Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant and Study Design
2.2. Data Collection and Clinical Measurement
2.3. Machine Learning-Based Decision Tree Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Dziadkowiak, E.; Waliszewska-Prosół, M.; Wieczorek, M.; Bladowska, J.; Budrewicz, S.; Ejma, M. Myasthenia Gravis—An Analysis of Multimodal Evoked Potentials. Brain Sci. 2021, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Rousseff, R.T. Diagnosis of Myasthenia Gravis. J. Clin. Med. 2021, 10, 1736. [Google Scholar] [CrossRef] [PubMed]
- Farmakidis, C.; Pasnoor, M.; Dimachkie, M.M.; Barohn, R.J. Treatment of Myasthenia Gravis. Neurol. Clin. 2018, 36, 311–337. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, D.B.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Wolfe, G.; Benatar, M.; Cea, G.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.; et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021, 96, 114–122. [Google Scholar] [CrossRef]
- Wakata, N.; Iguchi, H.; Sugimoto, H.; Nomoto, N.; Kurihara, T. Relapse of ocular symptoms after remission of myasthenia gravis—A comparison of relapsed and complete remission cases. Clin. Neurol. Neurosurg. 2003, 105, 75–77. [Google Scholar] [CrossRef]
- Jani-Acsadi, A.; Lisak, R.P. Myasthenic crisis: Guidelines for prevention and treatment. J. Neurol. Sci. 2007, 261, 127–133. [Google Scholar] [CrossRef]
- Thomas, C.E.; Mayer, S.A.; Gungor, Y.; Swarup, R.; Webster, E.A.; Chang, I.; Brannagan, T.H.; Fink, M.E.; Rowland, L.P. Myasthenic crisis: Clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology 1997, 48, 1253–1260. [Google Scholar] [CrossRef]
- Keesey, J.C. “Crisis” in myasthenia gravis: An historical perspective. Muscle Nerve 2002, 26, 1–3. [Google Scholar] [CrossRef]
- Spillane, J.; Taylor, C.; Hirsch, N.P.; Kullmann, D.M.; Howard, R.S. 171 Myasthenic crisis in the intensive care unit: A 10-year review. J. Neurol. Neurosurg. Psychiatry 2012, 83, e1. [Google Scholar] [CrossRef]
- Rabinstein, A.; Wijdicks, E.F. BiPAP in acute respiratory failure due to myasthenic crisis may prevent intubation. Neurology 2002, 59, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, J.; Mandrekar, J.; Wijdicks, E.F.; Rabinstein, A.A. Noninvasive ventilation in myasthenic crisis. Arch. Neurol. 2008, 65, 54–58. [Google Scholar] [CrossRef]
- Aggarwal, A.N.; Gupta, D.; Behera, D.; Prabhakar, S.; Jindal, S.K. Intensive respiratory care in patients with myasthenic crisis. Neurol. India 2002, 50, 348–351. [Google Scholar] [PubMed]
- Alshekhlee, A.; Miles, J.D.; Katirji, B.; Preston, D.C.; Kaminski, H.J. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009, 72, 1548–1554. [Google Scholar] [CrossRef]
- Spillane, J.; Hirsch, N.P.; Kullmann, D.M.; Taylor, C.; Howard, R.S. Myasthenia gravis—Treatment of acute severe exacerbations in the intensive care unit results in a favourable long-term prognosis. Eur. J. Neurol. 2014, 21, 171–173. [Google Scholar] [CrossRef]
- Sharma, S.; Lal, V.; Prabhakar, S.; Agarwal, R. Clinical profile and outcome of myasthenic crisis in a tertiary care hospital: A prospective study. Ann. Indian Acad. Neurol. 2013, 16, 203–207. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Yamashita, S.; Hirano, T.; Nakajima, M.; Kimura, E.; Maeda, Y.; Uchino, M. Myasthenic crisis patients who require intensive care unit management. Muscle Nerve 2012, 46, 440–442. [Google Scholar] [CrossRef]
- Lee, M.; Ha, J.; Son, Y.S.; Ahn, H.; Jung, K.B.; Son, M.Y.; Kim, J. Efficient exogenous DNA-free reprogramming with suicide gene vectors. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.H.C.; Krause, J.; Peng, L. How to Read Articles That Use Machine Learning: Users’ Guides to the Medical Literature. JAMA 2019, 322, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Chen, X. Myasthenic crisis treated in a Chinese neurological intensive care unit: Clinical features, mortality, outcomes, and predictors of survival. BMC Neurol. 2019, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Bedlack, R.S.; Simel, D.L.; Bosworth, H.; Samsa, G.; Tucker-Lipscomb, B.; Sanders, D.B. Quantitative myasthenia gravis score: Assessment of responsiveness and longitudinal validity. Neurology 2005, 64, 1968–1970. [Google Scholar] [CrossRef]
- Henrard, S.; Speybroeck, N.; Hermans, C. Classification and regression tree analysis vs. multivariable linear and logistic regression methods as statistical tools for studying haemophilia. Haemophilia 2015, 21, 715–722. [Google Scholar] [CrossRef]
- Peng, J.; Chen, C.; Zhou, M.; Xie, X.; Zhou, Y.; Luo, C.H. A machine-learning approach to forecast aggravation risk in patients with acute exacerbation of chronic obstructive pulmonary disease with clinical indicators. Sci. Rep. 2020, 10, 3118. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-C.; Lu, C.-J.; Chen, G.-D.; Chang, C.-C. Risk Prediction for Early Chronic Kidney Disease: Results from an Adult Health 384 Examination Program of 19,270 Individuals. Int. J. Environ. Res. Public Health 2020, 17, 4973. [Google Scholar] [CrossRef]
- Gulati, P.; Sharma, A.; Gupta, M. Theoretical study of decision tree algorithms to identify pivotal factors for performance improvement: A review. Int. J. Comput. Appl. 2016, 141, 975. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Routledge: Pacific Grove, CA, USA, 1984. [Google Scholar]
- Quinlan, J.R. C4.5: Programs for Machine Learning; Morgan Kaufmann Publishers: San Francisco, CA, USA, 1993. [Google Scholar]
- Quinlan, J.R. C5.0 and See 5: Illustrative Examples. Rule Quest Research. 1997. Available online: http://www.rulequest.com (accessed on 1 September 2021).
- Peng, C.-Y.J.; Lee, K.L.; Ingersoll, G.M. An introduction to logistic regression analysis and reporting. J. Educ. Res. 2002, 96, 3–14. [Google Scholar] [CrossRef]
- Hebbali, A. blorr: Tools for Developing Binary Logistic Regression Models. R package version 0.3.0. Available online: https://CRAN.R-project.org/package=blorr (accessed on 1 September 2021).
- Therneau, T.; Atkinson, B. rpart: Recursive Partitioning and Regression Trees. R Package Version, 4.1-15. Available online: https://CRAN.R-project.org/package=rpart (accessed on 1 September 2021).
- Hornik, K.; Buchta, C.; Hothorn, T.; Karatzoglou, A.; Meyer, D.; Zeileis, A. RWeka: R/Weka Interface. R package version 0.4-43. Available online: https://CRAN.R-project.org/package=RWeka (accessed on 1 September 2021).
- Kuhn, M.; Weston, S.; Culp, M.; Coulter, N.; Quinlan, R. C50: C5.0 Decision Trees and Rule-Based Models. R package version 0.1.5. Available online: https://CRAN.R-project.org/package=C50 (accessed on 1 September 2021).
- Kuhn, M. Caret: Classification and Regression Training. 2021. R Package Version, 6.0-88. Available online: https://CRAN.R-project.org/package=caret (accessed on 1 September 2021).
- Barnett, C.; Herbelin, L.; Dimachkie, M.M.; Barohn, R.J. Measuring clinical treatment response in myasthenia gravis. Neurol. Clin. 2018, 36, 339–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Y.; Jia, R.; Xue, L.; Liang, H. Myasthenia gravis in patients with thymoma affects survival rate following extended thymectomy. Oncol. Lett. 2016, 11, 4177–4182. [Google Scholar] [CrossRef]
- Margaritora, S.; Cesario, A.; Cusumano, G.; Meacci, E.; D’Angelillo, R.; Bonassi, S.; Carnassale, G.; Porziella, V.; Tessitore, A.; Vita, M.L.; et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann. Thorac. Surg. 2010, 89, 245–252. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Thymoma and myasthenia gravis: A clinical study of 1089 patients from Japan. Ann. Thorac. Surg. 2005, 79, 219–224. [Google Scholar] [CrossRef]
- Albert, M.L.; Darnell, R.B. Paraneoplastic neurological degenerations: Keys to tumour immunity. Nat. Rev. Cancer 2004, 4, 36–44. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, Y.Z.; Shi, Z.Y.; Qiu, Y.; Zhang, H.L.; Wang, Z.H.; Li, W.B.; Wang, Y. Different neurologic outcomes of myasthenia gravis with thymic hyperplasia and thymoma after extended thymectomy: A single center experience. J. Neurol. Sci. 2017, 383, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Palace, J.; Newsom-Davis, J.; Lecky, B.; Myasthenia Gravis Study Group. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology 1998, 50, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.H.; Ansher, M.; Horan, S.; Rutherford, R.B.; Ringel, S.P. The relationship of age to outcome in myasthenia gravis. Neurology 1990, 40, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Aarli, J.A. Late-onset myasthenia gravis: A changing scene. Arch. Neurol. 1999, 56, 25–27. [Google Scholar] [CrossRef]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; de Munain, A.L.; et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Sakai, W.; Matsui, N.; Ishida, M.; Furukawa, T.; Miyazaki, Y.; Fujita, K.; Miyamoto, R.; Yamamoto, N.; Sako, W.; Sato, K.; et al. Late-onset myasthenia gravis is predisposed to become generalized in the elderly. eNeurologicalSci 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Tiamkao, S.; Pranboon, S.; Thepsuthammarat, K.; Sawanyawisuth, K. Factors predicting the outcomes of elderly hospitalized myasthenia gravis patients: A national database study. Int. J. Gen. Med. 2017, 10, 131–135. [Google Scholar] [CrossRef][Green Version]
- Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Toubi, E.; Porat, B.S.; Shoenfeld, Y. Autoimmunity in the Elderly: Insights from Basic Science and Clinics—A Mini-Review. Gerontology 2017, 63, 515–523. [Google Scholar] [CrossRef]
- Citirak, G.; Cejvanovic, S.; Andersen, H.; Vissing, J. Effect of Gender, Disease Duration and Treatment on Muscle Strength in Myasthenia Gravis. PLoS ONE 2016, 11, e0164092. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Q.; Qiu, Z.; Lin, J.; Chen, B.; Li, Y.; Gui, M.; Zhang, M.; Yang, M.; Wang, W.; et al. Analysis of mortality and related factors in 2195 adult myasthenia gravis patients in a 10-year follow-up study. Neurol. India 2017, 65, 518–524. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Ding, M.; Ni, R.; Wang, J.; Qu, L.; Wang, W.; Wu, Y. Comparative analysis of three data mining techniques in diagnosis of lung cancer. Eur. J. Cancer Prev. 2021, 30, 15–20. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; He, M. Clinical predictors for the prognosis of myasthenia gravis. BMC Neurol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Tiamkao, S.; Pranboon, S.; Thepsuthammarat, K.; Sawanyawisuth, K. Prevalence of factors associated with poor outcomes of hospitalized myasthenia gravis patients in Thailand. Neurosciences 2014, 19, 286–290. [Google Scholar] [PubMed]
- Hügle, M.; Omoumi, P.; van Laar, J.M.; Boedecker, J.; Hügle, T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol. Adv. Pract. 2020, 4, rkaa005. [Google Scholar] [CrossRef]
- Vodenčarević, A.; Goes, M.; Medina, O.; Groot, M.; Haitjema, S.; Solinge, W.; Hoefer, I.; Peelen, L.; Laar, J.; Zimmermann-Rittereiser, M.; et al. Predicting flare probability in rheumatoid arthritis using machine learning methods. In Proceedings of the 7th International Conference on Data Science, Technology and Applications (DATA 2018), Porto, Portugal, 26–28 July 2018; pp. 187–192. [Google Scholar]
- Keesey, J.C. Clinical evaluation and management of myasthenia gravis. Muscle Nerve 2004, 29, 484–505. [Google Scholar] [CrossRef] [PubMed]
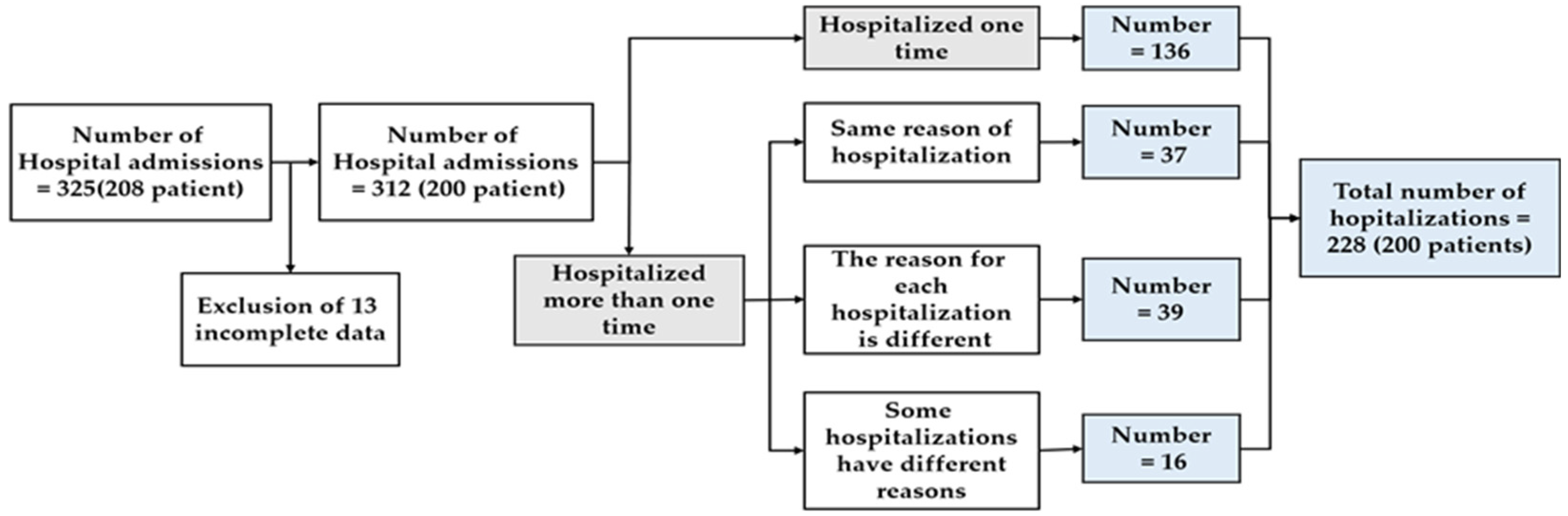
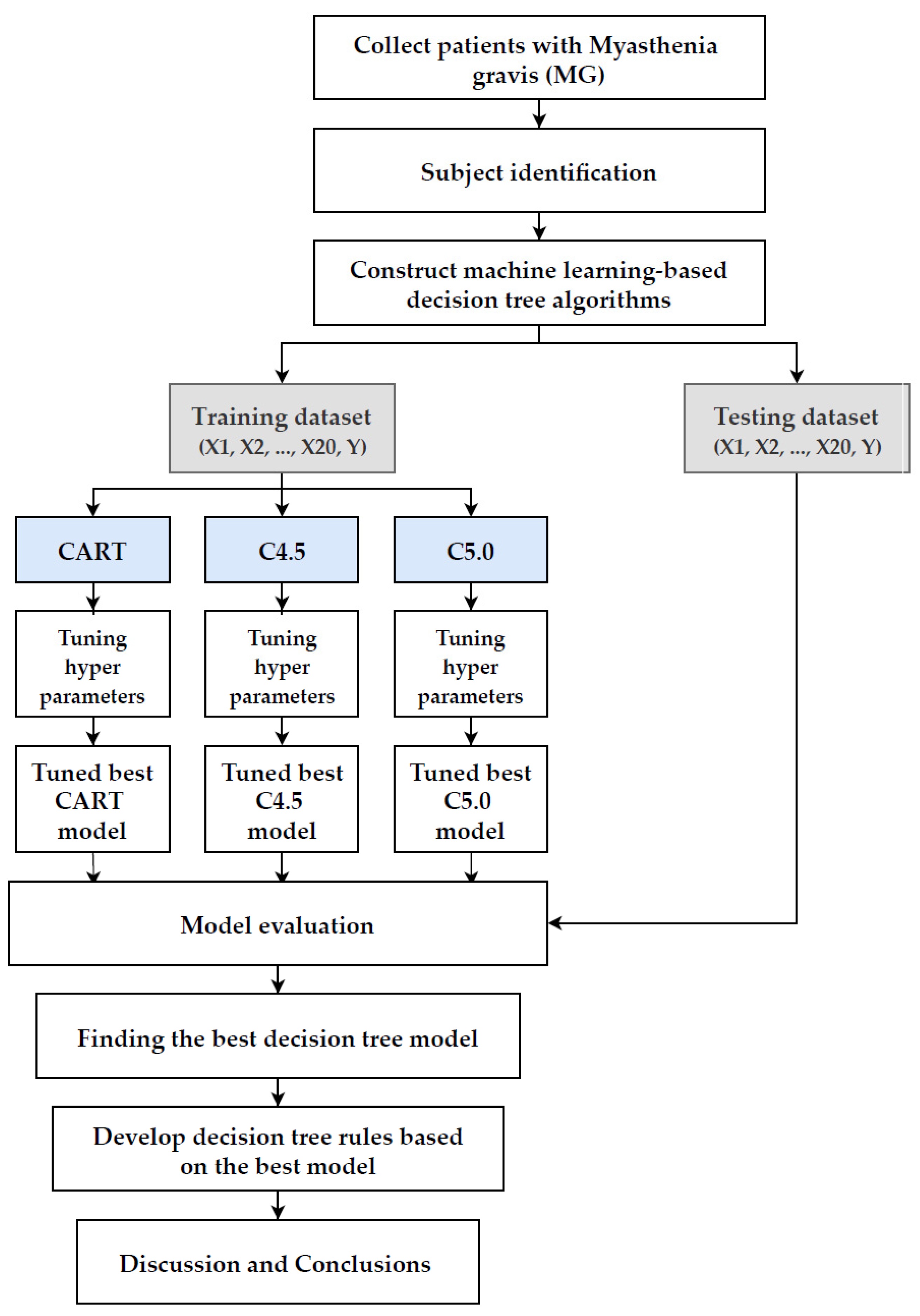
| Variables | Description | Unit | |
|---|---|---|---|
| X1 | Age at admission | Age of first visit after 1 December 2015 | Years |
| X2 | Disease duration | Time from the onset to the first visit after 1 December 2015 | Months |
| X3 | Age at onset | Age of MG symptoms onset | Years |
| X4 | Gender | Male/Female | — |
| X5 | MGFA clinical classification | The maximum MGFA clinical severity during enrollment period: 1: Class I: ocular muscle weakness 2: Class II: Mild limbs, axial, bulbar or respiratory weakness 3: Class III: Moderate limbs, axial, bulbar or respiratory weakness 4: Class IV: Severe limbs, axial, bulbar or respiratory weakness 5: Class V: Intubation | — |
| X6 | Thymoma | Thymus present with thymoma | Yes/No |
| X7 | Hyperplasia | Thymus present with thymic hyperplasia | Yes/No |
| X8 | Thymectomy | History of received thymectomy 0: No 1: Received thymectomy at presented hospitalization 2: Received thymectomy before | — |
| X9 | Anti-AChR Ab | Serology of autoantibody against Anti-AChR | Yes/No |
| X10 | Anti-MuSK Ab | Serology of autoantibody against Anti-MuSK Ab | Yes/No |
| X11 | dSN | Double seronegative | Yes/No |
| X12 | PSL Maximum daily dose | The maximum dose of corticosteroid from the first visit between December 2015 and October 2018 | mg |
| X13 | OI | Treatment with Oral Immunosuppressant during enrollment period | Yes/No |
| X14 | AZA | Treatment with Azathioprine during enrollment period | Yes/No |
| X15 | MMF | Treatment with Mycophenolate mofetil during enrollment period | Yes/No |
| X16 | OT | Treatment with Oral Tacrolimus during enrollment period | Yes/No |
| X17 | IVIG | Treatment with Intravenous immunoglobins during enrollment period | Yes/No |
| X18 | PP | Treatment with plasmapheresis during enrollment period 1: No 2: 5 sessions 3: >5 sessions | — |
| X19 | IC | Treatment with intravenous corticosteroid during enrollment period | Yes/No |
| X20 | RTX | Treatment with Rituximab during enrollment period | Yes/No |
| Y | ICU admission | ICU admission was defined as greater than 1 day 0: ≤1 day 1: >1 day | — |
| Characteristics | Metrics | |
|---|---|---|
| Basic Information: | Mean ± SD | |
| X1: | Age at admission | 49.14 ± 17.01 |
| X2: | Disease duration | 68.75 ± 84.40 |
| X3: | Age at onset | 43.22 ± 17.43 |
| X4: | Gender: | N (%) |
| Male | 88(38.60%) | |
| Female | 140(61.40%) | |
| X5: | MGFA clinical classification: | N (%) |
| Class I | 24(10.53%) | |
| Class II | 88(38.60%) | |
| Class III | 74(32.46%) | |
| Class IV | 26(11.40%) | |
| Class V | 16(7.02%) | |
| Thymus: | N (%) | |
| X6: | Thymoma: | |
| No | 118(51.75%) | |
| Yes | 110(48.25%) | |
| X7: | Hyperplasia: | |
| No | 161(70.61%) | |
| Yes | 67(29.39%) | |
| X8: | Thymectomy: | |
| No | 80(35.09%) | |
| Received thymectomy at presented | 93(40.79%) | |
| Received thymectomy before | 55(24.12%) | |
| Autoantibody: | N (%) | |
| X9: | Anti-AChR Ab: | |
| No | 27(11.84%) | |
| Yes | 201(88.16%) | |
| X10: | Anti-MuSK Ab: | |
| No | 217(95.18%) | |
| Yes | 11(4.82%) | |
| X11: | dSN: | |
| No | 211(92.54%) | |
| Yes | 17(7.46%) | |
| Treatment status: | Mean ± SD | |
| X12: | PSL Maximum daily dose | 14.60 ± 15.68 |
| X13: | OI: | N (%) |
| No | 91(39.91%) | |
| Yes | 137(60.09%) | |
| X14: | AZA: | N (%) |
| No | 152(66.67%) | |
| Yes | 76(33.33%) | |
| X15: | MMF: | N (%) |
| No | 219(96.05%) | |
| Yes | 9(3.95%) | |
| X16: | OT: | N (%) |
| No | 222(97.37%) | |
| Yes | 6(2.63%) | |
| X17: | IVIG: | N (%) |
| No | 213(93.42%) | |
| Yes | 15(6.58%) | |
| X18: | PP: | N (%) |
| No | 66(28.95%) | |
| 5 sessions | 131(57.46%) | |
| >5 sessions | 31(13.60%) | |
| X19: | IC: | N (%) |
| No | 185(81.14%) | |
| Yes | 43(18.86%) | |
| X20: | RTX: | N (%) |
| No | 222(97.37%) | |
| Yes | 6(2.63%) | |
| Y: | ICU admission: | N (%) |
| ≤1 day | 199(87.28%) | |
| >1 day | 29(12.72%) | |
| Methods | Hyperparameters | Value | Meaning |
|---|---|---|---|
| CART | minispilt | 20 | The minimum number of observations that must exist in a node for a split to be attempted. |
| minibucket | 20 | The minimum number of observations in any terminal node. | |
| maxdepth | 10 | The maximum depth of any node of the final tree. | |
| xval | 10 | Number of cross-validations. | |
| cp | 0.0781 | Complexity parameter: The minimum improvement in the model needed at each node. | |
| C4.5 | C | 0.5 | The confidence threshold tree size of pruning. |
| M | 3 | The minimum number of instances per leaf. | |
| C5.0 | trials | 20 | The number of boosting iterations. |
| model | Tree | The model growing of type. | |
| winnow | F | The tree be decomposed into a rule-based model. |
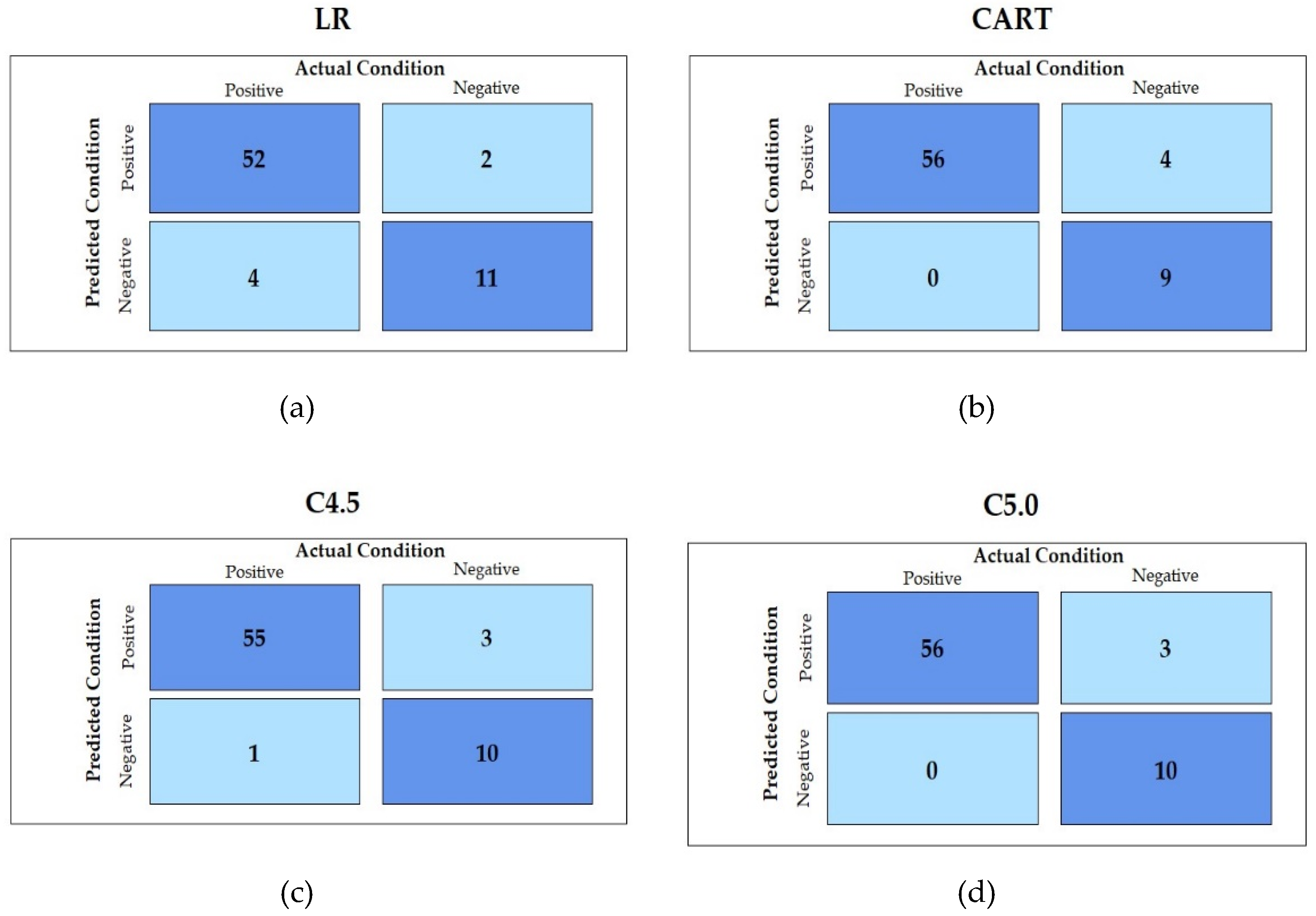
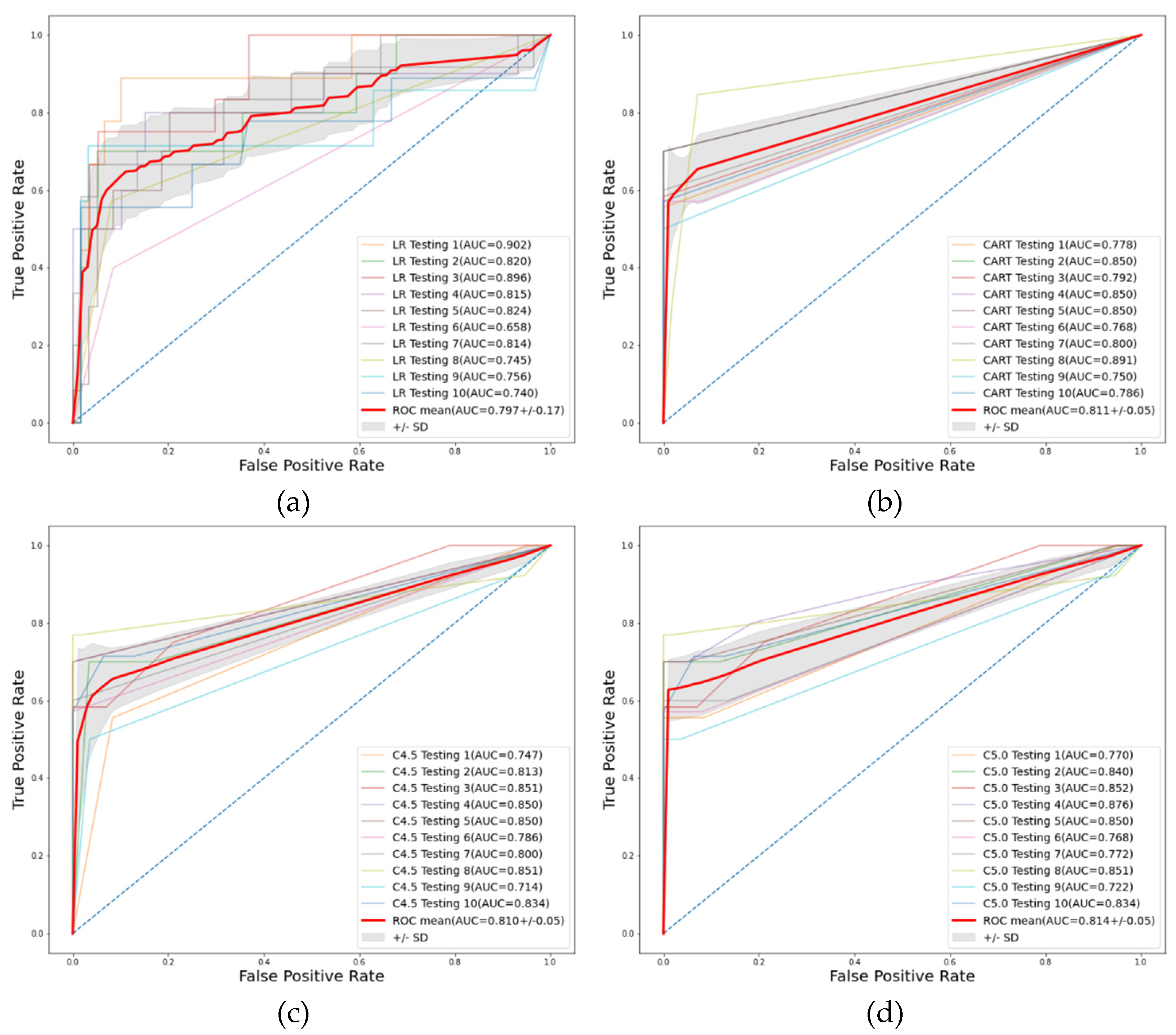
| Methods | Accuracy Mean (SD) | Sensitivity Mean (SD) | Specificity Mean (SD) | AUC Mean (SD) | F1 Score Mean (SD) |
|---|---|---|---|---|---|
| LR | 0.862(0.08) | 0.892(0.11) | 0.702(0.27) | 0.797(0.17) | 0.915(0.06) |
| CART | 0.942(0.02) | 0.993(0.02) | 0.633(0.10) | 0.811(0.05) | 0.967(0.01) |
| C4.5 | 0.929(0.03) | 0.978(0.03) | 0.639(0.09) | 0.810(0.05) | 0.959(0.02) |
| C5.0 | 0.942(0.02) | 0.994(0.02) | 0.639(0.09) | 0.814(0.05) | 0.967(0.01) |
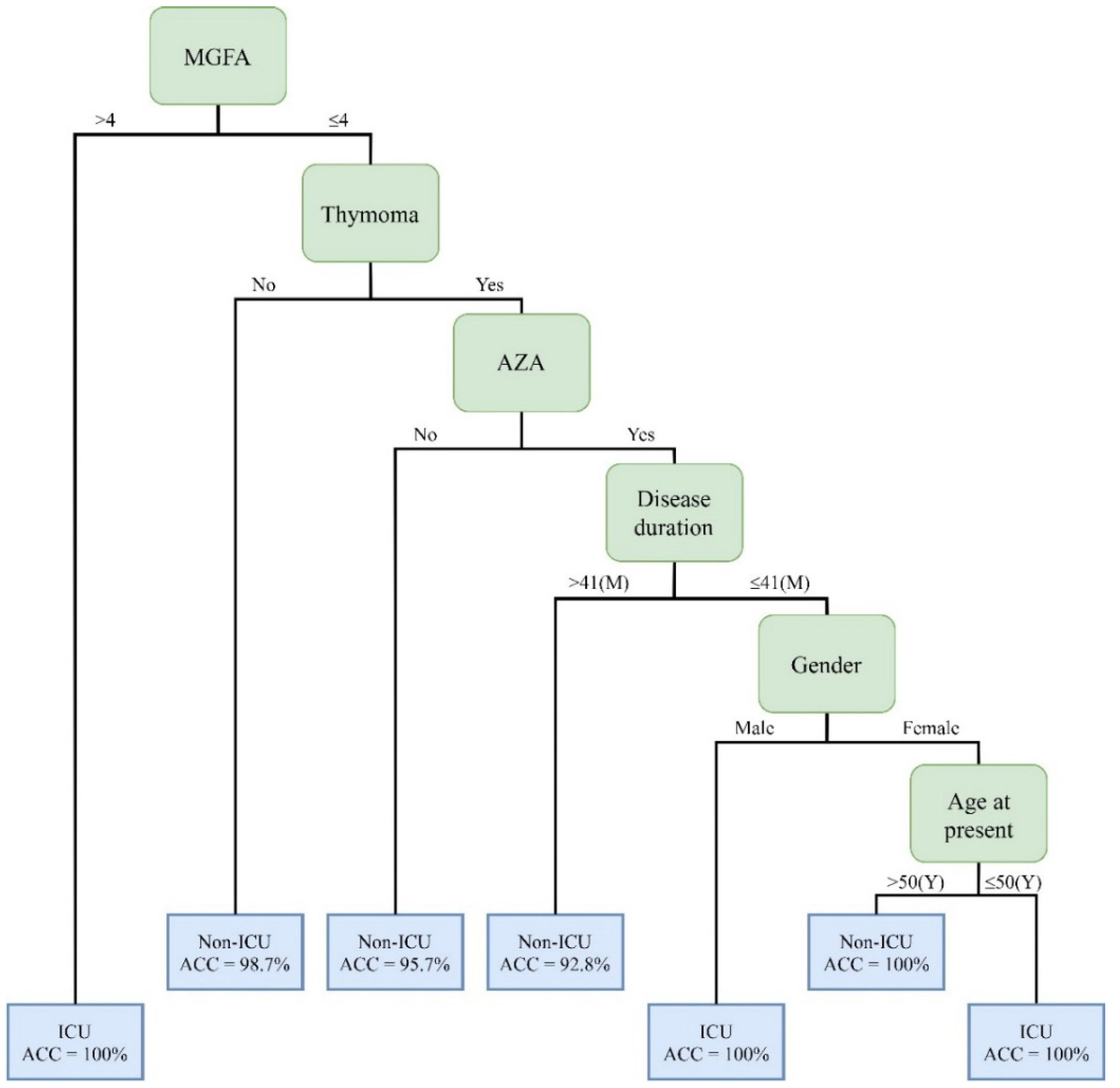
| Rules No. | Combinations of Clinical Factors | Cases | Positive/Negative | Accuracy |
|---|---|---|---|---|
| 1 | MGFA (>4) | 9 | Positive | 100% |
| 2 | MGFA (≤4) + Thymoma (No) | 81 | Negative | 98.7% |
| 3 | MGFA (≤4) + Thymoma (Yes) + AZA(No) | 47 | Negative | 95.7% |
| 4 | MGFA (≤4) + Thymoma (Yes) + AZA(Yes) + Disease duration (>41) | 14 | Negative | 92.8% |
| 5 | MGFA (≤4) + Thymoma (Yes) + AZA(Yes) + Disease duration (≤41) + Gender (Male) | 4 | Positive | 100% |
| 6 | MGFA (≤4) + Thymoma (Yes) + AZA(Yes) + Disease duration (≤41) + Gender (Female)+Age at present (≤50) | 2 | Positive | 100% |
| 7 | MGFA (≤4) + Thymoma (Yes) + AZA(Yes) + Disease duration (≤41 + Gender (Female)+Age at present (>50) | 2 | Negative | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Yeh, J.-H.; Chiu, H.-C.; Chen, Y.-M.; Jhou, M.-J.; Liu, T.-C.; Lu, C.-J. Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach. J. Pers. Med. 2022, 12, 32. https://doi.org/10.3390/jpm12010032
Chang C-C, Yeh J-H, Chiu H-C, Chen Y-M, Jhou M-J, Liu T-C, Lu C-J. Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach. Journal of Personalized Medicine. 2022; 12(1):32. https://doi.org/10.3390/jpm12010032
Chicago/Turabian StyleChang, Che-Cheng, Jiann-Horng Yeh, Hou-Chang Chiu, Yen-Ming Chen, Mao-Jhen Jhou, Tzu-Chi Liu, and Chi-Jie Lu. 2022. "Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach" Journal of Personalized Medicine 12, no. 1: 32. https://doi.org/10.3390/jpm12010032
APA StyleChang, C.-C., Yeh, J.-H., Chiu, H.-C., Chen, Y.-M., Jhou, M.-J., Liu, T.-C., & Lu, C.-J. (2022). Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach. Journal of Personalized Medicine, 12(1), 32. https://doi.org/10.3390/jpm12010032







