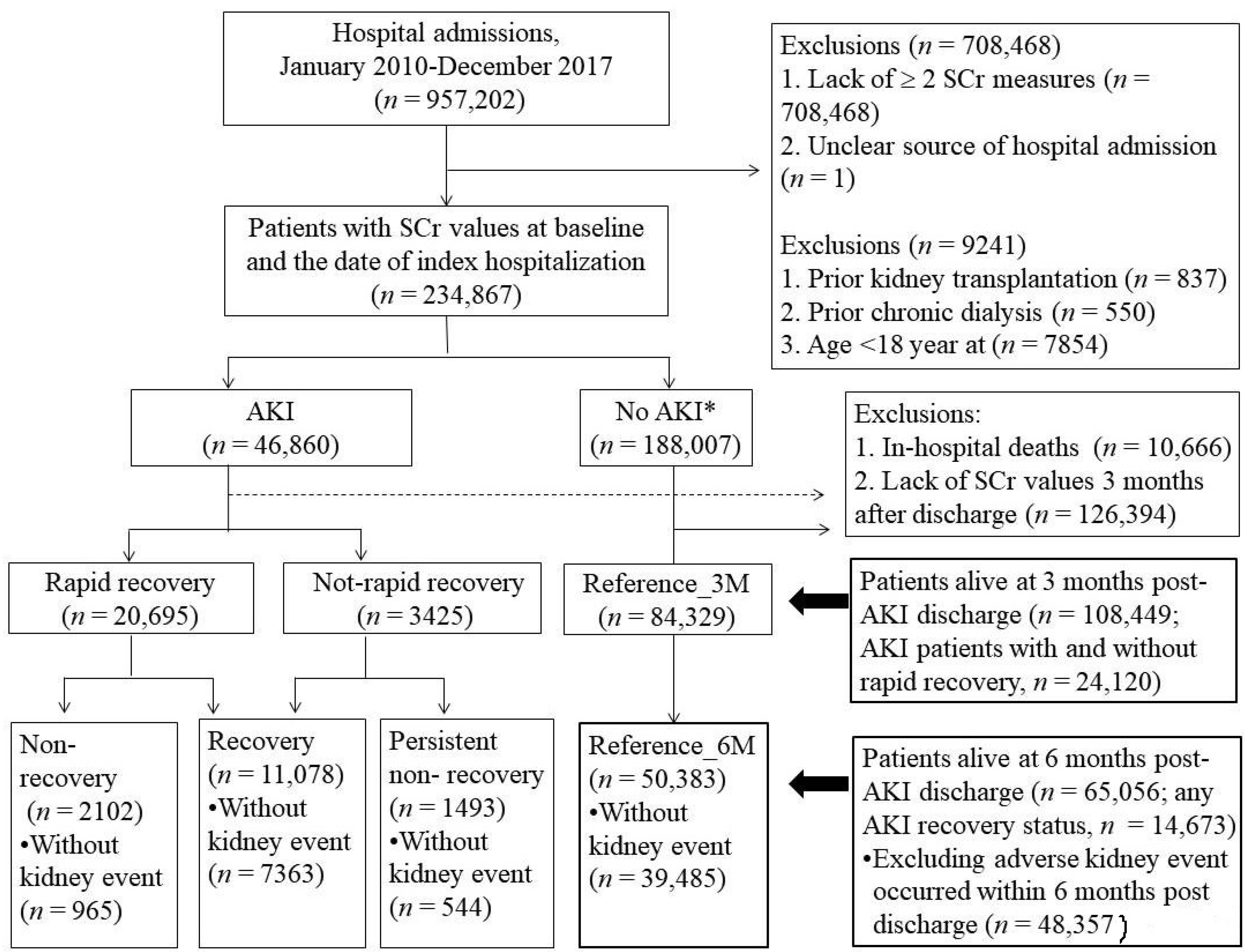
2.2. Study Cohort
2.3. Six-Month Kidney Function Trajectory after AKI Hospital Discharge
2.4. Adverse Kidney Outcomes
2.5. Baseline Covariates
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
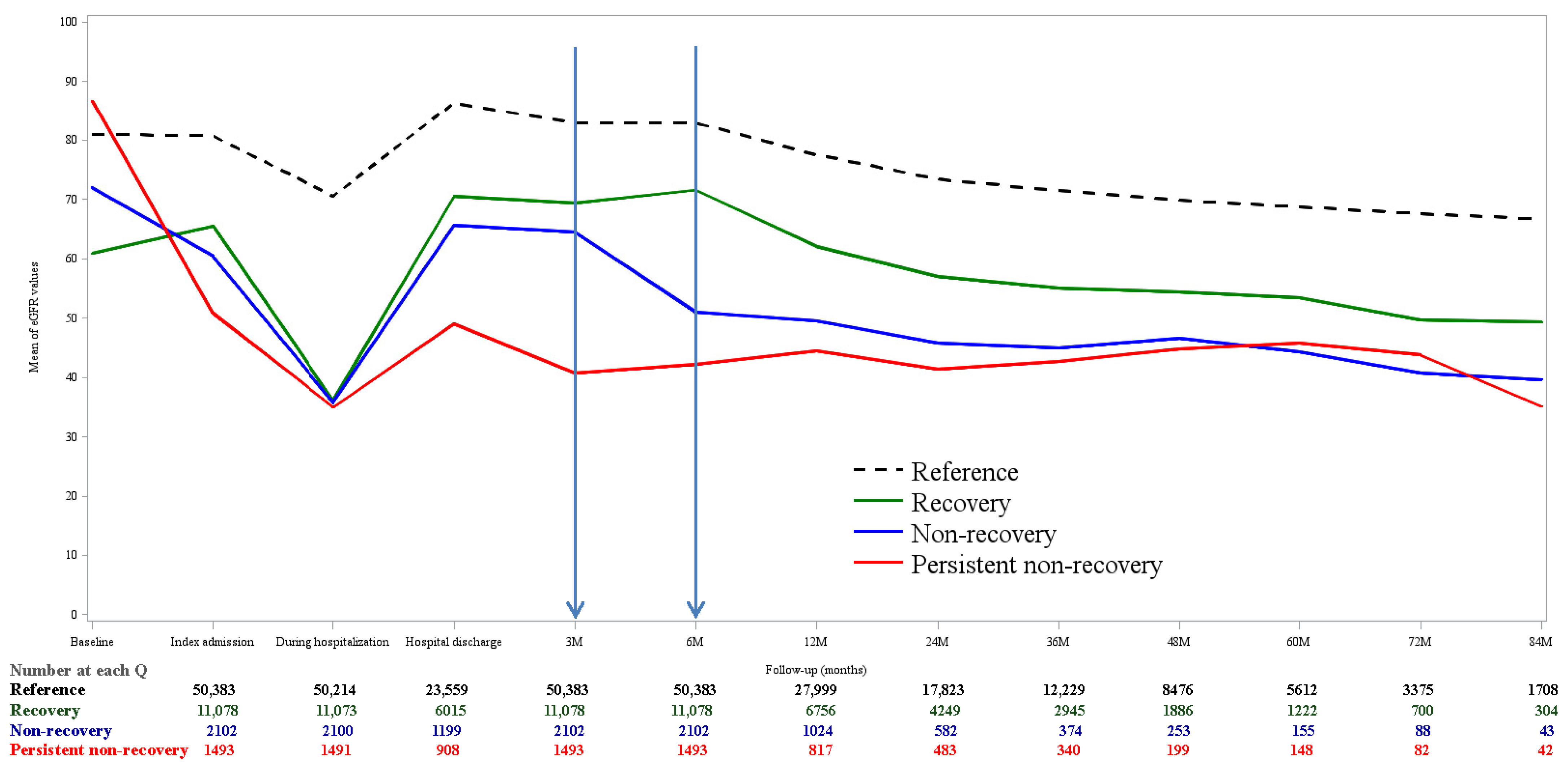
3.2. Kidney Function Trajectory
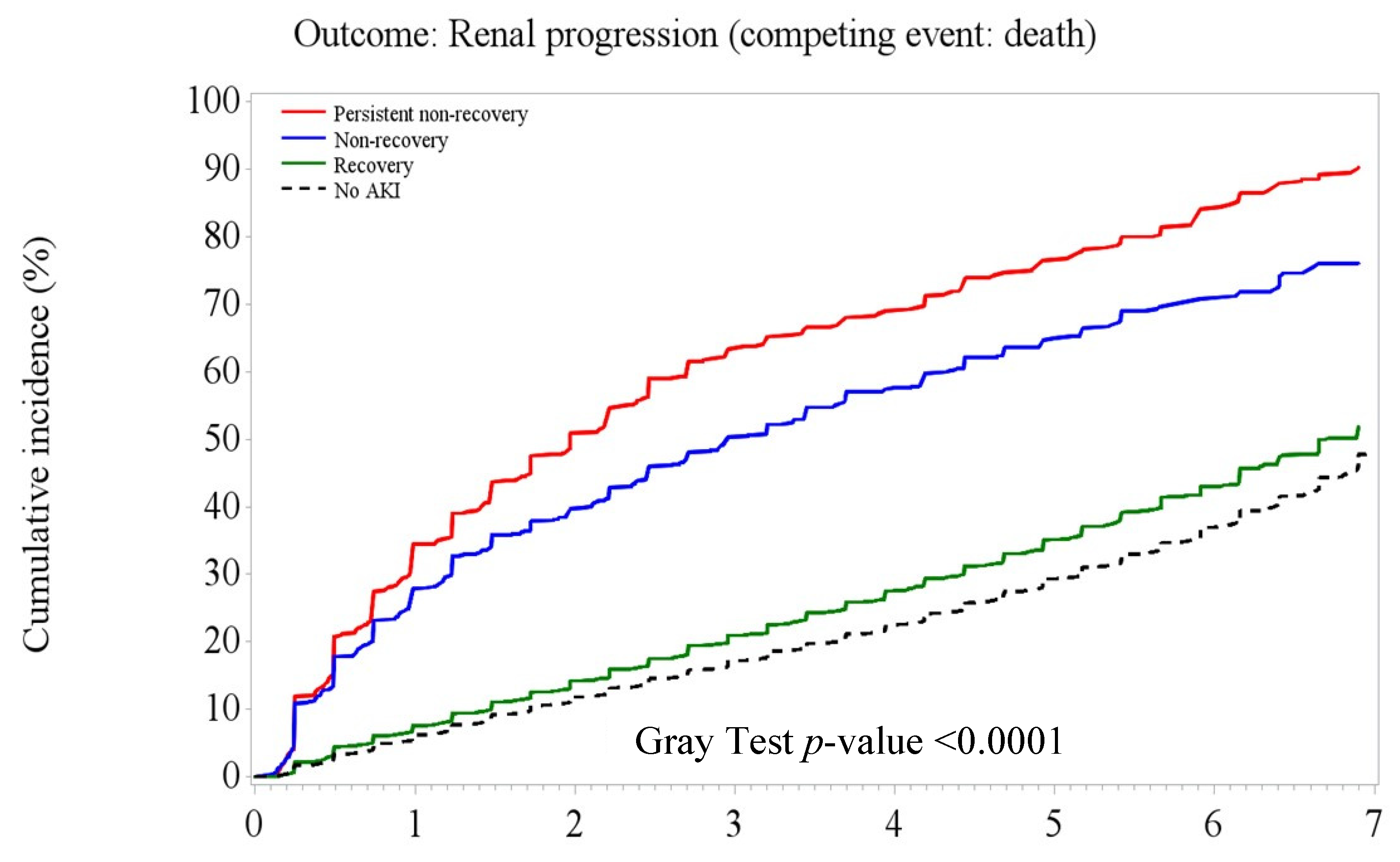
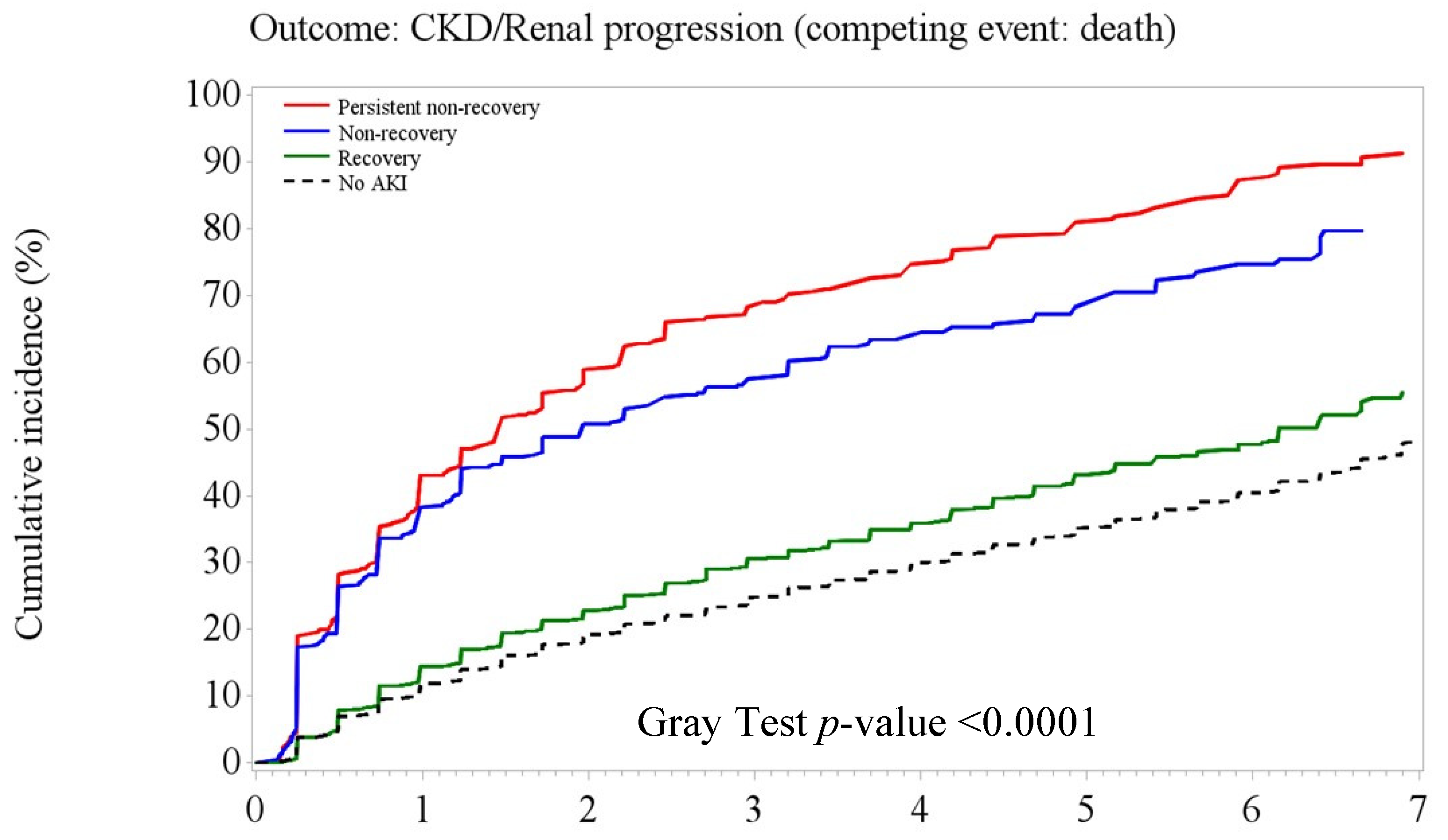
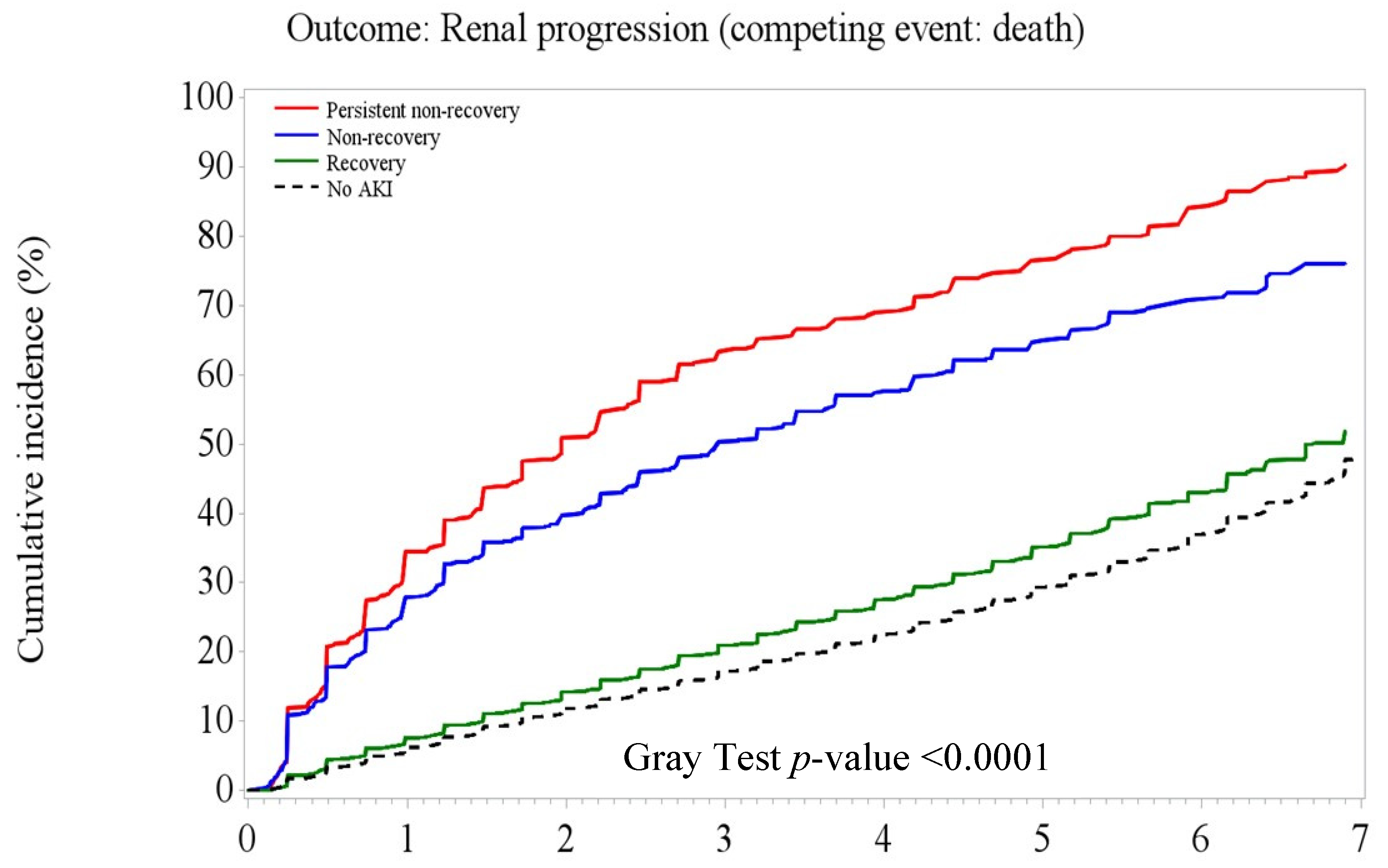
3.3. Post-AKI Kidney Function Recovery Patterns and Adverse Kidney Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.K.; Burdmann, E.A.; Mehta, R.L. Acute kidney injury: Global health alert. Intern. Med. J. 2013, 43, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Bhatt, M.; Pannu, N.; Tonelli, M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 2020, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.Y.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Macedo, E.; Mehta, R.L. Targeting recovery from acute kidney injury: Incidence and prevalence of recovery. Nephron Clin. Pract. 2014, 127, 4–9. [Google Scholar] [CrossRef]
- Pannu, N.; James, M.; Hemmelgarn, B.; Klarenbach, S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin. J. Am. Soc. Nephrol. 2013, 8, 94–202. [Google Scholar] [CrossRef]
- Sawhney, S.; Marks, A.; Fluck, N.; Levin, A.; Prescott, G.; Black, C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: A large population-based cohort study. Am. J. Kidney Dis. 2017, 69, 18–28. [Google Scholar] [CrossRef]
- Siew, E.D.; Abdel-Kader, K.; Perkins, A.M.; Greevy, R.A., Jr.; Parr, S.K.; Horner, J.; Vincz, A.J.; Denton, J.; Wilson, O.D.; Hung, A.M.; et al. Timing of Recovery From Moderate to Severe AKI and the Risk for Future Loss of Kidney Function. Am. J. Kidney Dis. 2020, 75, 204–213. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Zelnick, L.R.; Chinchilli, V.M.; Moledina, D.G.; Coca, S.G.; Parikh, C.R.; Garg, A.X.; Hsu, C.Y.; Go, A.S.; Liu, K.D. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw. Open 2020, 3, e202682. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lee, C.T.; Su, C.H.; Wang, Y.C.; Chen, H.L.; Chuang, J.H.; Tain, Y.L. Incidence, Outcomes, and Risk Factors of Community-Acquired and Hospital-Acquired Acute Kidney Injury: A Retrospective Cohort Study. Medicine 2016, 95, e3674. [Google Scholar] [CrossRef]
- Wonnacott, A.; Meran, S.; Amphlett, B.; Talabani, B.; Phillips, A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.; Campos, P.; Pinto, I.; Rodrigues, B.; Frade, F.; Papoila, A.L.; Devarajan, P. The risk of chronic kidney disease and mortality are increased after community-acquired acute kidney injury. Kidney Int. 2016, 90, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Haase, M.; Lewington, A.J.P.; O’Donoghue, D.J.; Wilson, F.P.; Nadim, M.K.; Silver, S.A.; Zarbock, A.; Ostermann, M.; et al. Quality Improvement Goals for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2019, 14, 941–953. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- National Health Insurance Administration. 2018 Annual Report of Health Services Claims, by Health Care Organizations. Available online: https://www.nhi.gov.tw/Content_List.aspx?n=8A5CA04F618E3364&topn=23C660CAACAA159D (accessed on 12 November 2021).
- Cheng, S.H.; Chiang, T.L. The effect of universal health insurance on health care utilization in Taiwan: Results from a natural experiment. JAMA 1997, 278, 89–93. [Google Scholar] [CrossRef]
- Hsu, C.N.; Liu, C.L.; Tain, Y.L.; Kuo, C.Y.; Lin, Y.C. Machine Learning Model for Risk Prediction of Community-Acquired Acute Kidney Injury Hospitalization From Electronic Health Records: Development and Validation Study. J. Med. Internet Res. 2020, 22, e16903. [Google Scholar] [CrossRef]
- Liu, C.L.; Tain, Y.L.; Lin, Y.C.; Hsu, C.N. Prediction and Clinically Important Factors of Acute Kidney Injury Non-recovery. Front. Med. 2022, 8, 789874. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after Acute Kidney Injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Vanmassenhove, J.; Vanholder, R.; Lameire, N. Points of concern in post acute kidney injury management. Nephron 2018, 138, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.I.; Guh, J.Y.; Wu, K.D.; Chen, Y.M.; Kuo, M.C.; Hwang, S.J.; Chen, T.H.; Chen, H.C. Modification of diet in renal disease (MDRD) study and CKD epidemiology collaboration (CKD-EPI) equations for Taiwanese adults. PLoS ONE 2014, 9, e99645. [Google Scholar]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Parikh, C.R.; Himmelfarb, J.; Chinchilli, V.M.; Liu, K.D.; Coca, S.G.; Garg, A.X.; Hsu, C.Y.; Siew, E.D.; Wurfel, M.M. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021, 99, 456–465. [Google Scholar] [CrossRef]
- Lee, B.J.; Hsu, C.Y.; Parikh, R.; McCulloch, C.E.; Tan, T.C.; Liu, K.D.; Hsu, R.K.; Pravoverov, L.; Zheng, S.; Go, A.S. Predicting Renal Recovery After Dialysis-Requiring Acute Kidney Injury. Kidney Int. Rep. 2019, 4, 571–581. [Google Scholar] [CrossRef]
- James, M.T.; Pannu, N.; Hemmelgarn, B.R.; Austin, P.C.; Tan, Z.; McArthur, E.; Manns, B.J.; Tonelli, M.; Wald, R.; Quinn, R.R.; et al. Derivation and External Validation of Prediction Models for Advanced Chronic Kidney Disease Following Acute Kidney Injury. JAMA 2017, 318, 1787–1797. [Google Scholar] [CrossRef]
- Siew, E.D.; Parr, S.K.; Wild, M.G.; Levea, S.L.; Mehta, K.G.; Umeukeje, E.M.; Silver, S.A.; Ikizler, T.A.; Cavanaugh, K.L. Kidney disease awareness and knowledge among survivors ofacute kidney injury. Am. J. Nephrol. 2019, 49, 449–459. [Google Scholar] [CrossRef]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. 30-day readmissions after an acute kidney injury hospitalization. Am. J. Med. 2017, 130, 163–172.e4. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.; Marks, A.; Fluck, N.; McLernon, D.J.; Prescott, G.J.; Black, C. Acute kidney injury as an independent risk factor for unplanned 90-day hospital readmissions. BMC Nephrol. 2017, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Goldstein, S.J.; Harel, Z.; Harvey, A.; Rompies, E.J.; Adhikari, N.K.; Acedillo, R.; Jain, A.K.; Richardson, R.; Chan, C.T. Ambulatory care after acute kidney injury: An opportunity to improve patient outcomes. Can. J. Kidney Health Dis. 2015, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E.; Caskey, F.J.; Farmer, C.K.; Fuentes, A.F. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 48,357) | Persistent Non-Recovery (n = 544) | Non-Recovery (n = 965) | Recovery (n = 7363) | No AKI (n = 39,485) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at index admission, year | <0.001 | |||||||||
| <65 | 27,952 | 342 | (62.87) | 521 | (53.99) | 3704 | (50.31) | 23,385 | (59.23) | |
| ≥65 | 20,405 | 202 | (37.13) | 444 | (46.01) | 3659 | (49.69) | 16,100 | (40.77) | |
| Sex, n (%) | 0.01 | |||||||||
| Male | 26,898 | 275 | (50.55) | 502 | (52.02) | 4088 | (55.52) | 22,033 | (55.80) | |
| Female | 21,459 | 269 | (49.45) | 463 | (47.98) | 3275 | (44.48) | 17,452 | (44.20) | |
| eGFR at baseline, mL/min/1.73 m2 | ||||||||||
| >=60 | 33,543 | 376 | (69.12) | 617 | (63.94) | 4021 | (54.61) | 28,529 | (72.25) | <0.001 |
| <60 | 14,814 | 168 | (30.88) | 348 | (36.06) | 3342 | (45.39) | 10,956 | (27.75) | |
| Dialysis before index admission | 24 | 3 | (0.55) | 3 | (0.31) | 13 | (0.18) | 5 | (0.01) | <0.001 |
| Baseline comorbidity | ||||||||||
| CCI score | <0.001 | |||||||||
| 0 | 9002 | 66 | (12.13) | 85 | (8.81) | 870 | (11.82) | 7981 | (20.21) | |
| 1~3 | 24,701 | 234 | (43.01) | 443 | (45.91) | 3712 | (50.41) | 20,312 | (51.44) | |
| >3 | 14,654 | 244 | (44.85) | 437 | (45.28) | 2781 | (37.77) | 11,192 | (28.34) | |
| Acute myocardial infarction | 1086 | 11 | (2.02) | 35 | (3.63) | 278 | (3.78) | 762 | (1.93) | <0.001 |
| Congestive heart failure | 2790 | 45 | (8.27) | 115 | (11.92) | 730 | (9.91) | 1900 | (4.81) | <0.001 |
| Peripheral vascular diseases | 1049 | 14 | (2.57) | 36 | (3.73) | 261 | (3.54) | 738 | (1.87) | <0.001 |
| Cerebral vascular accident | 5268 | 69 | (12.68) | 139 | (14.40) | 1177 | (15.99) | 3883 | (9.83) | <0.001 |
| Dementia | 1096 | 10 | (1.84) | 36 | (3.73) | 288 | (3.91) | 762 | (1.93) | <0.001 |
| Pulmonary disease | 4636 | 51 | (9.38) | 139 | (14.40) | 915 | (12.43) | 3531 | (8.94) | <0.001 |
| Connective tissue disorder | 1063 | 12 | (2.21) | 33 | (3.42) | 208 | (2.82) | 810 | (2.05) | <0.001 |
| Peptic ulcer | 7934 | 96 | (17.65) | 211 | (21.87) | 1501 | (20.39) | 6126 | (15.51) | <0.001 |
| Liver diseases | 10,256 | 134 | (24.63) | 260 | (26.94) | 1654 | (22.46) | 8208 | (20.79) | <0.001 |
| Diabetes | 14,843 | 180 | (33.09) | 383 | (39.69) | 2856 | (38.79) | 11,424 | (28.93) | <0.001 |
| Diabetes complications | 4994 | 53 | (9.74) | 116 | (12.02) | 984 | (13.36) | 3841 | (9.73) | <0.001 |
| Paraplegia | 565 | 18 | (3.31) | 19 | (1.97) | 150 | (2.04) | 378 | (0.96) | <0.001 |
| Renal disease | 6144 | 109 | (20.04) | 216 | (22.38) | 1595 | (21.66) | 4224 | (10.70) | <0.001 |
| Cancer | 18,031 | 236 | (43.38) | 357 | (36.99) | 2298 | (31.21) | 15,140 | (38.34) | <0.001 |
| Severe liver diseases | 1207 | 39 | (7.17) | 80 | (8.29) | 288 | (3.91) | 800 | (2.03) | <0.001 |
| Metastatic cancer | 4996 | 81 | (14.89) | 114 | (11.81) | 633 | (8.60) | 4168 | (10.56) | <0.001 |
| Health service uses during hospitalization | ||||||||||
| Dialysis | 255 | 20 | (3.68) | 23 | (2.38) | 167 | (2.27) | 45 | (0.11) | <0.001 |
| Intensive care unit | 4819 | 87 | (15.99) | 165 | (17.10) | 1164 | (15.81) | 3403 | (8.62) | <0.001 |
| Timing of AKI occurred | ||||||||||
| No AKI | 39,485 | - | 39,485 | |||||||
| CA-AKI only (at admission) | 2781 | 321 | (59.01) | 419 | (43.42) | 2041 | (27.72) | - | ||
| HA-AKI only (during hospitalization) | 5848 | 164 | (30.15) | 507 | (52.54) | 5177 | (70.31) | - | ||
| CA- and HA-AKI | 243 | 59 | (10.85) | 39 | (4.04) | 145 | (1.97) | - | ||
| Kidney function recovery within 3 months after discharge, n (%) | ||||||||||
| Not-rapid recovery | 811 | 544 | - | 267 | (3.63) | - | ||||
| Rapid recovery | 8061 | - | 965 | 7096 | (96.37) | - | ||||
| Not-rapid recovery | 811 | 544 | - | 267 | (3.63) | - | ||||
| Rapid recovery | 8061 | - | 965 | 7096 | (96.37) | - | ||||
| Mean (SD) value (n = 48,357) | ||||||||||
| Age at index admission, year | 59.20 | (15.40) | 62.43 | (15.41) | 63.46 | (15.23) | 60.91 | (14.13) | <0.001 | |
| Baseline eGFR, mL/min/1.73 m2 | 119.62 | (67.92) | 90.49 | (53.29) | 70.87 | (40.82) | 81.11 | (33.37) | <0.001 | |
| Number of outpatient visit | 5.47 | (4.61) | 5.39 | (4.22) | 5.31 | (4.63) | 5.32 | (4.12) | 0.79 | |
| Number of Emergency department visit | 0.64 | (1.16) | 0.72 | (1.01) | 0.74 | (1.12) | 0.42 | (0.91) | 0.001 | |
| Number of hospitalizations | 0.57 | (0.72) | 0.54 | (0.73) | 0.42 | (0.65) | 0.39 | (0.65) | <0.001 | |
| Overall | Persistent Non-Recovery | Non-Recovery | Recovery | No AKI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| per 100 PY | n (%) | per 100 PY | n (%) | per 100 PY | n (%) | per 100 PY | n (%) | per 100 PY | n (%) | ||||||
| Patients at risk, n | 48,357 | 544 | 965 | 7363 | 39,485 | ||||||||||
| Composite adverse kidney outcome (including incident CKD), n (%) | 9.04 | 12,415 | (25.67) | 34.26 | 435 | (79.96) | 27.84 | 563 | (58.34) | 9.51 | 1888 | (25.64) | 8.35 | 9529 | (24.13) |
| eGFR reduction ³30% baseline | 6.56 | 9754 | (20.17) | 28.05 | 422 | (77.57) | 21.38 | 511 | (52.95) | 6.77 | 1461 | (19.84) | 5.98 | 7360 | (18.64) |
| eGFR < 15 | 1.35 | 1981 | (4.10) | 4.63 | 68 | (12.50) | 4 | 94 | (9.74) | 2.1 | 444 | (6.03) | 1.13 | 1375 | (3.48) |
| Kidney replacement therapy | 0.25 | 377 | (0.78) | 0.76 | 12 | (2.21) | 0.28 | 7 | (0.73) | 0.38 | 82 | (1.11) | 0.22 | 276 | (0.70) |
| Chronic dialysis | 0.24 | 362 | (0.75) | 0.76 | 12 | (2.21) | 0.24 | 6 | (0.62) | 0.35 | 76 | (1.03) | 0.22 | 268 | (0.68) |
| Kidney transplantation | 0.012 | 18 | (0.04) | 0 | 0.041 | 1 | (0.10) | 0.027 | 6 | (0.08) | 0.01 | 11 | (0.03) | ||
| Death | 5.9 | 8881 | (18.37) | 9.08 | 145 | (26.65) | 11.84 | 293 | (30.36) | 6.66 | 1457 | (19.79) | 5.61 | 6986 | (17.69) |
| Patients without CKD at baseline, n | 32,208 | 355 | 559 | 3734 | 27,580 | ||||||||||
| Composite adverse kidney outcome (including incident CKD) n (%) | 8.89 | 7995 | (24.82) | 34.94 | 267 | (79.70) | 28.3 | 334 | (59.75) | 10.38 | 1017 | (27.24) | 8.16 | 6377 | (23.12) |
| eGFR reduction ³30% baseline | 5.73 | 5637 | (17.50) | 28.77 | 256 | (76.42) | 20.31 | 292 | (52.24) | 6.49 | 708 | (18.96) | 5.15 | 4381 | (15.88) |
| eGFR < 15 | 0.09 | 86 | (0.27) | 0.11 | 1 | (0.30) | 0.14 | 2 | (0.36) | 0.18 | 20 | (0.54) | 0.07 | 63 | (0.23) |
| Incident CKD | 5.48 | 4968 | (15.42) | 10.34 | 84 | (25.07) | 13.16 | 160 | (28.62) | 6.22 | 614 | (16.44) | 5.22 | 4110 | (14.90) |
| Kidney replacement therapy | 0.02 | 24 | (0.07) | 0 | 0 | 0 | 0 | 0.02 | 2 | (0.05) | 0.03 | 22 | (0.08) | ||
| Chronic dialysis | 0.02 | 20 | (0.06) | 0 | 0 | 0 | 0 | 0.02 | 2 | (0.05) | 0.02 | 18 | (0.07) | ||
| Kidney transplantation | 0.004 | 4 | (0.01) | 0 | 0 | 0 | 0 | 0 | 0 | 0.005 | 4 | (0.01) | |||
| Death | 5.85 | 5825 | (18.09) | 9.19 | 87 | (25.97) | 11.46 | 170 | (30.41) | 6.53 | 721 | (19.31) | 5.63 | 4847 | (17.57) |
| Patients with CKD at baseline, n | 16,149 | 209 | 406 | 3629 | 11,905 | ||||||||||
| Composite adverse kidney outcome n (%) | 9.33 | 4420 | (27.37) | 33.25 | 168 | (80.38) | 27.19 | 229 | (56.40) | 8.66 | 871 | (24.00) | 8.76 | 3152 | (26.48) |
| eGFR reduction ³30% baseline | 8.2 | 4117 | (25.49) | 26.995 | 166 | (79.43) | 22.99 | 219 | (53.94) | 7.05 | 753 | (20.75) | 7.84 | 2979 | (25.02) |
| eGFR < 15 | 3.97 | 1895 | (11.73) | 12.78 | 67 | (32.06) | 10.61 | 92 | (22.66) | 4.19 | 424 | (11.68) | 3.62 | 1312 | (11.02) |
| Kidney replacement therapy | 0.7 | 353 | (2.19) | 1.9 | 12 | (5.74) | 0.71 | 7 | (1.72) | 0.75 | 80 | (2.20) | 0.67 | 254 | (2.13) |
| Chronic dialysis | 0.68 | 342 | (2.12) | 1.9 | 12 | (5.74) | 0.61 | 6 | (1.48) | 0.69 | 74 | (2.04) | 0.66 | 250 | (2.10) |
| Kidney transplantation | 0.027 | 14 | (0.09) | 0 | 0 | 0.1 | 1 | (0.25) | 0.056 | 6 | (0.17) | 0.02 | 7 | (0.06) | |
| Death | 5.995 | 3056 | (18.92) | 8.93 | 58 | (27.75) | 12.41 | 123 | (30.30) | 6.8 | 736 | (20.28) | 5.56 | 2139 | (17.97) |
| Overall (n = 48,357) | Without CKD (n = 32,208) * | With CKD (n = 16,149) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p-Value | SHR | 95% CI | p-Value | SHR | 95% CI | p-Value | |
| Composite adverse kidney outcomes * | |||||||||
| At 6 months | |||||||||
| Persistent non-recovery | 4.55 | (4.05–5.11) | <0.001 | 4.85 | (4.20–5.61) | <0.001 | 5.38 | (4.46–6.49) | <0.001 |
| Non-recovery | 3.54 | (3.18–3.94) | <0.001 | 3.45 | (3.02–3.93) | <0.001 | 3.73 | (3.15–4.41) | <0.001 |
| Recovery | 1.30 | (1.20–1.40) | <0.001 | 1.36 | (1.24–1.50) | <0.001 | 1.12 | (1.00–1.25) | 0.045 |
| At 3 months | |||||||||
| Not-rapid recovery | 3.85 | (3.48–4.26) | <0.001 | 4.18 | (3.69–4.74) | <0.001 | 4.12 | (3.53–4.81) | <0.001 |
| Rapid recovery | 1.54 | (1.43–1.66) | <0.001 | 1.63 | (1.49–1.79) | <0.001 | 1.31 | (1.18–1.46) | <0.001 |
| Incident CKD # | |||||||||
| Persistent/non-recovery | 6.23 | (5.25–7.39) | <0.001 | ||||||
| Recovery | 1.58 | (1.39–1.80) | <0.001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Liu, C.-L.; Kuo, H.-C.; Hsu, C.-N. Kidney Function Trajectory within Six Months after Acute Kidney Injury Inpatient Care and Subsequent Adverse Kidney Outcomes: A Retrospective Cohort Study. J. Pers. Med. 2022, 12, 1606. https://doi.org/10.3390/jpm12101606
Tain Y-L, Liu C-L, Kuo H-C, Hsu C-N. Kidney Function Trajectory within Six Months after Acute Kidney Injury Inpatient Care and Subsequent Adverse Kidney Outcomes: A Retrospective Cohort Study. Journal of Personalized Medicine. 2022; 12(10):1606. https://doi.org/10.3390/jpm12101606
Chicago/Turabian StyleTain, You-Lin, Chien-Liang Liu, Hsiao-Ching Kuo, and Chien-Ning Hsu. 2022. "Kidney Function Trajectory within Six Months after Acute Kidney Injury Inpatient Care and Subsequent Adverse Kidney Outcomes: A Retrospective Cohort Study" Journal of Personalized Medicine 12, no. 10: 1606. https://doi.org/10.3390/jpm12101606
APA StyleTain, Y.-L., Liu, C.-L., Kuo, H.-C., & Hsu, C.-N. (2022). Kidney Function Trajectory within Six Months after Acute Kidney Injury Inpatient Care and Subsequent Adverse Kidney Outcomes: A Retrospective Cohort Study. Journal of Personalized Medicine, 12(10), 1606. https://doi.org/10.3390/jpm12101606








