Risk Factors for Pancreatic Cancer: Emerging Role of Viral Hepatitis
Abstract
:1. Introduction
- High-penetrance genes: BRCA2, STK11, CDKN2A, PALB2;
- Patients with genetic syndromes at risk of developing malignancies, including pancreatic cancer (e.g., Li-Fraumeni syndrome, ataxia-telangiectasia syndrome, Peutz-Jeghers syndrome, Lynch II syndrome, etc.);
- Patients at risk of familial pancreatic cancer, without a specific molecular basis [12].
2. Viral Hepatitis: Virology and Epidemiology
3. Hepatotropic Viruses and Pancreatic Cancer-Pathophysiological Links
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-De-Mesquita, H.B.; Olsen, A.; et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 2020, 35, 975–986. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr (accessed on 3 December 2021).
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C.C. Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef]
- Ghiorzo, P. Genetic predisposition to pancreatic cancer. World J. Gastroenterol. 2014, 20, 10778–10789. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Solomon, S.; Das, S.; Brand, R.; Whitcomb, D.C. Inherited pancreatic cancer syndromes. Cancer J. 2012, 18, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.H.; Kurtz, R.C. Epidemiology of pancreatic cancer and the role of family history. J. Surg. Oncol. 2013, 107, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Chan, A.T.; Hartge, P.; Chanock, S.J.; Kraft, P.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 2009, 101, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duell, E.J.; Lucenteforte, E.; Olson, S.H.; Bracci, P.M.; Li, D.; Risch, H.A.; Silverman, D.T.; Ji, B.T.; Gallinger, S.; Holly, E.A.; et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2012, 23, 2964. [Google Scholar] [CrossRef] [PubMed]
- Bang, U.C.; Benfield, T.; Hyldstrup, L.; Bendtsen, L.; Beck, J.F. Mortality, cancer, and comorbidities associated with chronic pancreatitis: A Danish nationwide matched-cohort study. Gastroenterology 2014, 146, 989. [Google Scholar] [CrossRef] [PubMed]
- Ekbom, A.; McLaughlin, J.K.; Karlsson, B.M.; Nyren, O.; Gridley, G.; Adami, H.O.; Fraumeni, J.F. Pancreatitis and pancreatic cancer: A population-based study. J. Natl. Cancer Inst. 1994, 86, 625. [Google Scholar] [CrossRef]
- Kirkegard, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic pancreatitis and pancreatic cancer risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Guo, L.; Zhang, S.; Wang, J.; Lin, X.; Gao, F. PRSS1 mutation:a possible pathomechanism of pancreatic carcinogenesis and pancreatic cancer. Mol. Med. 2019, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wei, J.; Na, R.; Resurreccion, W.K.; Zheng, S.L.; Hulick, P.J.; Helfand, B.T.; Talamonti, M.S.; Xu, J. Cystic fibrosis F508del carriers and cancer risk: Results from the UK Biobank. Int. J. Cancer 2021, 148, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, I.; Sahora, K.; Ferrone, C.R.; Morales-Oyarvide, V.; Wolpin, B.M.; Mucci, L.A.; Brugge, W.R.; Mino-Kenudson, M.; Patino, M.; Dushyant, V.S.; et al. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017, 153, 1284. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 1253, 30416–30419. [Google Scholar] [CrossRef]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Smith, G.D.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109, djx012. [Google Scholar] [CrossRef] [Green Version]
- Wolpin, B.M.; Bao, Y.; Qian, Z.R.; Wu, C.; Kraft, P.; Ogino, S.; Stampfer, M.J.; Sato, K.; Ma, J.; Buring, J.E.; et al. Hyperglycemia, insulin resistance, impaired pancreatic β-cell function, and risk of pancreatic cancer. J. Natl. Cancer Inst. 2013, 105, 1027. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, P.; Hoorn, V.S.; Christophi, C.; Nikfarjam, M. Association of diabetes mellitus and pancreatic adenocarcinoma: A meta-analysis of 88 studies. Ann. Surg. Oncol. 2014, 21, 2453. [Google Scholar] [CrossRef]
- Hank, T.; Sandini, M.; Qadan, M.; Weniger, M.; Ciprani, D.; Li, A.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Fernandez-Del Castillo, C. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology 2020, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef] [Green Version]
- Rebours, V.; Gaujoux, S.; d’Assignies, G.; Sauvanet, A.; Ruszniewski, P.; Phillipe, L.; Paradis, V.; Bedossa, P.; Couvelard, A. Obesity and fatty pancreatic infiltration are risk factors for pancreatic precancerous lesions (PanIN). Clin. Cancer Res. 2015, 21, 3522–3528. [Google Scholar] [CrossRef] [Green Version]
- Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Fuchs, C.S. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001, 286, 921. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Bao, Y.; Wu, C.; Kraft, P.; Ogino, S.; Ng, K.; Qian, Z.R.; Rubinson, D.A.; Stampfer, M.J.; Giovannucci, E.L.; et al. Prediagnostic body mass index and pancreatic cancer survival. J. Clin. Oncol. 2013, 31, 4229. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br. J. Cancer 2012, 106, 603. [Google Scholar] [CrossRef]
- Arem, H.; Reedy, J.; Sampson, J.; Jiao, L.; Hollenbeck, A.R.; Risch, H.; Mayne, S.T.; Stolzenberg-Solomon, R.Z. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J. Natl. Cancer Inst. 2013, 105, 1298. [Google Scholar] [CrossRef] [Green Version]
- Mario, C.; Marilisa, F.; Kryssia, I.R.C.; Pellegrino, C.; Ginevra, C.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018, 89, 141–146. [Google Scholar]
- Han, X.; Li, J.; Brasky, T.M.; Xun, P.; Stevens, J.; White, E.; Gammon, M.D.; He, K. Antioxidant intake and pancreatic cancer risk: The Vitamins and Lifestyle (VITAL) Study. Cancer 2013, 119, 1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackford, A.; Parmigiani, G.; Kensler, T.W.; Wolfgang, C.; Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.H.; Leary, J.; Eshleman, J.R.; et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009, 69, 3681–3688. [Google Scholar] [CrossRef] [Green Version]
- Lowenfels, A.B.; Maisonneuve, P. Epidemiology and risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 197–209. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2015, 44, 186. [Google Scholar] [CrossRef]
- Duell, E.J.; Holly, E.A.; Bracci, P.M.; Liu, M.; Wiencke, J.K.; Kelsey, K.T. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J. Natl. Cancer Inst. 2002, 94, 297. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Dunn, J.A.; Hoover, R.N.; Schiffman, M.; Lillemoe, K.D.; Schoenberg, J.B.; Brown, L.M.; Greenberg, R.S.; Hayes, R.B.; Swanson, G.M. Cigarette smoking and pancreas cancer: A case-control study based on direct interviews. J. Natl. Cancer Inst. 1994, 86, 1510. [Google Scholar] [CrossRef]
- Wang, Y.T.; Gou, Y.W.; Jin, W.W.; Xiao, M.; Fang, H.Y. Association between alcohol intake and the risk of pancreatic cancer: A dose–response meta-analysis of cohort studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Vrieling, A.; Jiao, L.; Mendelsohn, J.B.; Steplowski, E.; Lynch, S.M.; Wactawski-Wende, J.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; et al. Alcohol intake and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium (PanScan). Cancer Causes Control 2010, 21, 1213–1225. [Google Scholar] [CrossRef]
- Lucenteforte, E.; La Vecchia, C.; Silverman, D.; Petersen, G.M.; Bracci, P.M.; Ji, B.T.; Bosetti, C.; Li, D.; Gallinger, S.; Miller, A.B.; et al. Alcohol consumption and pancreatic cancer: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2012, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Galeone, C.; Edefonti, V.; Ferraroni, M.; Lagiou, P.; La Vecchia, C.; Tavani, A. A meta-analysis of coffee consumption and pancreatic cancer. Ann. Oncol. 2012, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Kang, J.H.; Chan, A.T.; Michaud, D.S.; Skinner, H.G.; Giovannucci, E.; Colditz, G.A.; Fuchs, C.S. A prospective study of aspirin use and the risk of pancreatic cancer in women. J. Natl. Cancer Inst. 2004, 96, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, E.J.; Connell, C.J.; Rodriguez, C.; Patel, A.V.; Calle, E.E.; Thun, M.J. Aspirin use and pancreatic cancer mortality in a large United States cohort. J. Natl. Cancer Inst. 2004, 96, 524. [Google Scholar] [CrossRef] [Green Version]
- Risch, H.A.; Yu, H.; Lu, L.; Kidd, M.S. ABO Blood Group. Helicobacter pylori seropositivity, and risk of pancreatic cancer: A case-control study. J. Natl. Cancer Inst. 2010, 102, 502–505. [Google Scholar] [PubMed] [Green Version]
- Shin, E.J.; Canto, M.I. Pancreatic cancer screening. Gastroenterol. Clin. N. Am. 2012, 41, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Joshipura, K.; Giovannucci, E.; Fuchs, C.S. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J. Natl. Cancer Inst. 2007, 99, 171–175. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Dodd, K.W.; Blaser, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Tooth loss, pancreatic cancer and Helicobacter Pylori. Am. J. Clin. Nutr. 2003, 78, 176–181. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Xu, J.H.; Fu, J.J.; Wang, X.L.; Zhu, J.Y.; Ye, X.H.; Chen, S.D. Hepatitis B or C viral infection and risk of pancreatic cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013, 19, 4234–4341. [Google Scholar] [CrossRef] [PubMed]
- Lanini, S.; Ustianowski, A.; Pisapia, R.; Zumla, A.; Ippolito, G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Hepatitis Report. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=DC3616B5BDF94FA8B6AC2FDBC71E5B51?sequence=1 (accessed on 17 August 2021).
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 25 August 2021).
- Lin, C.L.; Kao, J.H. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Lanini, S.; Gudiol, C.; Drgona, L.; Ippolito, G.; Fernandez-Ruiz, M.; Salzberger, B. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin. Microbiol. Infect. 2018, 24, S71–S82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Park, H.; Henry, L.; Adeyemi, A.; Stepanova, M. Extrahepatic manifestations of hepatitis C: A meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016, 150, 1599–1608. [Google Scholar] [CrossRef]
- Lanini, S.; Easterbrook, P.J.; Zumla, A.; Ippolito, G. Hepatitis C: Global epidemiology and strategies for control. Clin. Microbiol. Infect. 2016, 22, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Gasim, G.I.; Bella, A.; Adam, I. Schistosomiasis, hepatitis B and hepatitis C co-infection. Virol. J. 2015, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, G.; Goletti, D.; Lanini, S.; Girardi, E.; Loiacono, O. HIV/HCV co-infection: Putting the pieces of the puzzle together. Cell Death Differ. 2003, 10, S25–S26. [Google Scholar] [CrossRef]
- Dandri, M.; Locarnini, S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012, 61, i6–i17. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Charlotte, F.; Goodman, Z.; McHutchison, J.; Albrecht, J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J. Hepatol. 2001, 34, 730–739. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H. HBV infection as a risk factor for non-Hodgkin lymphoma. Lancet Oncol. 2010, 11, 806. [Google Scholar] [CrossRef]
- Fiorilli, M.; Mecucci, C.; Farci, P.; Casato, M. HCV-associated lymphomas. Rev. Clin. Exp. Hematol. 2003, 7, 406–423. [Google Scholar] [PubMed]
- Tian, T.; Song, C.; Jiang, L.; Dai, J.; Lin, Y.; Xu, X.; Yu, C.; Ge, Z.; Ding, Y.; Wen, Y.; et al. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int. J. Cancer 2020, 147, 3075–3084. [Google Scholar] [CrossRef]
- Yan, F.M.; Chen, A.S.; Hao, F.; Zhao, X.P.; Gu, C.H.; Bin Zhao, L.; Yang, D.L.; Hao, L.J. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J. Gastroenterol. 2000, 6, 805–811. [Google Scholar] [CrossRef]
- Mason, A.; Wick, M.; White, H.; Perrillo, R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology 1993, 18, 781–789. [Google Scholar] [CrossRef]
- Hoefs, J.C.; Renner, I.G.; Askhcavai, M.; Redeker, A.G. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology 1980, 79, 191–194. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sakurai, I.; Shimoda, T.; Abe, K.; Okano, T.; Shikata, T. Detection of HBsAg in the pancreas. Pathol. Int. 1981, 31, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Dejean, A.; Lugassy, C.; Zafrani, S.; Tiollais, P.; Brechot, C. Detection of Hepatitis B Virus DNA in Pancreas, Kidney and Skin of Two Human Carriers of the Virus. J. Gen. Virol. 1984, 65, 651–655. [Google Scholar] [CrossRef]
- Olivera-Martínez, M.A.; Gallegos-Orozco, J.F. Recurrent Viral Liver Disease (Hepatitis B and C) after Liver Transplantation. Arch. Med Res. 2007, 38, 691–701. [Google Scholar] [CrossRef]
- Montalbano, M.; Neff, G.W. Management of recurrent viral hepatitis B and C after liver transplantation. Curr. Gastroenterol. Rep. 2006, 8, 60–66. [Google Scholar] [CrossRef]
- Hassan, M.M.; Li, D.; El-Deeb, A.S.; Wolff, R.A.; Bondy, M.L.; Davila, M.; Abbruzzese, J.L. Association Between Hepatitis B Virus and Pancreatic Cancer. J. Clin. Oncol. 2008, 26, 4557–4562. [Google Scholar] [CrossRef] [PubMed]
- Katakura, Y.; Yotsuyanagi, H.; Hashizume, K.; Okuse, C.; Okuse, N.; Nishikawa, K.; Suzuki, M.; Iino, S.; Itoh, F. Pancreatic involvement in chronic viral hepatitis. World J. Gastroenterol. 2005, 11, 3508–3513. [Google Scholar] [CrossRef]
- Yoffe, B.; Bagri, A.S.; Tran, T.; Dural, A.T.; Shtenberg, K.M.; Khaoustov, V.I. Hyperlipasemia associated with hepatitis C virus. Dig. Dis. Sci. 2003, 48, 1648–1653. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Chan, T.M.; Hui, C.-K.; Chan, A.O.-O.; Ng, I.O.-L.; Lai, C.-L. Acute pancreatitis complicating acute exacerbation of chronic hepatitis B infection carries a poor prognosis. J. Viral Hepat. 2001, 8, 459–464. [Google Scholar] [CrossRef]
- Jain, P.; Nijhawan, S. Acute viral hepatitis with pancreatitis: Is it due to the viruses or sludge? Pancreatology 2007, 7, 544–545. [Google Scholar] [CrossRef]
- Parsa, I.; Longnecker, D.S.; Scarpelli, D.G.; Pour, P.; Reddy, J.K.; Lefkowitz, M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985, 45, 1285–1290. [Google Scholar]
- Huang, J.; Magnusson, M.K.; Törner, A.; Ye, W.; Duberg, A.-S. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: A nationwide study in Sweden. Br. J. Cancer 2013, 109, 2917–2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panic, P.; Mihajlovic, S.; Vujasinovic, M.; Bulajic, M.; Lohr, J.M. Pancreatitis associated with viral hepatitis: Systematic Review. J. Clin. Med. 2020, 9, 3309. [Google Scholar] [CrossRef]
- Alvares-Da-Silva, M.R.; Francisconi, C.F.; Waechter, F.L. Acute hepatitis C complicated by pancreatitis: Another extrahepatic manifestation of hepatitis C virus? J. Viral Hepat. 2000, 7, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Kusterer, K.; Enghofer, M.; Zendler, S.; Blöchle, C.; Usadel, K.H. Microcirculatory changes in sodium taurocholate-induced pancreatitis in rats. Am. J. Physiol. 1991, 260, G346–G351. [Google Scholar] [CrossRef]
- Rajesh, G.; Nair, A.S.; A Narayanan, V.; Balakrishnan, V. Acute pancreatitis in viral infections, with possible progression to chronic pancreatitis. Indian J. Gastroenterol. 2008, 27, 162–164. [Google Scholar]
- Zaret, K.S. Genetic programming of liver and pancreas progenitors: Lessons for stem-cell differentiation. Nat. Rev. Genet. 2008, 9, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Dabeva, M.D.; Hwang, S.-G.; Vasa, S.R.G.; Hurston, E.; Novikoff, P.M.; Hixson, D.C.; Gupta, S.; Shafritz, D.A. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc. Natl. Acad. Sci. USA 1997, 94, 7356–7361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.Y.-L.; Shen, C.-N.; Lin, M.-H.; Tosh, D.; Shih, C. Hepatocyte-Like Cells Transdifferentiated from a Pancreatic Origin Can Support Replication of Hepatitis B Virus. J. Virol. 2005, 79, 13116–13128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechot, C.; Pourcel, C.; Louise, A.; Rain, B.; Tiollais, P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature 1980, 286, 533–535. [Google Scholar] [CrossRef]
- Rossner, M.T. Review: Hepatitis B virus X-gene product—A promiscuous transcriptional activator. J. Med. Virol. 1992, 36, 101–117. [Google Scholar] [CrossRef]
- Simonetti, R.G.; Camma, C.; Fiorello, F.; Cottone, M.; Rapicetta, M.; Marino, L.; Fiorentino, G.; Craxi, A.; Ciccaglione, A.; Giuseppeti, R. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis: A case-control study. Ann. Intern. Med. 1992, 116, 97–102. [Google Scholar] [CrossRef]
- Song, C.; Lv, J.; Liu, Y.; Chen, J.-G.; Ge, Z.; Zhu, J.; Dai, J.; Du, L.-B.; Yu, C.; Guo, Y.; et al. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw. Open 2019, 2, e195718. [Google Scholar] [CrossRef]
- Wei, X.-L.; Qiu, M.-Z.; Jin, Y.; Huang, Y.-X.; Wang, R.-Y.; Chen, W.-W.; Wang, D.-S.; Wang, F.-H.; Luo, H.-Y.; Zhang, D.-S.; et al. Hepatitis B virus infection is associated with gastric cancer in China: An endemic area of both diseases. Br. J. Cancer 2015, 112, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Song, C.; Zhang, Y.; Siyin, S.T.; Zhang, Q.; Song, M.; Cao, L.; Shi, H. Hepatitis B virus infection and the risk of gastrointestinal cancers among Chinese population: A prospective cohort study. Int. J. Cancer 2021. [Google Scholar] [CrossRef]
- Lam, J.O.; Hurley, L.B.; Lai, J.B.; Saxena, V.; Seo, S.; Chamberland, S.; Quesenberry, C.P., Jr.; Champsi, J.H.; Ready, J.; Chiao, E.Y.; et al. Cancer in People with and without Hepatitis C Virus Infection: Comparison of Risk Before and After Introduction of Direct-Acting Antivirals. Cancer Epidemiol. Biomark. Prev. 2021, 12, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.Z.; Chen, X.L.; Zhang, W.H.; Liu, K.; Wang, Y.J.; Tang, H.R.; Hu, J.K. SIGES research group. Associations between hepatitis B virus exposure and the risk of extrahepatic digestive system cancers: A hospital-based, case-control study (SIGES). Cancer Med. 2021, 11, 3741–3755. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Găman, M.A.; Saad, A.M.; Al-Husseini, M.J.; Shehata, O.A.; Saleh, M.A.; Nelson, A.D.; Simons-Linares, C.R. Temporal trends of incidence and mortality in Asian-Americans with pancreatic adenocarcinoma: An epidemiological study. Ann. Gastroenterol. 2020, 33, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, A.; Muula, G.; Ekouevi, D.K.; Wandeler, G. Elimination of Viral Hepatitis in Low and Middle-Income Countries: Epidemiological Research Gaps. Curr. Epidemiol. Rep. 2021, 8, 89–96. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Yan, L.; Liu, Y.; Yang, H.; Li, H. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990-2019. J. Hematol. Oncol. 2021, 14, 197. [Google Scholar] [CrossRef]
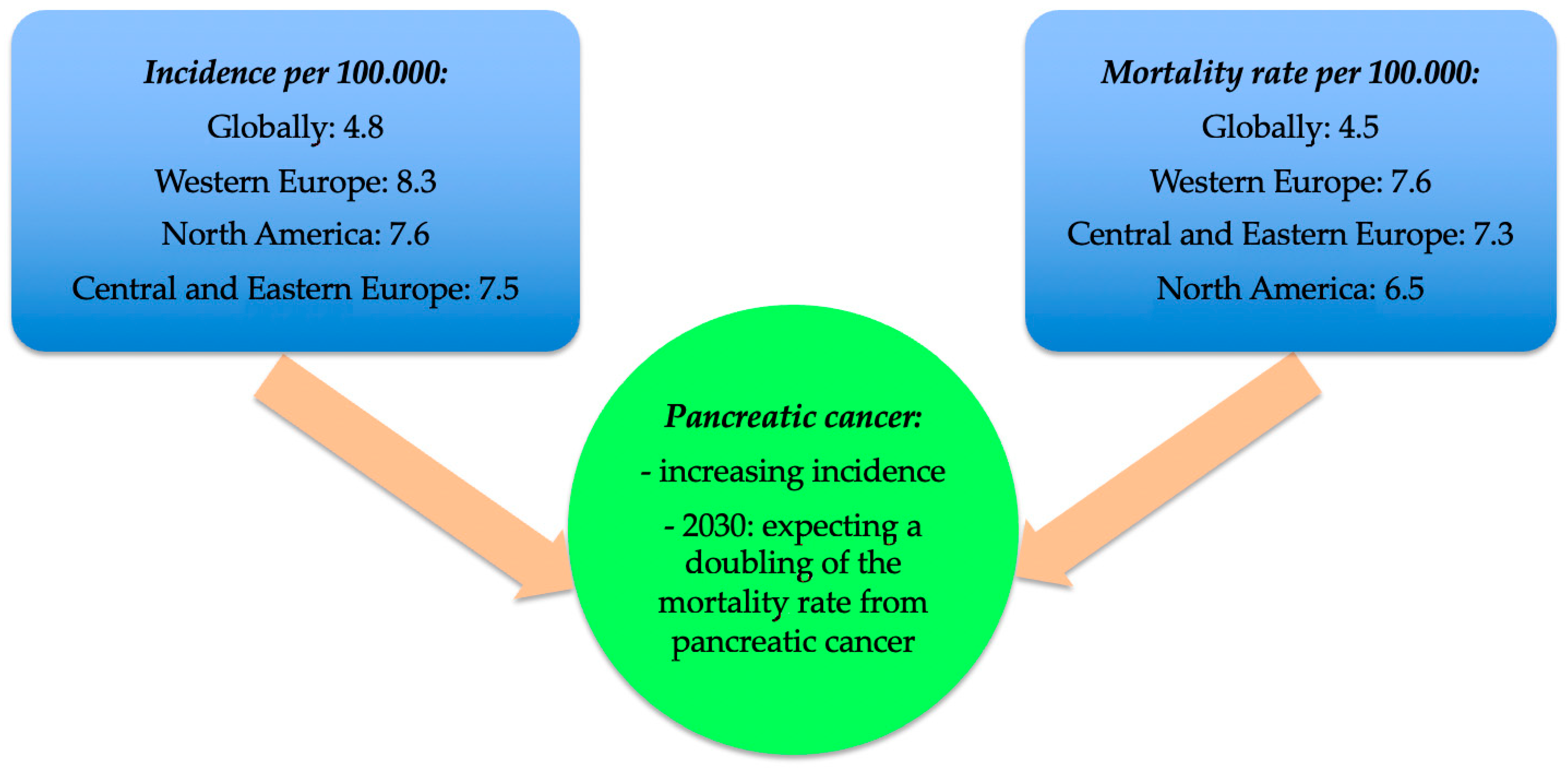
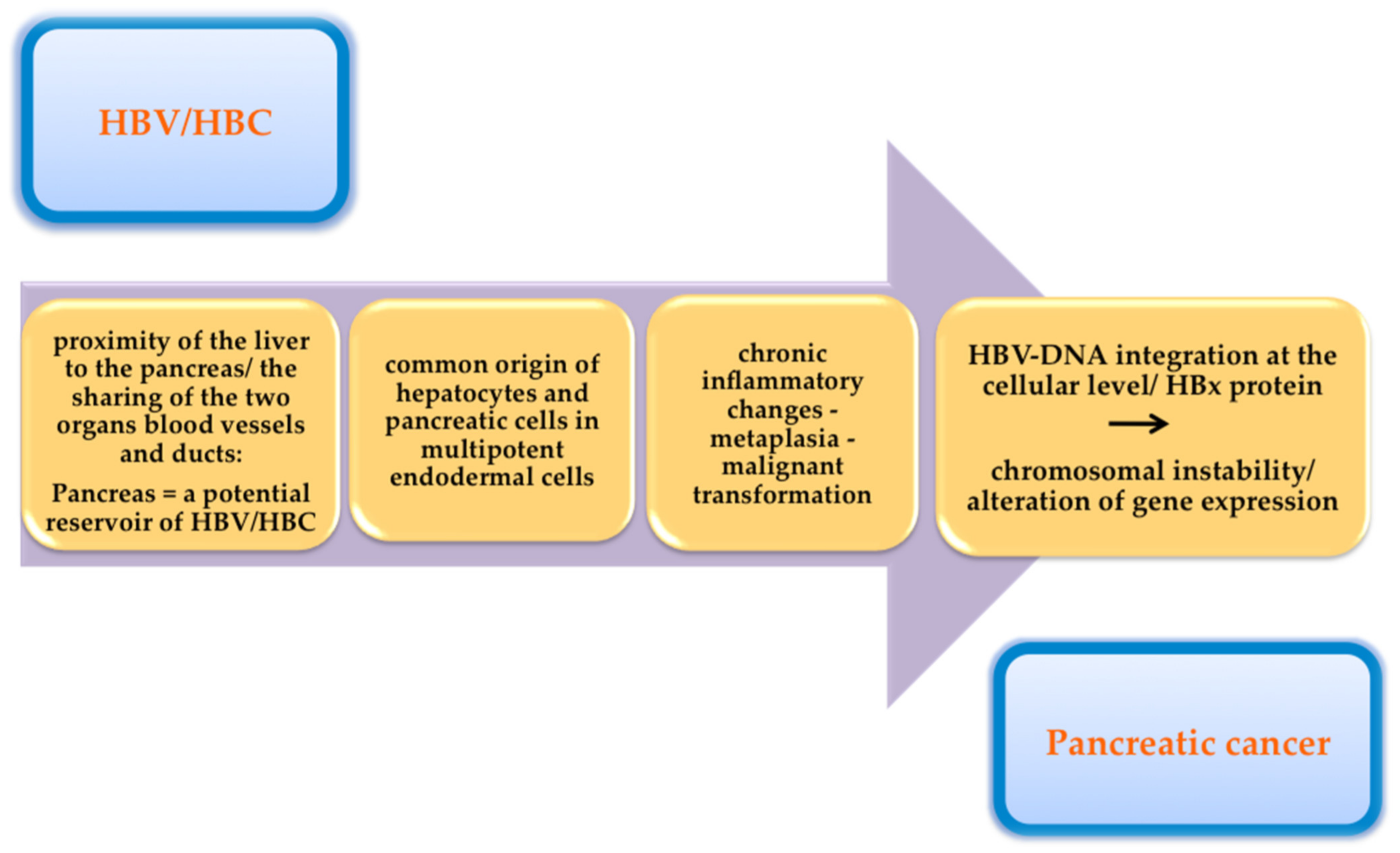
| Intrinsic Risk Factors | Extrinsic Risk Factors |
|---|---|
| Hereditary | Diet |
| AB0 blood group | Obesity |
| Chronic pancreatitis | Tobacco |
| Cystic fibrosis | Coffee and alcohol consumption |
| Pancreatic cysts | Helicobacter pylori infection |
| Diabetes mellitus and insulin resistance | Infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, G.; Diaconu, C.C.; Ionescu, V.; Constantinescu, G.; Bacalbasa, N.; Bungau, S.; Gaman, M.-A.; Stan-Ilie, M. Risk Factors for Pancreatic Cancer: Emerging Role of Viral Hepatitis. J. Pers. Med. 2022, 12, 83. https://doi.org/10.3390/jpm12010083
Gheorghe G, Diaconu CC, Ionescu V, Constantinescu G, Bacalbasa N, Bungau S, Gaman M-A, Stan-Ilie M. Risk Factors for Pancreatic Cancer: Emerging Role of Viral Hepatitis. Journal of Personalized Medicine. 2022; 12(1):83. https://doi.org/10.3390/jpm12010083
Chicago/Turabian StyleGheorghe, Gina, Camelia Cristina Diaconu, Vlad Ionescu, Gabriel Constantinescu, Nicolae Bacalbasa, Simona Bungau, Mihnea-Alexandru Gaman, and Madalina Stan-Ilie. 2022. "Risk Factors for Pancreatic Cancer: Emerging Role of Viral Hepatitis" Journal of Personalized Medicine 12, no. 1: 83. https://doi.org/10.3390/jpm12010083
APA StyleGheorghe, G., Diaconu, C. C., Ionescu, V., Constantinescu, G., Bacalbasa, N., Bungau, S., Gaman, M.-A., & Stan-Ilie, M. (2022). Risk Factors for Pancreatic Cancer: Emerging Role of Viral Hepatitis. Journal of Personalized Medicine, 12(1), 83. https://doi.org/10.3390/jpm12010083











