Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- Inclusion criteria were:
- Apparently healthy patients without a history of CV disease and aged between 18 and 55 years to minimize the possibility of age-induced CV alteration;
- The presence of a SARS-CoV2 infection, certified by a positive result of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs within 4–8 weeks before the first cardiologic examination;
- The availability of a discharge summary or an initial COVID-19 assessment with CCT and laboratory tests, documenting the presence of a mild/moderate viral pneumonia during the acute phase, where pulmonary injury under 30% was considered mild and between 30% and 60% was considered of moderate severity;
- An initial diagnosis of post-acute COVID-19 syndrome, established based on some long-lasting symptoms such as exhaustion, tiredness, breathing difficulties/dyspnea, palpitations, chest discomfort or angina, reduced effort capacity;
- A previous assessment, by TTE, at 4–8 weeks after the acute phase of COVID-19, revealing alterations of cardiac performance, associated or not with elevated sPAP values, and/or the presence of pericardial exudate or thickened pericardium and the patients’ agreement to undergo further cardiologic controls with TTE at 3 and 6 months after the COVID-19 episode.
- -
- Exclusion criteria:
- Individuals not capable or not willing to sign an informed consent, as well as those aged under 18 years;
- Patients already diagnosed with significant pre-existing cardiovascular diseases or other chronic pathologies, including those aged over 55 years who could have age-related abnormalities;
- Subjects not able to provide a baseline COVID-19 evaluation, with CCT describing the severity of the pulmonary injury and laboratory tests;
- Patients who suffered from severe/critical forms of COVID-19, with severe respiratory insufficiency, or CV complications requiring intensive care unit hospitalization;
- Subjects diagnosed during the study with significant previously unknown cardiac pathology and/or those not willing to undergo all assessments required by the study protocol.
2.2. Methods
- The assessment of LV function (LVF) was realized from apical 4-chamber view and included: (a) the assessment of the LV ejection fraction (LVEF) according to the modified Simpson rule, with values under 50% being considered abnormal; (b) the measurement of the lateral mitral annular plane systolic excursion (MAPSE), normal values being over 10 mm, lower values being pathological; (c) from apical 2-, 3-, and 4-chamber view, we employed strain techniques to evaluate the LV global longitudinal strain (LV-GLS), the region of interest (ROI) being automatically generated, with manual corrections if needed, to adjust the thickness of the LV myocardial wall after tracing the LV endocardial border [16,17]. Values under −18% strongly indicated an impaired LV systolic function.
- The evaluation of LV diastolic dysfunction (DD) included: (a) LV mass index (LVMI), determined from parasternal long axes, with LV hypertrophy (LVH) being confirmed by values of over 115 g/m2 for males and 95 g/m2 for females; (b) left atrial volume index (LAVI) was assessed from apical 4 chambers view, and values higher than 34 mL/m2 were considered abnormal; (c) from the same view, at the level of the mitral valve, we used pulsed Doppler to register the mitral inflow and measured the peak early diastolic velocity (E), the late diastolic velocity (A), and the E/A ratio; (d) at the level of the septal and lateral mitral annulus, tissue Doppler imaging (TDI) was employed to record the early diastolic velocity (e’) and the late diastolic velocity (a’), and an average and E/e’ ratio were calculated. DD of type I was considered if E/A ratio ≤ 0.8 and E < 50 cm/s, and type III DD was certified by an E/A ratio of over 2. In the situation of an E/A ratio ≤ 0.8, with E ˃ 50 cm/sec, or in case of an E/A between 0.8 and 2, DD of type II was assumed, being confirmed by at least two of the following three criteria: an average E/e’ > 14, LAVI > 34 mL/m2, and/or TRV > 2.8 m/s. If only one of the three previously mentioned criteria were present, DD of type I was considered [18].
- Right ventricular (RV) function (RVF) was assessed from 4-chamber view and comprised: (a) the measurement, in M-Mode, at the level of the lateral tricuspid valve annulus, of the tricuspid annular plane systolic excursion (TAPSE), with values under 17 mm being pathological; (b) the fractional area change (FAC), from apical view, with levels under 35% being significant for RV dysfunction (RVD); (c) from the same view, by strain techniques, we determined the RV global longitudinal strain (RV-GLS) [19,20], RVD being certified by values < −28%; (d) the estimated systolic PAP (sPAP) was determined based on the peak tricuspid regurgitation velocity (TRV) recorded by continuous Doppler and considering the right atrial pressure, appreciated by measuring the inferior vena cava diameter and its respiratory variations. In our research, we considered that sPAP values of ≥35 mmHg at rest indicate PH [14,16], with severity ranging from mild (35–44 mmHg) to moderate (45–60 mmHg) to severe (>60 mmHg) [17,19];
- The thickness of the pericardial exudate (PE), and/or of the thickened posterior pericardium (PT) were measured from standard views [21].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu-Ray, I.; Almaddah, N.K.; Adeboye, A.; Soos, M.P. Cardiac Manifestations Of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Naeini, M.B.; Sahebi, M.; Nikbakht, F.; Jamshidi, Z.; Ahmadimanesh, M.; Hashemi, M.; Ramezani, J.; Miri, H.H.; Yazdian-Robati, R. A meta-meta-analysis: Evaluation of meta-analyses published in the effectiveness of cardiovascular comorbidities on the severity of COVID-19. Obes. Med. 2021, 22, 100323. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Java, A.; Apicelli, A.J.; Liszewski, M.K.; Coler-Reilly, A.; Atkinson, J.P.; Kim, A.H.J.; Kulkarni, H.S. The complement system in COVID-19: Friend and foe? JCI Insight 2020, 5, e140711. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Intermediate and Long-Term Impact of COVID-19 on Cardiovascular Disease. Available online: https://www.acc.org/latest-in-cardiology/articles/2021/04/21/13/08/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2021%2f04%2f21%2f13%2f08%2fintermediate-and-long-term-impact-of-covid-19-on-cardiovascular-disease (accessed on 1 October 2021).
- Ranard, L.S.; Fried, J.A.; Abdalla, M.; Anstey, D.E.; Givens, R.C.; Kumaraiah, D.; Kodali, S.K.; Takeda, K.; Karmpaliotis, D.; Rabbani, L.E.; et al. Approach to Acute Cardiovascular Complications in COVID-19 Infection. Circ. Heart Fail. 2020, 13, e007220. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Iqubal, M.K.; Hoda, F.; Najmi, A.K.; Haque, S.E. COVID-19 and cardiovascular complications: An update from the underlying mechanism to consequences and possible clinical intervention. Expert Rev. Anti-Infect. Ther. 2021, 19, 1083–1092. [Google Scholar] [CrossRef]
- Lewek, J.; Jatczak-Pawlik, I.; Maciejewski, M.; Jankowski, P.; Banach, M. COVID-19 and cardiovascular complications—The preliminary results of the LATE-COVID study. Arch. Med. Sci. 2021, 17, 818–822. [Google Scholar] [CrossRef]
- Jain, S.S.; Liu, Q.; Raikhelkar, J.; Fried, J.; Elias, P.; Poterucha, T.J.; DeFilippis, E.M.; Rosenblum, H.; Wang, E.Y.; Redfors, B.; et al. Indications for and Findings on Transthoracic Echocardiography in COVID-19. J. Am. Soc. Echocardiogr. 2020, 33, 1278–1284. [Google Scholar] [CrossRef]
- Tudoran, M.; Tudoran, C.; Lazureanu, V.; Marinescu, A.; Pop, G.; Pescariu, A.; Enache, A.; Cut, T. Alterations of Left Ventricular Function Persisting during Post-Acute COVID-19 in Subjects without Previously Diagnosed Cardiovascular Pathology. J. Pers. Med. 2021, 11, 225. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Pop, G.N.; Giurgi-Oncu, C.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Parv, F.; Ciocarlie, T.; Bende, F. Associations between the Severity of the Post-Acute COVID-19 Syndrome and Echocardiographic Abnormalities in Previously Healthy Outpatients Following Infection with SARS-CoV-2. Biology 2021, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status (PCFS) Scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Altman, M.; Stanton, T.; Thomas, L. Echocardiographic Strain in Clinical Practice. Heart Lung Circ. 2019, 28, 1320–1330. [Google Scholar] [CrossRef] [Green Version]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef] [Green Version]
- Baycan, O.F.; Barman, H.A.; Atici, A.; Tatlisu, A.; Bolen, F.; Ergen, P.; Icten, S.; Gungor, B.; Caliskan, M. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2020, 37, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients with COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef]
- Sauer, F.; Dagrenat, C.; Couppie, P.; Jochum, G.; Leddet, P. Pericardial effusion in patients with COVID-19: Case series. Eur. Heart J.-Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, R.C. Anticipating the long-term cardiovascular effects of COVID-19. J. Thromb. Thrombolysis 2020, 50, 512–524. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Deng, K.Q.; Li, C.; Yang, Z.; Hu, H.; Cai, H.; Zhang, C.; He, T.; Zheng, F.; Wang, H.; et al. Cardiac Involvement in Recovered Patients From COVID-19: A Preliminary 6-Month Follow-Up Study. Front. Cardiovasc. Med. 2021, 8, 654405. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.A.; Bhatt, D.L.; Gersh, B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. Epub ahead of print. 2021. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, H.; Ma, F.; Cui, G.; Peng, L.; Li, C.; Zeng, H.; Marian, A.J.; Wang, D. Widespread myocardial dysfunction in COVID-19 patients detected by myocardial strain imaging using 2-D speckle-tracking echocardiography. Acta Pharmacol. Sin. 2021, 42, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Xiong, T.-Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; et al.; The Task Force for the management of COVID-19 of the European Society of Cardiology ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2—Care pathways, treatment, and follow-up. Eur. Heart J. 2021, ehab697. [Google Scholar] [CrossRef]
- Singh, T.; Kite, T.A.; Joshi, S.S.; Spath, N.B.; Kershaw, L.; Baker, A.; Jordan, H.; Gulsin, G.S.; Williams, M.C.; Van Beek, E.J.; et al. MRI and CT coronary angiography in survivors of COVID-19 Heart. Heart 2022, 108, 46–53. [Google Scholar] [CrossRef] [PubMed]
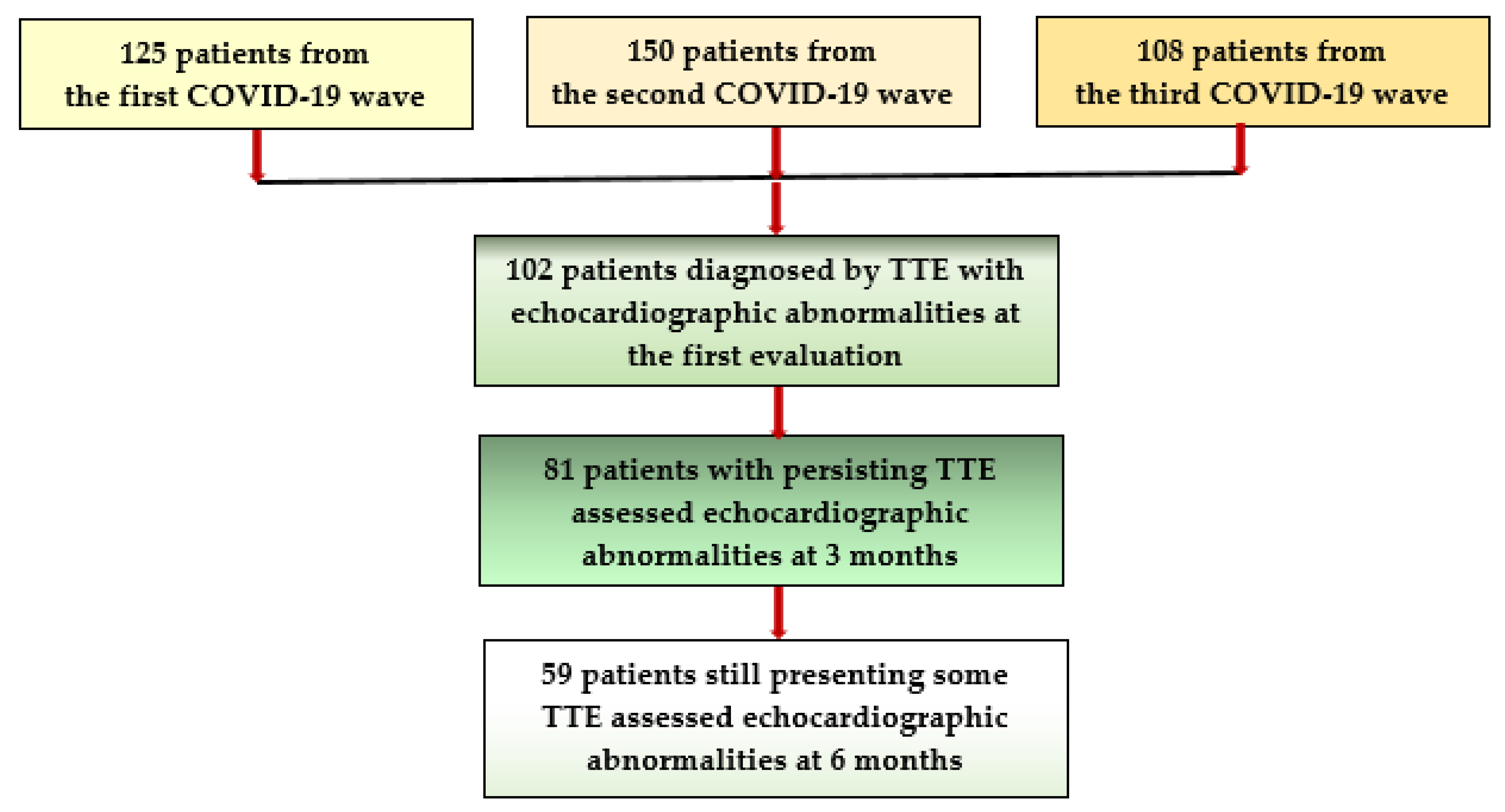
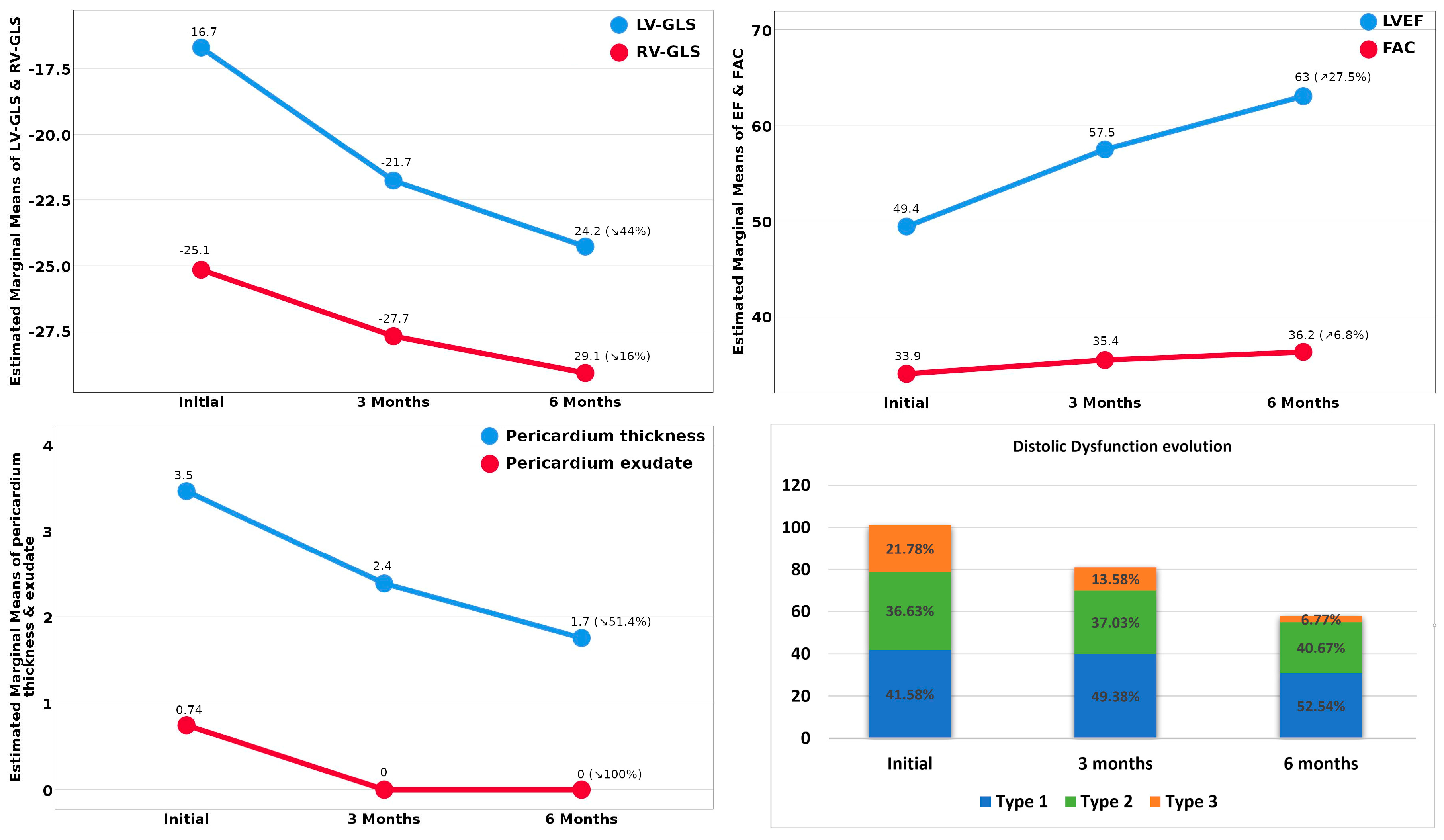
| Patients’ Characteristics | Group A 35 Patients | Group B 51 Patients | Group C 66 Patients | Group D 23 Patients |
|---|---|---|---|---|
| Age (years) | 50.2 (±4.89) | 49.29 (±5.74) | 47.63 (±5.06) | 48.78 (±5.41) |
| Gender: male | 22 (62.85%) | 28 (54.90%) | 25 (37.87%) | 15 (65.21%) |
| 13 (37.14%) | 23 (45.09%) | 41 (62.12%) | 8 (34.78%) |
| Initial pulmonary injury assessed on CCT | 38 (35–40) | 35 (31–40) | 23.5 (14.75–30) | 40 (35–40) |
| 3 (8.57%) | 8 (15.68%) | 43 (65.15%) | 3 (13.04%) |
| 32 (91.42%) | 43 (84.31%) | 23 (34.84%) | 20 (86.95%) |
| Initial CRP (mg/dL) | 45.6 (40.1–56.3) | 42.5(39.1–50.8) | 35.7 (28.5–39.7) | 47.9 (42.1–57.9) |
| Clinical findings at the inclusion in the study | ||||
| BMI (Kg/m2) | 31.1 (27.7–32.9) | 30.5 (27.5–31.8) | 27.6 (25.6–30.5) | 30.5 (27.1–31.7) |
| Heart rate (b/min) | 85 (80–90) | 80 (80–86) | 75 (75–80) | 85 (80–90) |
| Blood pressure (mmHg) | ||||
| 135 (130–140) | 130 (130–140) | 130 (120–130) | 130 (130–140) |
| 80 (80–90) | 80 (70–85) | 70 (70–80) | 80 (75–90) |
| PCFS scale | 3 (3–3) | 3 (2–3) | 2 (2–2) | 3 (3–3) |
| Weeks since COVID-19 | 5 (4–6) | 6 (4–7) | 8 (7–8) | 5 (4–6) |
| Electrocardiography | ||||
| Sinus tachycardia (˃ 80 b/min) | 18 (51.42%) | 25 (49.01%) | 13 (19.69%) | 12 (52.17%) |
| Non-specific ST/T changes | 13 (37.14%) | 15 (29.41%) | 11 (16.66%) | 11 (47.82%) |
| Isolated PSVB/PVB | 17 (48.57%) | 17 (33.33%) | 13 (19.69%) | 7 (30.43%) |
| TTE Results at the First Evaluation | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| LVMI (g/m2) | 110 (94.56–118) | 98.13 (94–116.73) | 98.69(94.48–112.83) | 109.12 (96.7–117.65) |
| LAVI (mL/m2) | 30.45 (22.9–35.4) | 29.7 (21.59–34.85) | 29.51 (20.12–34.05) | 30.45 (22.9–35.4) |
| Pericardial exudate (mm) | 4 (3.67–4.15) | 4 (3.6–4.1) | 3.2 in 1 patient | 4 (3.6–4.1) |
| Pericardial thickness | 4.3 (3.6–6) | 3.8 (3.4–5.2) | 2.85 (2.15–3.52) | 5.6 (4.3–6) |
| MAPSE (mm) | 8 (7–8) | 8 (7–10) | 12 (11–14.25) | 7 (7–8) |
| LVEF (%) | 42 (39–44) | 43 (40–50) | 54.5 (50–56.25) | 41 (38–43) |
| LV-GLS (%) | −13 (−15–−11) | −15 (−17–−12) | −19 (−21–−18) | −13 (−15–−11) |
| TAPSE (mm) | 15.3 (13–16) | 16 (15–17) | 19 (18–20.25) | 15.3 (13–16) |
| FAC (%) | 31.78 (30–33.56) | 33.11(30.24–34.02) | 35.29 (34.5–35.91) | 31.23 (29–33.56) |
| RV-GLS (%) | −20(−24–−19) | −22 (−25–−19) | −28 (−29–−27) | −20 (−22–−19) |
| TRV (m/sec) | 3.23 (3.12–3.35) | 3.15 (2.98–3.3) | 2.7 (2.68–2.74) | 3.28 (3.17–3.39) |
| sPAP (mmHg) | 46.73 (43.93–49.9) | 44.68 (40.52–40.56) | 34.16 (33.72–34.98) | 48.03 (45.19–50.96) |
| E/A | 2.03 (0.96–2.11) | 1.4 (0.94–2.1) | 0.94 (0.74–1.26) | 2.05 (1.27–2.12) |
| E/e’ | 14.44 (14.18–15.1) | 14.35 (14.12–14.79) | 14.13 (13.38–14.32) | 14.52 (14.15–15.0.9) |
| Age | LVEF | MAPSE | sPAP | RV-GLS | E/e’ | Weeks | CCT Score | CRP | PCFS Scale | |
|---|---|---|---|---|---|---|---|---|---|---|
| R | 0.248 | −0.851 | −0.745 | 0.699 | 0.678 | 0.433 | −0.588 | 0.525 | 0.522 | 0.529 |
| 95%CI | 0.055 | −0.919 | −0.825 | 0.560 | 0.534 | 0.231 | −0.708 | 0.369 | 0.343 | 0.365 |
| 0.434 | −0.747 | −0.627 | 0.800 | 0.784 | 0.609 | −0.441 | 0.646 | 0.674 | 0.674 | |
| p | 0.012 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Age | LVEF | MAPSE | sPAP | RV-GLS | BMI | PT | CCT Score | CRP | PCFS Scale | |
|---|---|---|---|---|---|---|---|---|---|---|
| R | 0.358 | −0.499 | −0.426 | 0.454 | 0.474 | 0.222 | 0.423 | 0.376 | 0.547 | 0.364 |
| 95%CI | 0.172 | −0.603 | −0.603 | 0.249 | 0.293 | 0.028 | 0.225 | 0.180 | 0.353 | 0.144 |
| 0.520 | −0.311 | −0.213 | 0.633 | 0.638 | 0.401 | 0.589 | 0.573 | 0.699 | 0.539 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.025 | <0.001 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Marinescu, A.R.; Pescariu, S.A.; Pop, G.N.; Bende, F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. J. Pers. Med. 2022, 12, 46. https://doi.org/10.3390/jpm12010046
Tudoran C, Tudoran M, Cut TG, Lazureanu VE, Oancea C, Marinescu AR, Pescariu SA, Pop GN, Bende F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. Journal of Personalized Medicine. 2022; 12(1):46. https://doi.org/10.3390/jpm12010046
Chicago/Turabian StyleTudoran, Cristina, Mariana Tudoran, Talida Georgiana Cut, Voichita Elena Lazureanu, Cristian Oancea, Adelina Raluca Marinescu, Silvius Alexandru Pescariu, Gheorghe Nicusor Pop, and Felix Bende. 2022. "Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19" Journal of Personalized Medicine 12, no. 1: 46. https://doi.org/10.3390/jpm12010046
APA StyleTudoran, C., Tudoran, M., Cut, T. G., Lazureanu, V. E., Oancea, C., Marinescu, A. R., Pescariu, S. A., Pop, G. N., & Bende, F. (2022). Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. Journal of Personalized Medicine, 12(1), 46. https://doi.org/10.3390/jpm12010046








