Transcatheter Versus Surgical Valve Repair in Patients with Severe Mitral Regurgitation
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Echocardiography
2.3. Mitral Valve Procedures
2.4. Outcome Measures
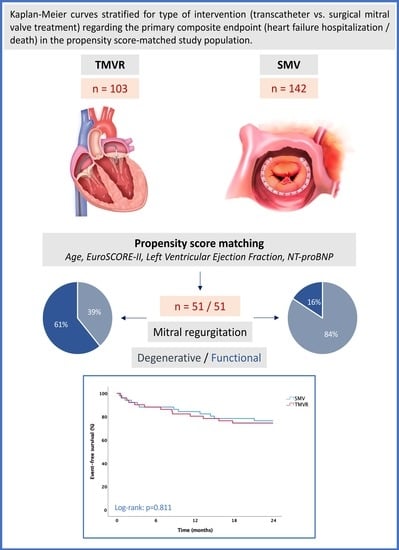
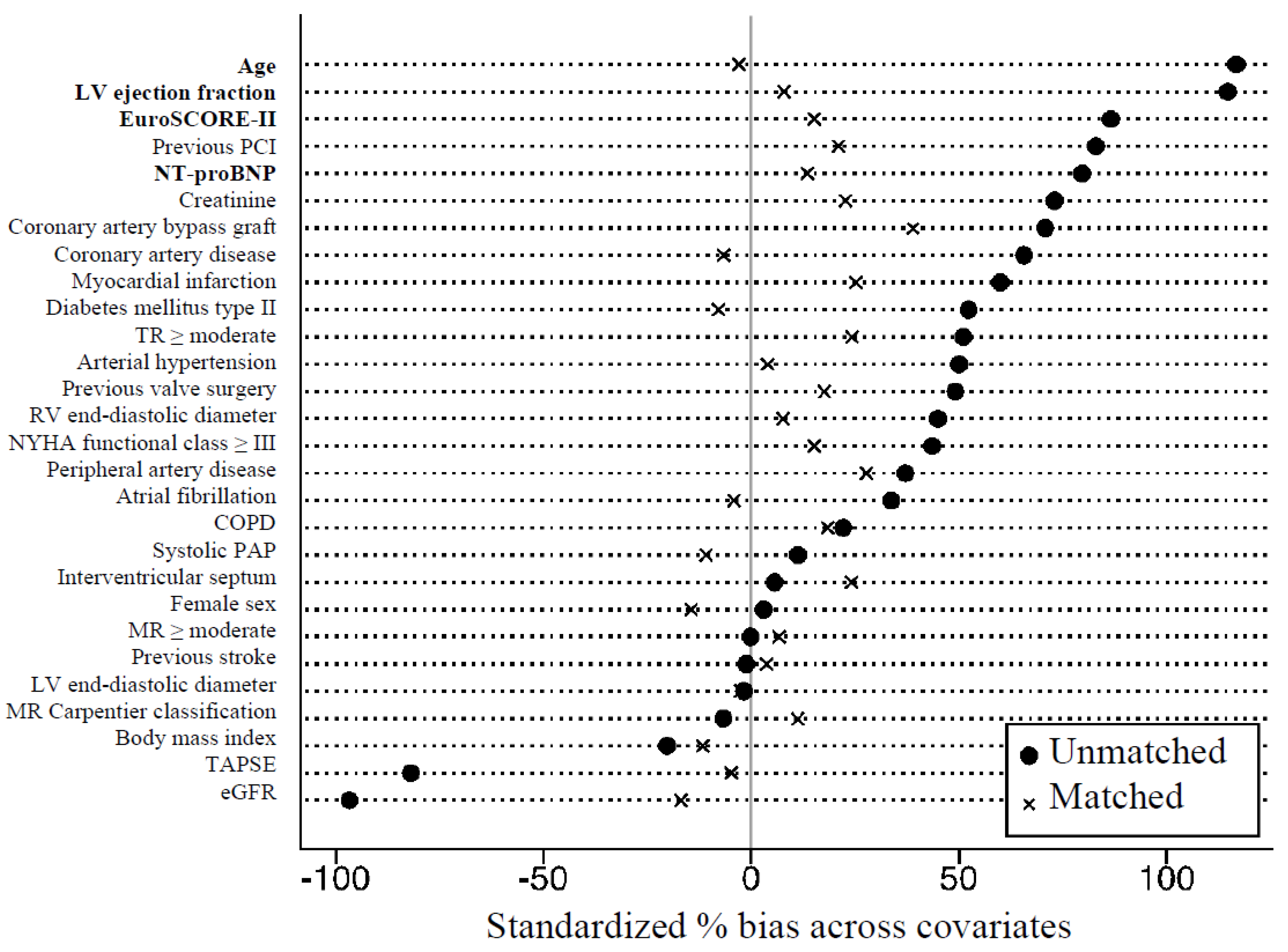
2.5. Statistical Analysis and Propensity Matching
3. Results
3.1. Baseline Characteristics
3.2. Procedural Data
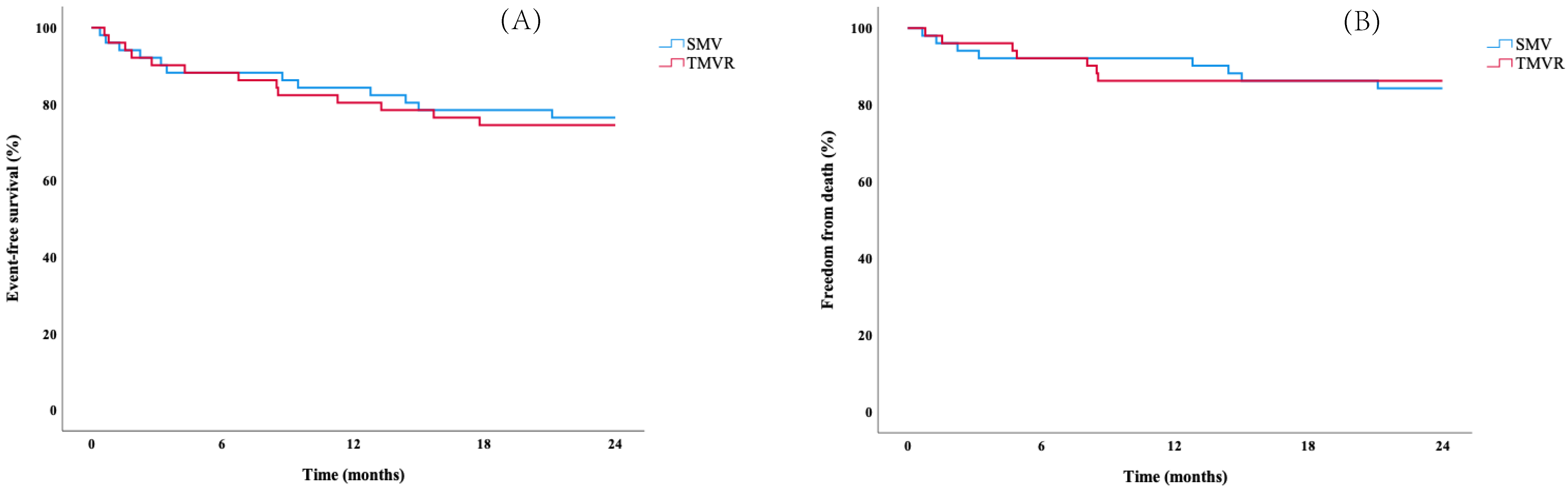
3.3. Cardiovascular Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| LVEF | Left ventricular ejection fraction |
| MR | Mitral regurgitation |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| SMV | Surgical mitral valve treatment |
| TMVR | Transcatheter edge-to-edge mitral valve repair |
References
- Trichon, B.H.; Felker, G.; Shaw, L.K.; Cabell, C.H.; O’Connor, C.M. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am. J. Cardiol. 2003, 91, 538–543. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, 932. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation (EVEREST II). N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofer, J.; Siminiak, T.; Haude, M.; Herrman, J.P.; Vainer, J.; Wu, J.C.; Levy, W.C.; Mauri, L.; Feldman, T.; Kwong, R.Y.; et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: Results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009, 120, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Kalbacher, D.; Schäfer, U.; Bardeleben, R.S.V.; Eggebrecht, H.; Sievert, H.; Nickenig, G.; Butter, C.; May, A.E.; Bekeredjian, R.; Ouarrak, T.; et al. Long-term outcome, survival and predictors of mortality after MitraClip therapy: Results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int. J. Cardiol. 2019, 277, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Vemulapalli, S.; Feldman, T.; Mack, M.; Holmes, D.R.; Stebbins, A.; Kar, S.; Thourani, V.; Ailawadi, G. Outcomes With Transcatheter Mitral Valve Repair in the United States: An STS/ACC TVT Registry Report. J. Am. Coll. Cardiol. 2017, 70, 2315–2327. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure (COAPT). N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Glower, D.D.; Kar, S.; Trento, A.; Lim, D.S.; Bajwa, T.; Quesada, R.; Whitlow, P.L.; Rinaldi, M.J.; Grayburn, P.; Mack, M.J.; et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: Results of the EVEREST II study. J. Am. Coll. Cardiol. 2014, 64, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Pleger, S.T.; Schulz-Schönhagen, M.; Geis, N.; Mereles, D.; Chorianopoulos, E.; Antaredja, M.; Lewening, M.; Katus, H.A.; Bekeredjian, R. One year clinical efficacy and reverse cardiac remodelling in patients with severe mitral regurgitation and reduced ejection fraction after MitraClip© implantation. Eur. J. Hear Fail. 2013, 15, 919–927. [Google Scholar] [CrossRef]
- Mauri, L.; Foster, E.; Glower, D.D.; Apruzzese, P.; Massaro, J.; Herrmann, H.C.; Hermiller, J.; Gray, W.; Wang, A.; Pedersen, W.R.; et al. 4-Year Results of a Randomized Controlled Trial of Percutaneous Repair Versus Surgery for Mitral Regurgitation. J. Am. Coll. Cardiol. 2013, 62, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzatti, N.; Maisano, F.; Latib, A.; Taramasso, M.; Denti, P.; La Canna, G.; Colombo, A.; Alfieri, O. Comparison of outcomes of percutaneous MitraClip versus surgical repair or replacement for degenerative mitral regurgitation in octogenarians. Am. J. Cardiol. 2015, 115, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Swaans, M.J.; Bakker, A.L.; Alipour, A.; Post, M.C.; Kelder, J.C.; de Kroon, T.L.; Eefting, F.D.; Rensing, B.J.; Van der Heyden, J.A. Survival of Transcatheter Mitral Valve Repair Compared With Surgical and Conservative Treatment in High-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2014, 7, 875–881. [Google Scholar] [CrossRef] [Green Version]
- De Bonis, M.; Taramasso, M.; Lapenna, E.; Denti, P.; La Canna, G.; Buzzatti, N.; Pappalardo, F.; Di Giannuario, G.; Cioni, M.; Giacomini, A.; et al. MitraClip therapy and surgical edge-to-edge repair in patients with severe left ventricular dysfunction and secondary mitral regurgitation: Mid-term results of a single-centre experience. Eur. J. Cardiothorac. Surg. 2016, 49, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [Green Version]
- Praz, F.; Braun, D.; Unterhuber, M.; Spirito, A.; Orban, M.; Brugger, N.; Brinkmann, I.; Spring, K.; Moschovitis, A.; Nabauer, M.; et al. Edge-to-Edge Mitral Valve Repair With Extended Clip Arms: Early Experience From a Multicenter Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1356–1365. [Google Scholar]
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc. Interv. 2019, 12, 1369–1378. [Google Scholar] [CrossRef]
- Praz, F.; Spargias, K.; Chrissoheris, M.; Büllesfeld, L.; Nickenig, G.; Deuschl, F.; Schueler, R.; Fam, N.P.; Moss, R.; Makar, M.; et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: A multicentre, prospective, observational, first-in-man study. Lancet 2017, 390, 773–780. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.-L.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Moura, L.; Popescu, B.A.; Agricola, E.; Monin, J.-L.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleger, S.T.; Mereles, D.; Schulz-Schönhagen, M.; Krumsdorf, U.; Chorianopoulos, E.; Rottbauer, W.; Katus, H.A.; Bekeredjian, R. Acute Safety and 30-Day Outcome After Percutaneous Edge-to-Edge Repair of Mitral Regurgitation in Very High-Risk Patients. Am. J. Cardiol. 2011, 108, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Sannino, A.; Cohen, D.J.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Rinaldi, M.J.; Kapadia, S.R.; Rajagopal, V.; et al. Predictors of Clinical Response to Transcatheter Reduction of Secondary Mitral Regurgitation: The COAPT Trial. J. Am. Coll. Cardiol. 2020, 76, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- McMurry, T.L.; Hu, Y.; Blackstone, E.H.; Kozower, B.D. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J. Thorac. Cardiovasc. Surg. 2015, 150, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef] [PubMed]
- Kortlandt, F.; Velu, J.; Schurer, R.; Hendriks, T.; Branden, B.V.D.; Bouma, B.; Feldman, T.; Kelder, J.; Bakker, A.; Post, M.; et al. Survival After MitraClip Treatment Compared to Surgical and Conservative Treatment for High-Surgical-Risk Patients With Mitral Regurgitation. Circ. Cardiovasc. Interv. 2018, 11, e005985. [Google Scholar] [CrossRef]
- Takagi, H.; Ando, T.; Umemoto, T.; for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. A review of comparative studies of MitraClip versus surgical repair for mitral regurgitation. Int. J. Cardiol. 2017, 228, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Külling, M.; Corti, R.; Noll, G.; Küest, S.; Hürlimann, D.; Wyss, C.; Reho, I.; Tanner, F.C.; Külling, J.; Meinshausen, N.; et al. Heart team approach in treatment of mitral regurgitation: Patient selection and outcome. Open Hear 2020, 7, e001280. [Google Scholar] [CrossRef]
- Mack, M.J.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Grayburn, P.A.; Rinaldi, M.J.; Kapadia, S.R.; et al. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 1029–1040. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 102) | SMV (n = 51) | TMVR (n = 51) | p Value | Super Responders (n = 75) | Non-Responders (n = 27) | p Value | |
|---|---|---|---|---|---|---|---|
| Clinical parameters | |||||||
| Age (years) | 72.5 ± 9.7 | 71.1 ± 10.1 | 74.0 ± 9.0 | 0.123 | 72.4 ± 9.9 | 73.0 ± 9.1 | 0.937 |
| Female sex, n (%) | 62 (61) | 29 (57) | 33 (65) | 0.417 | 42 (56) | 20 (74) | 0.099 |
| Body mass index (kg/m2) | 27.1 ± 5.0 | 27.9 ± 4.9 | 26.2 ± 4.8 | 0.149 | 27.2 ± 5.1 | 27.1 ± 4.9 | 0.850 |
| EuroSCORE-II (%) | 5.7 ± 5.7 | 5.0 ± 3.9 | 6.8 ± 5.7 | 0.241 | 5.0 ± 3.5 | 7.6 ± 9.2 | 0.203 |
| NYHA functional class ≥ III, n (%) | 83 (81) | 39 (77) | 44 (86) | 0.255 | 61 (81) | 19 (70) | 0.235 |
| NT-proBNP (pg/mL) | 2900 ± 4442 | 2192 ± 2504 | 3608 ± 5707 | 0.535 | 2158 ± 2543 | 4960 ± 7228 | 0.017 |
| Creatinine (mg/dL) | 1.3 ± 0.7 | 1.2 ± 0.7 | 1.4 ± 0.7 | 0.137 | 1.32 ± 0.7 | 1.5 ± 0.8 | 0.094 |
| eGFR (mL/min/1.73m2) | 63.6 ± 28.9 | 68.6 ± 28.2 | 58.6 ± 27.0 | 0.068 | 66.2 ± 29.0 | 56.4 ± 23.7 | 0.144 |
| Co-morbidities | |||||||
| Coronary artery disease, n (%) | 42 (41) | 17 (33) | 25 (49) | 0.108 | 30 (40) | 12 (44) | 0.687 |
| Myocardial infarction, n (%) | 14 (14) | 2 (4) | 12 (24) | 0.008 | 10 (13) | 4 (15) | 0.848 |
| Percutaneous coronary intervention, n (%) | 19 (19) | 2 (4) | 17 (33) | <0.001 | 12 (16) | 7 (26) | 0.256 |
| CABG, n (%) | 9 (9) | 0 (0) | 9 (18) | 0.003 | 7 (9) | 2 (7) | 1.000 |
| Previous valve surgery, n (%) | 8 (8) | 2 (4) | 6 (12) | 0.269 | 4 (5) | 4 (15) | 0.203 |
| Previous pacemaker implantation, n (%) | 14 (14) | 2 (4) | 12 (24) | 0.008 | 9 (12) | 5 (19) | 0.399 |
| Atrial fibrillation, n (%) | 69 (68) | 33 (65) | 36 (71) | 0.525 | 47 (63) | 22 (82) | 0.073 |
| Arterial hypertension, n (%) | 100 (98) | 50 (98) | 50 (98) | 1.000 | 73 (97) | 27 (100) | 1.000 |
| Diabetes mellitus type II, n (%) | 23 (23) | 6 (12) | 17 (33) | 0.009 | 17 (23) | 6 (22) | 0.962 |
| Hyperlipidemia, n (%) | 61 (60) | 23 (45) | 38 (75) | 0.002 | 44 (59) | 17 (63) | 0.696 |
| Previous stroke, n (%) | 8 (8) | 5 (10) | 3 (6) | 0.715 | 2 (3) | 6 (22) | 0.004 |
| Cerebral artery disease, n (%) | 9 (9) | 3 (6) | 6 (12) | 0.487 | 4 (5) | 5 (19) | 0.053 |
| Peripheral artery disease, n (%) | 5 (5) | 1 (2) | 4 (8) | 0.362 | 3 (4) | 2 (7) | 0.606 |
| COPD, n (%) | 23 (23) | 9 (18) | 14 (28) | 0.236 | 14 (19) | 9 (33) | 0.118 |
| Concomitant medication | |||||||
| Beta blockers, n (%) | 80 (78) | 42 (82) | 38 (75) | 0.336 | 56 (75) | 24 (89) | 0.174 |
| ACE inhibitors, n (%) | 36 (35) | 21 (41) | 15 (29) | 0.214 | 28 (37) | 8 (30) | 0.473 |
| Angiotensin receptor blockers, n (%) | 27 (27) | 16 (31) | 11 (22) | 0.262 | 21 (28) | 6 (22) | 0.560 |
| ARNIs, n (%) | 8 (8) | 0 (0) | 8 (16) | 0.006 | 7 (9) | 1 (4) | 0.678 |
| Calcium channel blockers, n (%) | 16 (16) | 8 (16) | 8 (16) | 1.000 | 14 (19) | 2 (7) | 0.225 |
| Loop diuretics, n (%) / daily dose (mg) | 58 (57)/46 ± 27 | 25 (49)/44 ± 29 | 33 (65)/48 ± 26 | 0.110 | 39 (52)/44 ± 27 | 19 (70)/50 ± 27 | 0.098 |
| Thiazide diuretics, n (%) / daily dose (mg) | 26 (26)/21 ± 13 | 19 (37)/20 ± 12 | 7 (14)/24 ± 15 | 0.006 | 21 (28)/20 ± 13 | 5 (19)/26 ± 14 | 0.332 |
| Spironolactone, n (%) / daily dose (mg) | 48 (47)/45 ± 21 | 18 (35)/54 ± 20 | 30 (59)/40 ± 21 | 0.017 | 32 (43)/40 ± 21 | 16 (59)/53 ± 20 | 0.139 |
| Oral anticoagulants, n (%) | 41 (40) | 11 (22) | 30 (59) | <0.001 | 29 (39) | 12 (44) | 0.600 |
| Vitamin-K-Antagonists, n (%) | 24 (24) | 19 (37) | 5 (10) | 0.002 | 16 (21) | 8 (30) | 0.384 |
| Statins, n (%) | 53 (52) | 19 (37) | 34 (67) | 0.003 | 39 (52) | 14 (52) | 0.989 |
| All Patients (n = 102) | SMV (n = 51) | TMVR (n = 51) | p Value | Super Responders (n = 75) | Non-Responders (n = 27) | p Value | |
|---|---|---|---|---|---|---|---|
| Echocardiographic parameters | |||||||
| LV end-diastolic diameter (mm) | 51.9 ± 9.8 | 51.7 ± 7.8 | 52.0 ± 11.5 | 0.914 | 51.9 ± 10.6 | 51.6 ± 7.5 | 0.686 |
| RV end-diastolic diameter (mm) | 36.5 ± 6.5 | 36.1 ± 6.5 | 36.9 ± 6.5 | 0.549 | 35.9 ± 6.3 | 38.4 ± 6.9 | 0.171 |
| Interventricular septum (mm) | 13.0 ± 2.1 | 13.1 ± 1.8 | 12.8 ± 2.4 | 0.536 | 12.8 ± 2.3 | 13.4 ± 1.5 | 0.045 |
| Aorta ascendens (mm) | 34.5 ± 4.2 | 34.2 ± 4.4 | 34.7 ± 4.0 | 0.600 | 34.7 ± 4.3 | 33.7 ± 3.8 | 0.573 |
| LV ejection fraction | 55.1 ± 15.2 | 57.9 ± 13.8 | 52.3 ± 16.1 | 0.065 | 54.9 ± 15.5 | 55.8 ± 14.6 | 0.644 |
| LV ejection fraction < 50% | 29 (32) | 13 (28) | 16 (36) | 0.415 | 19 (26) | 8 (30) | 0.719 |
| LV ejection fraction < 30% | 11 (12) | 3 (6) | 8 (16) | 0.116 | 8 (11) | 2 (7) | 1.000 |
| Systolic PAP (mmHg) | 57.7 ± 17.5 | 59.9 ± 20.2 | 55.5 ± 14.1 | 0.245 | 57.1 ± 18.2 | 59.2 ± 15.8 | 0.549 |
| TAPSE (mm) | 19.2 ± 5.5 | 19.9 ± 6.2 | 18.4 ± 4.6 | 0.177 | 19.6 ± 5.7 | 18.0 ± 4.7 | 0.283 |
| MR ≥ moderate, n (%) | 102 (100) | 51 (100) | 51 (100) | 1.000 | 75 (100) | 27 (100) | 1.000 |
| MR etiology | <0.001 | 0.755 | |||||
| Degenerative, n (%) | 63 (62) | 43 (84) | 20 (39) | 47 (63) | 16 (59) | ||
| Functional, n (%) | 39 (38) | 8 (16) | 31 (61) | 28 (37) | 11 (41) | ||
| Carpentier classification | |||||||
| Type I, n (%) | 28 (27) | 5 (10) | 23 (45) | 22 (29) | 6 (22) | ||
| Type II, n (%) | 52 (51) | 33 (65) | 19 (37) | 41 (55) | 11 (41) | ||
| Type IIIa, n (%) | 11 (11) | 10 (20) | 1 (2) | 6 (8) | 5 (19) | ||
| Type IIIb, n (%) | 11 (11) | 3 (6) | 8 (16) | 6 (8) | 5 (19) | ||
| TR ≥ moderate, n (%) | 51 (51) | 23 (46) | 28 (55) | 0.371 | 40 (54) | 11 (41) | 0.236 |
| Procedural data | |||||||
| No. of clips implanted | |||||||
| 1 (%), 2 (%), or 3 (%) | N/A | N/A | (72), (23), (5) | (74), (24), (2) | (68), (21), (11) | ||
| NTR (n), XTR (n), or PASCAL (n) | N/A | N/A | (46), (35), (5) | (27), (27), (5) | (19), (8), (0) | ||
| Type of surgery | 0.822 | ||||||
| MV repair, n (%) | N/A | 34 (67) | N/A | 25/37 (68) | 9/14 (64) | ||
| MV replacement, n (%) | N/A | 17 (33) | N/A | 12/37 (32) | 5/14 (36) | ||
| Concomitant TV procedure, n (%) | 35 (34) | 27 (53) | 8 (16) | <0.001 | 26 (35) | 9 (33) | 0.900 |
| Concomitant AV procedure, n (%) | N/A | 8 (16) | N/A | 3/37 (8) | 5/14 (36) | ||
| Concomitant CABG, n (%) | N/A | 11 (22) | N/A | 7/37 (19) | 3/14 (21) | ||
| MR postprocedural < moderate, n (%) | 93 (91) | 48 (94) | 45 (88) | 0.487 | 73 (97) | 20 (74) | 0.001 |
| MV meanPG postprocedural (mmHg) | 4.5 ± 2.0 | 5.0 ± 2.6 | 4.2 ± 1.3 | 0.113 | 4.4 ± 2.0 | 5.0 ± 2.1 | 0.173 |
| Re-intervention/surgery, n (%) | 4 (4) | 1 (2) | 3 (6) | 0.308 | 3 (4) | 1 (4) | 0.946 |
| HR | 95% CI | p Value | Adj. HR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||||
| Clinical parameters | ||||||
| Age | 1.02 | 0.98–1.06 | 0.409 | |||
| Female sex | 0.53 | 0.22–1.25 | 0.145 | |||
| Body mass index | 1.00 | 0.93–1.08 | 0.999 | |||
| EuroSCORE-II | 1.08 | 1.03–1.13 | 0.001 | 1.07 | 1.00–1.13 | 0.027 |
| NYHA functional class ≥ III | 0.74 | 0.32–1.68 | 0.467 | |||
| NT-proBNP (logarithmized) | 2.88 | 1.35–6.12 | 0.006 | 1.42 | 0.61–3.30 | 0.422 |
| Creatinine | 1.40 | 0.96–2.03 | 0.079 | |||
| eGFR | 0.99 | 0.97–1.00 | 0.067 | |||
| Co-morbidities | ||||||
| Coronary artery disease | 1.31 | 0.61–2.82 | 0.486 | |||
| Myocardial infarction | 1.46 | 0.50–4.27 | 0.494 | |||
| Percutaneous coronary intervention | 2.17 | 0.90–5.23 | 0.086 | |||
| CABG | 1.30 | 0.30–5.57 | 0.724 | |||
| Previous valve surgery | 3.36 | 1.14–9.87 | 0.028 | |||
| Previous pacemaker implantation | 2.12 | 0.78–5.66 | 0.140 | |||
| Atrial fibrillation | 2.89 | 1.09–7.68 | 0.033 | 2.36 | 0.82–6.79 | 0.112 |
| Diabetes mellitus type II | 1.15 | 0.46–2.87 | 0.760 | |||
| Hyperlipidemia | 1.43 | 0.65–3.16 | 0.379 | |||
| Previous stroke | 4.87 | 1.96–12.11 | 0.001 | |||
| Cerebral artery disease | 3.71 | 1.37–10.05 | 0.010 | |||
| Peripheral artery disease | 2.37 | 0.55–10.20 | 0.245 | |||
| COPD | 2.08 | 0.93–4.65 | 0.074 | |||
| Echocardiographic parameters | ||||||
| LV end-diastolic diameter | 0.99 | 0.95–1.04 | 0.739 | |||
| RV end-diastolic diameter | 1.06 | 1.00–1.14 | 0.072 | |||
| Interventricular septum | 1.11 | 0.93–1.33 | 0.255 | |||
| Aorta ascendens | 0.96 | 0.86–1.08 | 0.479 | |||
| LV ejection fraction | 1.00 | 0.97–1.02 | 0.934 | |||
| LV ejection fraction < 50% | 1.28 | 0.56–2.94 | 0.559 | |||
| LV ejection fraction < 30% | 0.81 | 0.19–3.44 | 0.779 | |||
| Systolic PAP | 1.00 | 0.98–1.02 | 0.862 | |||
| TAPSE | 0.95 | 0.88–1.02 | 0.948 | |||
| MR etiology | 1.58 | 0.72–3.45 | 0.256 | |||
| TR ≥ moderate | 0.77 | 0.36–1.66 | 0.499 | |||
| Procedural data | ||||||
| Type of procedure | 1.65 | 0.73–3.72 | 0.225 | |||
| Concomitant TV procedure | 0.91 | 0.41–2.04 | 0.913 | |||
| MR postprocedural | 2.28 | 1.46–3.56 | <0.001 | 1.85 | 1.17–2.92 | 0.009 |
| MV meanPG postprocedural | 1.07 | 0.91–1.25 | 0.443 | |||
| Re-intervention/surgery | 1.97 | 0.26–14.97 | 0.514 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koschutnik, M.; Dannenberg, V.; Donà, C.; Nitsche, C.; Kammerlander, A.A.; Koschatko, S.; Zimpfer, D.; Hülsmann, M.; Aschauer, S.; Schneider, M.; et al. Transcatheter Versus Surgical Valve Repair in Patients with Severe Mitral Regurgitation. J. Pers. Med. 2022, 12, 90. https://doi.org/10.3390/jpm12010090
Koschutnik M, Dannenberg V, Donà C, Nitsche C, Kammerlander AA, Koschatko S, Zimpfer D, Hülsmann M, Aschauer S, Schneider M, et al. Transcatheter Versus Surgical Valve Repair in Patients with Severe Mitral Regurgitation. Journal of Personalized Medicine. 2022; 12(1):90. https://doi.org/10.3390/jpm12010090
Chicago/Turabian StyleKoschutnik, Matthias, Varius Dannenberg, Carolina Donà, Christian Nitsche, Andreas A. Kammerlander, Sophia Koschatko, Daniel Zimpfer, Martin Hülsmann, Stefan Aschauer, Matthias Schneider, and et al. 2022. "Transcatheter Versus Surgical Valve Repair in Patients with Severe Mitral Regurgitation" Journal of Personalized Medicine 12, no. 1: 90. https://doi.org/10.3390/jpm12010090
APA StyleKoschutnik, M., Dannenberg, V., Donà, C., Nitsche, C., Kammerlander, A. A., Koschatko, S., Zimpfer, D., Hülsmann, M., Aschauer, S., Schneider, M., Bartko, P. E., Goliasch, G., Hengstenberg, C., & Mascherbauer, J. (2022). Transcatheter Versus Surgical Valve Repair in Patients with Severe Mitral Regurgitation. Journal of Personalized Medicine, 12(1), 90. https://doi.org/10.3390/jpm12010090