Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Class Definition
2.3. Machine Learning Algorithm and Statistical Analysis
3. Results
3.1. Study Population
3.2. Model Prediction Ability
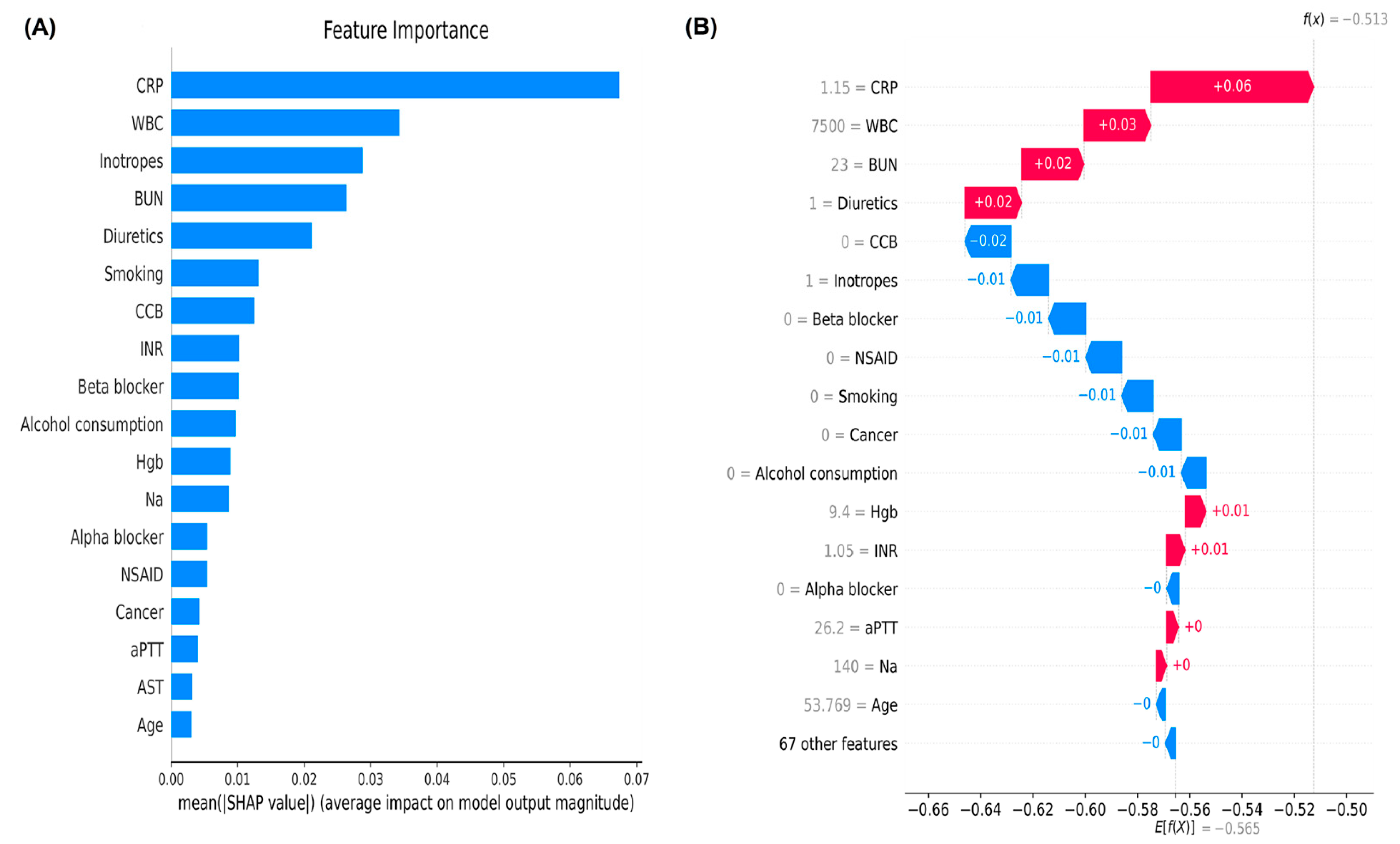
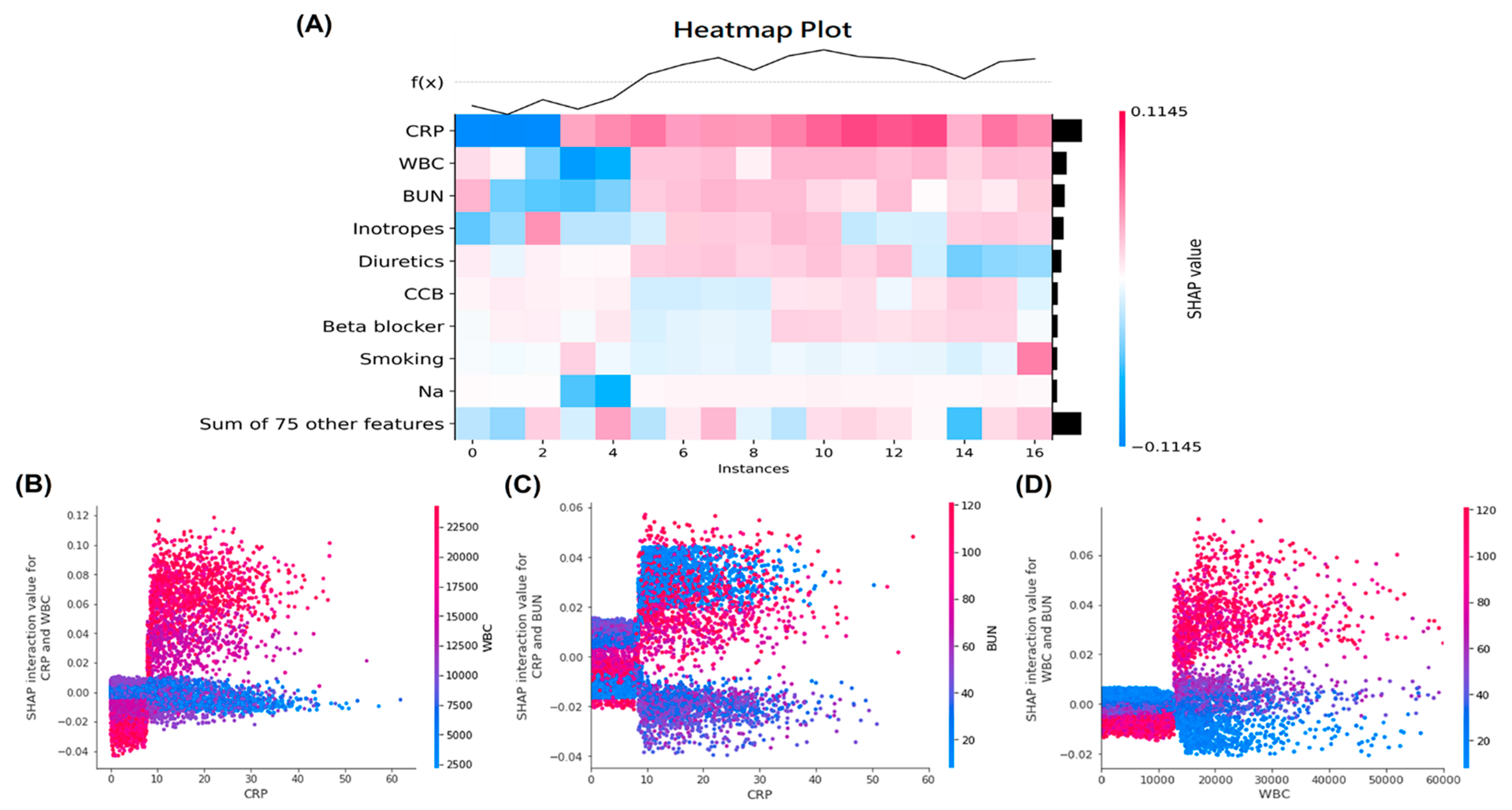
3.3. Ranks of Feature Importance and SHAP Value in the Machine Learning Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wu, M.H.; Tsou, P.Y.; Wang, Y.H.; Lee, M.G.; Chao, C.C.T.; Lee, W.C.; Lee, S.-H.; Hu, J.-R.; Wu, J.-Y.; Chang, S.-S.; et al. Impact of post-sepsis cardiovascular complications on mortality in sepsis survivors: A population-based study. Crit. Care 2019, 23, 293. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Chen, J.; Hillman, K.; Flabouris, A.; Parr, M.; Assareh, H.; Bellomo, R. The impact of post-operative sepsis on mortality after hospital dis-charge among elective surgical patients: A population-based cohort study. Crit. Care 2017, 21, 34. [Google Scholar] [CrossRef] [Green Version]
- Prescott, H.C.; Langa, K.M.; Liu, V.; Escobar, G.J.; Iwashyna, T.J. Increased 1-Year Healthcare Use in Survivors of Severe Sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.M.; Chu, H.; Chao, P.W.; Lee, Y.J.; Kuo, S.C.; Chen, T.J.; Tseng, C.-M.; Shih, C.-J.; Chen, Y.-T. Long-Term Mortality and Major Adverse Cardiovascular Events in Sepsis Survivors. A Nationwide Population-based Study. Am. J. Respir. Crit. Care Med. 2016, 194, 209–217. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Yugeesh, L.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Murugan, R.; Karajala-Subramanyam, V.; Lee, M.; Yende, S.; Kong, L.; Carter, M.; Angus, D.C.; Kellum, J.A. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010, 77, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Godin, M.; Murray, P.; Mehta, R.L. Clinical Approach to the Patient with AKI and Sepsis. Semin. Nephrol. 2015, 35, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.; Lameire, N.H.; Vanholder, R.C.; Benoit, D.D.; Decruyenaere, J.M.; Colardyn, F.A. Acute Renal Failure in Patients with Sepsis in a Surgical ICU: Predictive Factors, Incidence, Comorbidity, and Outcome. J. Am. Soc. Nephrol. 2003, 14, 1022–1030. [Google Scholar] [CrossRef] [Green Version]
- Bagshaw, S.M.; George, C.; Bellomo, R. Early acute kidney injury and sepsis: A multicentre evaluation. Crit. Care 2008, 12, R47. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.P.; Hohmann, S.F.; Wang, H.E. Unplanned Readmissions After Hospitalization for Severe Sepsis at Academic Medical Center-Affiliated Hospitals. Crit. Care Med. 2015, 43, 1916–1927. [Google Scholar] [CrossRef]
- Singh, A.; Bhagat, M.; George, S.V.; Gorthi, R.; Chaturvedula, C. Factors Associated with 30-day Unplanned Readmissions of Sepsis Patients: A Retrospective Analysis of Patients Admitted with Sepsis at a Community Hospital. Cureus 2019, 11, e5118. [Google Scholar] [CrossRef] [Green Version]
- Shamout, F.; Zhu, T.; Clifton, D.A. Machine Learning for Clinical Outcome Prediction. IEEE Rev. Biomed. Eng. 2021, 14, 116–126. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning—Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Lai, C.C.; Huang, W.H.; Chang, B.C.; Hwang, L.C. Development of Machine Learning Models for Prediction of Smoking Cessation Outcome. Int. J. Environ. Res. Public Health 2021, 18, 2584. [Google Scholar] [CrossRef]
- Yang, S.; Su, T.; Huang, L.; Feng, L.H.; Liao, T. A novel risk-predicted nomogram for sepsis associated-acute kidney injury among critically ill patients. BMC Nephrol. 2021, 22, 173. [Google Scholar] [CrossRef]
- Fan, C.; Ding, X.; Song, Y. A new prediction model for acute kidney injury in patients with sepsis. Ann. Palliat. Med. 2021, 10, 1772–1778. [Google Scholar] [CrossRef]
- Luo, X.Q.; Yan, P.; Zhang, N.Y.; Luo, B.; Wang, M.; Deng, Y.H.; Wu, T.; Wu, X.; Liu, Q.; Wang, H.-S.; et al. Machine learning for early discrimination between transient and persistent acute kidney injury in critically ill patients with sepsis. Sci. Rep. 2021, 11, 20269. [Google Scholar] [CrossRef]
- Kuan, A.S.; Chen, T.J. Healthcare data research: The inception of the Taipei Veterans General Hospital Big Data Center. J. Chin. Med. Assoc. 2019, 82, 679. [Google Scholar] [CrossRef]
- Jolley, R.J.; Sawka, K.J.; Yergens, D.W.; Quan, H.; Jetté, N.; Doig, C.J. Validity of administrative data in recording sepsis: A systematic review. Crit. Care. 2015, 19, 139. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Ochoa, I.; Bustamante-Munguira, J.; Mendiluce-Herrero, A.; Bustamante-Bustamante, J.; Coca-Rojo, A. Impact on Outcomes across KDIGO-2012 AKI Criteria According to Baseline Renal Function. J. Clin. Med. 2019, 8, 1323. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.R.; Suyundikov, A.; Slattery, M.L. Accounting for Missing Data in Clinical Research. JAMA 2016, 315, 517–518. [Google Scholar] [CrossRef]
- Liao, S.G.; Lin, Y.; Kang, D.D.; Chandra, D.; Bon, J.; Kaminski, N.; Sciurba, F.C.; Tseng, G.C. Missing value imputation in high-dimensional phenomic data: Imputable or not, and how? BMC Bioinform. 2014, 15, 346. [Google Scholar] [CrossRef] [Green Version]
- Rigatti, S.J. Random Forest. J. Insurance Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Geurts, P.; Ernstr, D.; Wehenkel, L. Extremely Randomized Trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, I.B.; Saeed, F. Bioactive Molecule Prediction Using Extreme Gradient Boosting. Molecules 2016, 21, 983. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Irfan, M.; Raza, M.; Ghori, K.; Yaqoob, S.; Awais, M. Performance Analysis of Boosting Classifiers in Recognizing Activities of Daily Living. Int. J. Environ. Res. Public Health 2020, 17, 1082. [Google Scholar] [CrossRef] [Green Version]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of machine learning models using shapley values: Application to compound potency and multi-target activity predictions. J. Comput. Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Parsa, A.B.; Movahedi, A.; Taghipour, H.; Derrible, S.; Mohammadian, A.K. Toward safer highways, application of XGBoost and SHAP for real-time accident detection and feature analysis. Accid. Anal. Prev. 2020, 136, 105405. [Google Scholar] [CrossRef]
- Bloch, L.; Friedrich, C.M. Data analysis with Shapley values for automatic subject selection in Alzheimer’s disease data sets using interpretable machine learning. Alzheimer’s Res. Ther. 2021, 13, 155. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Rubenfeld, G.D. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr. Infect. Dis. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [Green Version]
- Shankar-Hari, M.; Harrison, D.A.; Ferrando-Vivas, P.; Rubenfeld, G.D.; Rowan, K. Risk Factors at Index Hospitalization Associated with Longer-term Mortality in Adult Sepsis Survivors. JAMA Netw. Open 2019, 2, e194900. [Google Scholar] [CrossRef] [Green Version]
- Meurer, W.J.; Losman, E.D.; Smith, B.L.; Malani, P.N.; Younger, J.G. Short-term functional decline of older adults admitted for suspected sepsis. Am. J. Emerg. Med. 2011, 29, 936–942. [Google Scholar] [CrossRef]

| All Patients | Training Set | Testing Set | |
|---|---|---|---|
| (n = 23,761) | (n = 16,632) | (n = 7129) | |
| Demographic and clinical characteristics | |||
| Age, years | 76.4 (61.4, 85.2) | 76.4 (61.2, 85.2) | 76.4 (61.9, 85.2) |
| Male sex, n (%) | 8557 (36.0) | 5995 (36.0) | 2562 (35.9) |
| Smoking, n (%) | 5373 (22.6) | 3744 (22.5) | 1629 (22.9) |
| Alcohol consumption, n (%) | 3945 (16.6) | 2739 (16.5) | 1206 (16.9) |
| Underlying Comorbidities | |||
| Hypertension, n (%) | 13,238 (55.7) | 9271 (55.7) | 3967 (55.6) |
| Transient ischemic attack, n (%) | 579 (2.4) | 399 (2.4) | 180 (2.5) |
| Ischemic stroke, n (%) | 3343 (14.1) | 2358 (14.2) | 985 (13.8) |
| Hemorrhagic stroke, n (%) | 1084 (4.6) | 774 (4.7) | 310 (4.3) |
| Dementia, n (%) | 3190 (13.4) | 2218 (13.3) | 972 (13.6) |
| Diabetes mellitus, n (%) | 7803 (32.8) | 5432 (32.7) | 2371 (33.3) |
| Gout, n (%) | 2443 (10.3) | 1737 (10.4) | 706 (9.9) |
| Myocardial infarction, n (%) | 1852 (7.8) | 1272 (7.6) | 580 (8.1) |
| Coronary artery disease, n (%) | 6260 (26.3) | 4308 (25.9) | 1952 (27.4) |
| CHF, n (%) | 4759 (20.0) | 3282 (19.7) | 1477 (20.7) |
| Atrial fibrillation, n (%) | 2394 (10.1) | 1667 (10.0) | 727 (10.2) |
| Chronic liver disease, n (%) | 3875 (16.3) | 2729 (16.4) | 1146 (16.1) |
| Cirrhosis, n (%) | 1395 (5.9) | 996 (6.0) | 399 (5.6) |
| Peptic ulcer disease, n (%) | 5632 (23.7) | 3957 (23.8) | 1675 (23.5) |
| COPD, n (%) | 4469 (18.8) | 3110 (18.7) | 1359 (19.1) |
| Asthma, n (%) | 1192 (5.0) | 835 (5.0) | 357 (5.0) |
| PAOD, n (%) | 192 (0.8) | 130 (0.8) | 62 (0.9) |
| Autoimmune disease, n (%) | 821 (3.5) | 591 (3.6) | 230 (3.2) |
| Cancer, n (%) | 11,592 (48.8) | 8145 (49.0) | 3447 (48.4) |
| Valvular heart disease, n (%) | 1303 (5.5) | 908 (5.5) | 395 (5.5) |
| Critical conditions | |||
| ICU admission, n (%) | 12,962 (54.6) | 9041 (54.4) | 3921 (55.0) |
| Use of mechanical ventilators, n (%) | 8740 (36.8) | 6083 (36.6) | 2657 (37.3) |
| Use of inotropes, n (%) | 11,343 (47.7) | 7933 (47.7) | 3410 (47.8) |
| Laboratory data | |||
| Blood urea nitrogen, mg/dL | 24.0 (14.0, 51.0) | 24.0 (14.0, 51.0) | 24.0 (14.0, 50.0) |
| Creatinine, mg/dL | 1.1 (0.7, 2.1) | 1.1 (0.7, 2.2) | 1.1 (0.7, 2.1) |
| White blood cells, /mm3 | 8100 (5700, 11,900) | 8100 (5700, 11,900) | 8100 (5700, 12,000) |
| Hemoglobin, g/dL | 10.1 (8.9, 11.5) | 10.1 (8.9, 11.5) | 10.1 (9.0, 11.6) |
| Sodium, mmol/L | 139.0 (135.0, 142.0) | 139.0 (135.0, 142.0) | 139.0 (135.0, 142.0) |
| Potassium, mmol/L | 4.1 (3.6, 4.6) | 4.1 (3.6, 4.6) | 4.1 (3.6, 4.6) |
| Chloride, mmol/L | 103.0 (98.0, 106.0) | 103.0 (98.0, 106.0) | 103.0 (98.0, 106.0) |
| Calcium, mg/dL | 8.5 (8.0, 9.0) | 8.5 (8.0, 9.0) | 8.5 (8.0, 9.0) |
| Phosphate, mg/dL | 3.3 (2.6, 4.0) | 3.3 (2.6, 4.0) | 3.3 (2.7, 4.1) |
| HCO3, mmol/L | 23.7 (19.3, 28.0) | 23.7 (19.3, 28.0) | 23.8 (19.4, 28.0) |
| C-reactive protein, mg/dL | 3.4 (1.2, 9.0) | 3.4 (1.2, 9.1) | 3.3 (1.1, 8.7) |
| Albumin, mg/dL | 3.0 (2.6, 3.4) | 3.0 (2.6, 3.4) | 3.0 (2.6, 3.4) |
| Alanine transaminase, U/L | 25.0 (15.0, 44.0) | 25.0 (15.0, 45.0) | 25.0 (15.0, 44.0) |
| Aspartate transaminase, U/L | 29.0 (20.0, 51.0) | 29.0 (20.0, 51.0) | 29.0 (20.0, 50.0) |
| Alkaline phosphatase, U/L | 95.0 (70.0, 147.0) | 95.0 (69.0, 147.0) | 94.0 (70.0, 147.0) |
| Gamma-glutamyl transferase, U/L | 54.0 (25.0, 125.0) | 53.0 (25.0, 125.0) | 54.0 (24.0, 126.0) |
| Total bilirubin, mg/dL | 0.6 (0.4, 1.1) | 0.6 (0.4, 1.1) | 0.6 (0.4, 1.1) |
| HbA1c, % | 6.4 (5.8, 7.4) | 6.4 (5.8, 7.4) | 6.4 (5.8, 7.4) |
| Glucose, mg/dL | 116.0 (95.0, 156.0) | 116.0 (94.0, 155.0) | 117.0 (95.0, 157.0) |
| Uric acid, mg/dL | 5.5 (4.1, 7.1) | 5.5 (4.1, 7.1) | 5.6 (4.1, 7.1) |
| Cholesterol, mg/dL | 151.0 (122.0, 182.0) | 152.0 (122.0, 183.0) | 150.0 (121.0, 181.0) |
| LDL-C, mg/dL | 91.0 (70.0, 114.0) | 91.0 (70.0, 115.0) | 91.0 (69.0, 113.0) |
| HDL-C, mg/dL | 41.0 (32.0, 51.0) | 41.0 (32.0, 51.0) | 41.0 (32.0, 51.0) |
| INR | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) |
| aPTT, seconds | 29.9 (27.1, 34.0) | 29.9 (27.2, 34.2) | 29.9 (27.1, 33.8) |
| D-Dimer, ug/mL | 3.6 (1.6, 8.1) | 3.6 (1.5, 7.7) | 3.9 (1.8, 9.3) |
| LDH, U/L | 253.0 (196.0, 361.0) | 252.0 (196.0, 361.0) | 255.0 (197.0, 361.0) |
| NT-proBNP, pg/mL | 3146.0 (836.5, 11,617.0) | 3142.0 (823.8, 11,648.5) | 3185.0 (856.8, 11,580.8) |
| Concomitant Medications | |||
| ACEI, n (%) | 2225 (9.4) | 1537 (9.2) | 688 (9.7) |
| ARB, n (%) | 6972 (29.3) | 4884 (29.4) | 2088 (29.3) |
| Alpha, blocker, n (%) | 6109 (25.7) | 4228 (25.4) | 1881 (26.4) |
| Beta blocker, n (%) | 8521 (35.9) | 5891 (35.4) | 2630 (36.9) |
| CCB, n (%) | 10,534 (44.3) | 7362 (44.3) | 3172 (44.5) |
| Warfarin, n (%) | 1263 (5.3) | 893 (5.4) | 370 (5.2) |
| DOAC, n (%) | 147 (0.6) | 106 (0.6) | 41 (0.6) |
| Aspirin, n (%) | 5445 (22.9) | 3743 (22.5) | 1702 (23.9) |
| Plavix, n (%) | 3267 (13.7) | 2242 (13.5) | 1025 (14.4) |
| Nitrate, n (%) | 6521 (27.4) | 4473 (26.9) | 2048 (28.7) |
| Statin, n (%) | 3446 (14.5) | 2387 (14.4) | 1059 (14.9) |
| Diuretic, n (%) | 14,714 (61.9) | 10,287 (61.9) | 4427 (62.1) |
| Spironolactone, n (%) | 4927 (20.7) | 3427 (20.6) | 1500 (21.0) |
| Metformin, n (%) | 3459 (14.6) | 2447 (14.7) | 1012 (14.2) |
| Sulfonylurea, n (%) | 2214 (9.3) | 1533 (9.2) | 681 (9.6) |
| Meglitinide, n (%) | 2150 (9.0) | 1495 (9.0) | 655 (9.2) |
| SGLT2 inhibitor, n (%) | 47 (0.2) | 33 (0.2) | 14 (0.2) |
| GLP1 receptor agonist, n (%) | 3 (0.0) | 3 (0.0) | 0 (0.0) |
| Dipeptidyl peptidase-4 inhibitor, n (%) | 2720 (11.4) | 1883 (11.3) | 837 (11.7) |
| Thiazolidinedione, n (%) | 283 (1.2) | 203 (1.2) | 80 (1.1) |
| Alpha-glucosidase inhibitor, n (%) | 1084 (4.6) | 744 (4.5) | 340 (4.8) |
| Insulin, n (%) | 11,163 (47.0) | 7810 (47.0) | 3353 (47.0) |
| NSAID, n (%) | 11,300 (47.6) | 7917 (47.6) | 3383 (47.5) |
| COX-2 inhibitor, n (%) | 3316 (14.0) | 2284 (13.7) | 1032 (14.5) |
| Proton pump inhibitor, n (%) | 13,642 (57.4) | 9506 (57.2) | 4136 (58.0) |
| Steroid, n (%) | 8227 (34.6) | 5781 (34.8) | 2446 (34.3) |
| Allopurinol, n (%) | 1583 (6.7) | 1110 (6.7) | 473 (6.6) |
| Febuxostat, n (%) | 1446 (6.1) | 1006 (6.0) | 440 (6.2) |
| Benzbromarone, n (%) | 1424 (6.0) | 1007 (6.1) | 417 (5.8) |
| Class/Outcome | |||
| Rehospitalization with AKI † | 8756 (36.9) | 6076 (36.5) | 2680 (37.6) |
| Demographics | Comorbidities | Laboratory Data | Containment Medications |
|---|---|---|---|
| Age | Hypertension | Blood urea nitrogen | ACEI |
| Gender | Transient ischemic attack | Creatinine | ARB |
| Smoking | Ischemic stroke | White blood cell counts | Alpha blocker |
| Alcohol consumption | Hemorrhagic stroke | Hemoglobin | Beta blocker |
| Dementia | Sodium | CCB | |
| Diabetes mellitus | Potassium | Warfarin | |
| Gout | Chloride | DOAC | |
| Myocardial infarction | Calcium | Aspirin | |
| Coronary artery disease | Phosphate | Plavix | |
| CHF | HCO3 | Nitrate | |
| Atrial fibrillation | C-reactive protein | Statin | |
| Chronic liver disease | Albumin | Diuretic | |
| Cirrhosis | Alanine transaminase | Spironolactone | |
| Peptic ulcer disease | Aspartate transaminase | Metformin | |
| COPD | Alkaline phosphatase | Sulfonylurea | |
| Asthma | Gamma-glutamyl transferase | Meglitinide | |
| PAOD | Total bilirubin | SGLT2 inhibitor | |
| Autoimmune disease | HbA1c | GLP1 receptor agonist | |
| Cancer | Glucose | DPP4 inhibitor | |
| Valvular heart disease | Uric acid | Thiazolidinedione | |
| ICU admission | Cholesterol | Alpha-glucosidase inhibitor | |
| Use of mechanical ventilators | LDL-C | Insulin | |
| Use of inotropes | HDL-C | NSAID | |
| INR | COX-2 inhibitor | ||
| aPTT | Proton pump inhibitor | ||
| D-dimer | Steroid | ||
| LDH | Allopurinol | ||
| NT-proBNP | Febuxostat | ||
| Benzbromarone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, S.-M.; Lee, K.-H.; Tsai, M.-T.; Tseng, W.-C.; Chu, Y.-C.; Tarng, D.-C. Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors. J. Pers. Med. 2022, 12, 43. https://doi.org/10.3390/jpm12010043
Ou S-M, Lee K-H, Tsai M-T, Tseng W-C, Chu Y-C, Tarng D-C. Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors. Journal of Personalized Medicine. 2022; 12(1):43. https://doi.org/10.3390/jpm12010043
Chicago/Turabian StyleOu, Shuo-Ming, Kuo-Hua Lee, Ming-Tsun Tsai, Wei-Cheng Tseng, Yuan-Chia Chu, and Der-Cherng Tarng. 2022. "Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors" Journal of Personalized Medicine 12, no. 1: 43. https://doi.org/10.3390/jpm12010043
APA StyleOu, S.-M., Lee, K.-H., Tsai, M.-T., Tseng, W.-C., Chu, Y.-C., & Tarng, D.-C. (2022). Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors. Journal of Personalized Medicine, 12(1), 43. https://doi.org/10.3390/jpm12010043







