Predictive Value of Ambulatory Objective Movement Measurement for Outcomes of Levodopa/Carbidopa Intestinal Gel Infusion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
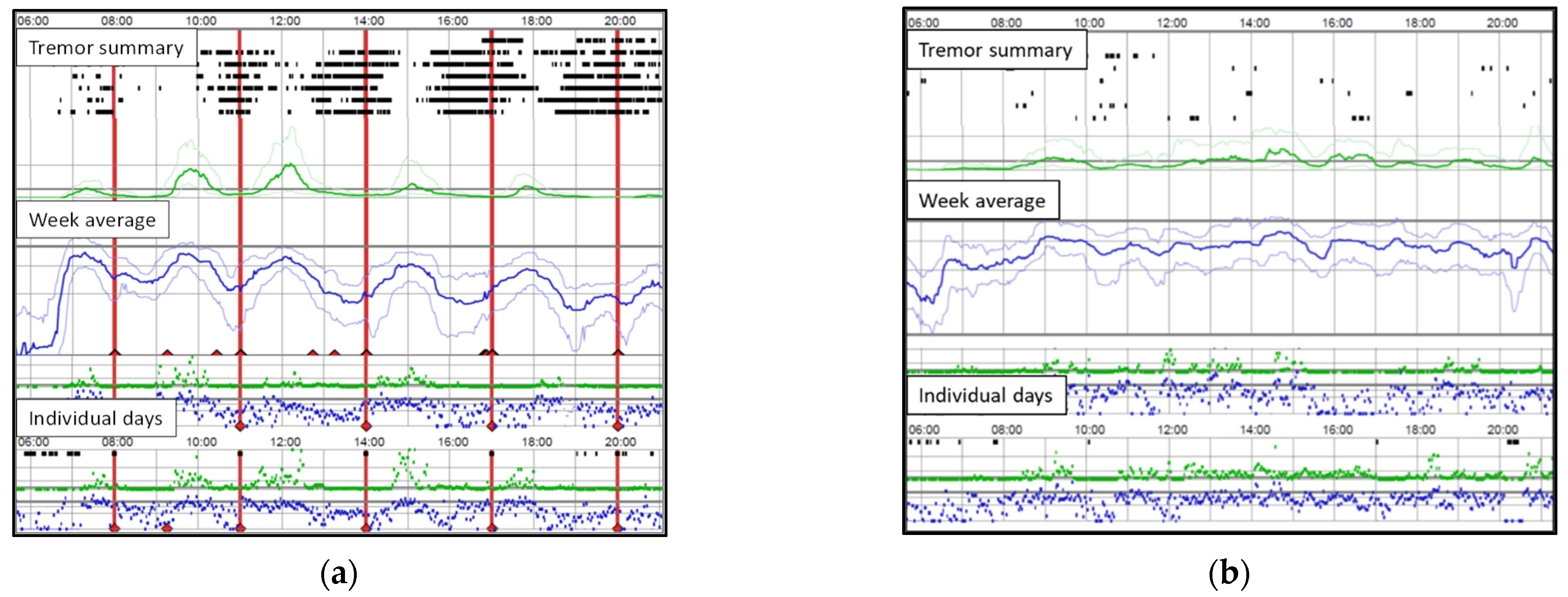
3.1. Study Population
3.2. Overall Treatment Outcome Assessment (CGI-I)
3.3. Outcome Predictors
3.4. Effect of LCIG Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeWitt, P.A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 2015, 30, 64–72. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kieburtz, K.; Odin, P.; Espay, A.J.; Standaert, D.G.; Fernandez, H.H.; Vanagunas, A.; Othman, A.A.; Widnell, K.L.; Robieson, W.Z.; et al. LCIG Horizon Study Group Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014, 13, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Nyholm, D.; Klangemo, K.; Johansson, A. Levodopa/carbidopa intestinal gel infusion long-term therapy in advanced Parkinson’s disease. Eur. J. Neurol. 2012, 19, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Bologna, M.; Miyasaki, J.M.; Colosimo, C. Parkinson’s disease advanced therapies—A systematic review: More unanswered questions than guidance. Parkinsonism Relat. Disord. 2021, 83, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, H.S.; Martinez-Martin, P.; Rizos, A.; Trost, M.; Dos Santos Ghilardi, M.G.; Reddy, P.; Sauerbier, A.; Petry-Schmelzer, J.N.; Kramberger, M.; Borgemeester, R.W.K.; et al. EUROPAR and the International Parkinson and Movement Disorders Society Non-Motor Parkinson’s Disease Study Group EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov. Disord. 2019, 34, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odin, P.; Ray Chaudhuri, K.; Slevin, J.T.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.K.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. National Steering Committees Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef]
- Udd, M.; Lyytinen, J.; Eerola-Rautio, J.; Kenttämies, A.; Lindström, O.; Kylänpää, L.; Pekkonen, E. Problems related to levodopa-carbidopa intestinal gel treatment in advanced Parkinson’s disease. Brain Behav. 2017, 7, e00737. [Google Scholar] [CrossRef]
- Galati, S.; Stefani, A. Deep brain stimulation of the subthalamic nucleus: All that glitters isn’t gold? Mov. Disord. 2015, 30, 632–637. [Google Scholar] [CrossRef]
- Sensi, M.; Cossu, G.; Mancini, F.; Pilleri, M.; Zibetti, M.; Modugno, N.; Quatrale, R.; Tamma, F.; Antonini, A.; on behalf of the ITALIAN LEVODOPA CARBIDOPA INTESTINAL GEL WORKING GROUP. Which patients discontinue? Issues on Levodopa/carbidopa intestinal gel treatment: Italian multicentre survey of 905 patients with long-term follow-up. Parkinsonism Relat. Disord. 2017, 38, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J. Parkinson’s Dis. 2012, 2, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayés, À.; Samá, A.; Prats, A.; Pérez-López, C.; Crespo-Maraver, M.; Moreno, J.M.; Alcaine, S.; Rodriguez-Molinero, A.; Mestre, B.; Quispe, P.; et al. A “HOLTER” for Parkinson’s disease: Validation of the ability to detect on-off states using the REMPARK system. Gait Posture 2018, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Heldman, D.A.; Espay, A.J.; LeWitt, P.A.; Giuffrida, J.P. Clinician versus machine: Reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 590–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulliam, C.L.; Heldman, D.A.; Orcutt, T.H.; Mera, T.O.; Giuffrida, J.P.; Vitek, J.L. Motion sensor strategies for automated optimization of deep brain stimulation in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Farzanehfar, P.; Woodrow, H.; Braybrook, M.; McGregor, S.; Evans, A.; Nicklason, F.; Horne, M. Objective measurement in routine care of people with Parkinson’s disease improves outcomes. NPJ Parkinsons Dis. 2018, 4, 10. [Google Scholar] [CrossRef]
- Woodrow, H.; Horne, M.K.; Fernando, C.V.; Kotschet, K.E. Treat to Target Study Group A blinded, controlled trial of objective measurement in Parkinson’s disease. NPJ Parkinsons Dis. 2020, 6, 35. [Google Scholar] [CrossRef]
- Khodakarami, H.; Farzanehfar, P.; Horne, M. The Use of Data from the Parkinson’s KinetiGraph to Identify Potential Candidates for Device Assisted Therapies. Sensors 2019, 19, 2241. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, F.; Ax, A.; Sjostrom, A.; Wallerstedt, S. West Sweden Parkinson Objective Measurement Registry Study (WestPORTS). Mov. Disord. 2018, 33, S356–S357. [Google Scholar]
- Schneider, L.S.; Olin, J.T. Clinical global impressions in Alzheimer’s clinical trials. Int. Psychogeriatr. 1996, 8, 277–288, discussion 288. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Standaert, D.G.; Hauser, R.A.; Lang, A.E.; Fung, V.S.C.; Klostermann, F.; Lew, M.F.; Odin, P.; Steiger, M.; Yakupov, E.Z.; et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: Final 12-month, open-label results. Mov. Disord. 2015, 30, 500–509. [Google Scholar] [CrossRef]
- Epstein, M.; Johnson, D.A.; Hawes, R.; Schmulewitz, N.; Vanagunas, A.D.; Gossen, E.R.; Robieson, W.Z.; Eaton, S.; Dubow, J.; Chatamra, K.; et al. Long-Term PEG-J Tube Safety in Patients With Advanced Parkinson’s Disease. Clin. Transl. Gastroenterol. 2016, 7, e159. [Google Scholar] [CrossRef]
- Nyholm, D.; Nilsson Remahl, A.I.M.; Dizdar, N.; Constantinescu, R.; Holmberg, B.; Jansson, R.; Aquilonius, S.M.; Askmark, H. Duodenal levodopa infusion monotherapy vs. oral polypharmacy in advanced Parkinson disease. Neurology 2005, 64, 216–223. [Google Scholar] [CrossRef]
- Antonini, A.; Poewe, W.; Chaudhuri, K.R.; Jech, R.; Pickut, B.; Pirtošek, Z.; Szasz, J.; Valldeoriola, F.; Winkler, C.; Bergmann, L.; et al. GLORIA study co-investigators Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat. Disord. 2017, 45, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poewe, W.; Bergmann, L.; Robieson, W.Z.; Antonini, A. Predictors of Response for “Off” Time Improvement with Levodopa-Carbidopa Intestinal Gel Treatment: An Analysis of the GLORIA Registry. Front. Neurol. 2020, 11, 419. [Google Scholar] [CrossRef]
- Kamel, W.A.; Al-Hashel, J.Y. LCIG in treatment of non-motor symptoms in advanced Parkinson’s disease: Review of literature. Brain Behav. 2020, 10, e01757. [Google Scholar] [CrossRef]
- Heldman, D.A.; Giuffrida, J.P.; Cubo, E. Wearable sensors for advanced therapy referral in parkinson’s disease. J. Parkinson’s Dis. 2016, 6, 631–638. [Google Scholar] [CrossRef]
- Johansson, D.; Ericsson, A.; Johansson, A.; Medvedev, A.; Nyholm, D.; Ohlsson, F.; Senek, M.; Spira, J.; Thomas, I.; Westin, J.; et al. Individualization of levodopa treatment using a microtablet dispenser and ambulatory accelerometry. CNS Neurosci. Ther. 2018, 24, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Khodakarami, H.; Shokouhi, N.; Horne, M. A method for measuring time spent in bradykinesia and dyskinesia in people with Parkinson’s disease using an ambulatory monitor. J. Neuroeng. Rehabil. 2021, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Ossig, C.; Gandor, F.; Fauser, M.; Bosredon, C.; Churilov, L.; Reichmann, H.; Horne, M.K.; Ebersbach, G.; Storch, A. Correlation of Quantitative Motor State Assessment Using a Kinetograph and Patient Diaries in Advanced PD: Data from an Observational Study. PLoS ONE 2016, 11, e0161559. [Google Scholar] [CrossRef] [PubMed]
- Morgante, F.; Oppo, V.; Fabbri, M.; Olivola, E.; Sorbera, C.; De Micco, R.; Ielo, G.C.; Colucci, F.; Bonvegna, S.; Novelli, A.; et al. Levodopa-carbidopa intrajejunal infusion in Parkinson’s disease: Untangling the role of age. J. Neurol. 2021, 268, 1728–1737. [Google Scholar] [CrossRef]
| Baseline Variables | Median (Min–Max) or n (%) |
|---|---|
| Age (years) | 73 (49–85) |
| LED24h (mg) | 1337 (677–3033) |
| Number of levodopa doses, 06:00–22:00 | 7 (5–9) |
| Levodopa dose interval (h) | 2.5 (1.5–3) |
| Single dose effect duration (h) 1 | 2.0 (1.0–3.0) |
| OM ON-time, 06:00–22:00 1 | 12 (4–16) |
| BK (median score 09:00–18:00) | 22.8 (8.9–37.3.1) |
| DK (median score 09:00–18:00) | 4.6 (0.6–46.3) |
| FDS | 11.7 (4.5–25.8) |
| PTT (09:00–18:00), % | 0.8 (0.1–46.5) |
| OM OFF episodes absent, n (%) 1 | 14 (31.1) |
| Responders (CGI-I = 2) n = 27 | Partial Responders (CGI-I = 3) n = 15 | Non-Responders (CGI-I > 3) n = 3 | Odds Ratio of a Good Outcome (95% Confidence Interval) | (Wald χ) p-Value | |
|---|---|---|---|---|---|
| Age | 70 (49–82) | 78 (68–85) | 73 (65–77) | 0.847 (0.730–0.982) | (4.856) 0.028 |
| Male, n (%) | 19 (70.4) | 8 (53.3) | 2 (66.7) | 6.194 (0.924–41.505) | (3.530) 0.060 |
| Number of doses | 7 (5–9) | 6 (5–8) | 7 (5–8) | 1.010 (0.472–2.159) | (0.001) 0.980 |
| BK | 23.2 (8.9–37.3) | 22.4 (13.4–30.4) | 28.3 (16.6–36.3) | 0.974 (0.783–1.212) | (0.057) 0.812 |
| DK | 4.5 (0.6–46.3) | 4.6 (1.1–17.9) | 5.5 (0.8–11.4) | 0.976 (0.764–1.246) | (0.039) 0.844 |
| FDS | 11.7 (4.5–25.8) | 12.2 (7.9–20.6) | 9.3 (5.7−10.6) | 1.124 (0.699–1.806) | (0.232) 0.630 |
| PTT (%) | 0.9 (0.1–46.5) | 0.5 (0.1–0.3) | 0.6 (0.1–0.9) | 1.255 (0.692–2.276) | (0.559) 0.455 |
| OM ON-time | 11 (8–16) | 12 (4−16) | 12 (12–16) | 1.449 (0.898–2.339) | (2.309) 0.129 |
| No OM OFF, n (%) | 5 (18.5) | 5 (31.1) | 3 (100) | 0.055 (0.003–0.884) | (4.188) 0.041 |
| LED24h, mg | 1235 (677–2267) | 1184 (712–3034) | 1514 (1308–2300) | 0.240 1 (0.018–3.278) | (1.144) 0.285 |
| Outcome Group | BK | DK | FDS | PTT (%) | OM ON Time | No OM OFF, n | LED24h | LED16h 2 | |
|---|---|---|---|---|---|---|---|---|---|
| Responders | Baseline | 24.1 | 4.3 | 10.3 | 1.3 | 11 | 5 | 1235 | 850 |
| (8.9–37.3) | (0.6–46.3) | (4.5–25.8) | (0.1–46.5) | (8–16) | (677–2267) | (499–1697) | |||
| Follow-up | 23.6 | 4.1 | 9.6 | 0.9 | 14 | 18 | 1479 | 1376 | |
| (10.2–35.5) | (0.9–36.5) | (5.7–23.0) | (0.0–13.8) | (4–16) | 767−2374 | (330–1964) | |||
| p-value 1 (n) | 0.412 | 0.893 | 0.333 | 0.213 | 0.045 | 0.000 | 0.005 | 0.000 | |
| (25) | (25) | (25) | (25) | (25) | (25) | (27) | (27) | ||
| Part. responders | Baseline | 18.4 | 9.8 | 14 | 0.5 | 13 | 5 | 1184 | 900 |
| (13.4–30.8) | (1.6–17.9) | (7.9–20.6) | (1.0–3.0) | (6–16) | (712–3034) | (450–1663) | |||
| Follow-up | 21.8 | 6.4 | 9.1 | 0.4 | 13 | 6 | 1382 | 1040 | |
| (10.3–33.9) | (0.6–17.6) | (5.7–19.8) | (0.2–10) | (6–16) | (588–2195) | (528–1764) | |||
| p-value 1 (n) | 0.093 | 0.197 | 0.155 | 0.266 | 0.345 | 1.000 | 0.156 | 0.009 | |
| (11) | (11) | (11) | (11) | (11) | (11) | (15) | (15) | ||
| Non-responders | Baseline | 28.3 | 5.5 | 9.3 | 0.6 | 12 | 3 | 1514 | 1614 |
| (16.6–36.3) | (0.8–11.4) | (5.7–10.6) | (0.1–0.9) | (12–16) | (1308–2300) | (1540–1620) | |||
| Follow-up | 35.6 | 0.6 | 5.7 | 0.7 | 7 | 3 | 1364 | 1420 | |
| (26.5–42.1) | (0.2–0.9) | (4.3–8.10) | (0.2–2.4) | (2–15.5) | (998–1600) | (1156–1454) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilinçalp, G.; Sjöström, A.-C.; Eriksson, B.; Holmberg, B.; Constantinescu, R.; Bergquist, F. Predictive Value of Ambulatory Objective Movement Measurement for Outcomes of Levodopa/Carbidopa Intestinal Gel Infusion. J. Pers. Med. 2022, 12, 27. https://doi.org/10.3390/jpm12010027
Kilinçalp G, Sjöström A-C, Eriksson B, Holmberg B, Constantinescu R, Bergquist F. Predictive Value of Ambulatory Objective Movement Measurement for Outcomes of Levodopa/Carbidopa Intestinal Gel Infusion. Journal of Personalized Medicine. 2022; 12(1):27. https://doi.org/10.3390/jpm12010027
Chicago/Turabian StyleKilinçalp, Gökçe, Anne-Christine Sjöström, Barbro Eriksson, Björn Holmberg, Radu Constantinescu, and Filip Bergquist. 2022. "Predictive Value of Ambulatory Objective Movement Measurement for Outcomes of Levodopa/Carbidopa Intestinal Gel Infusion" Journal of Personalized Medicine 12, no. 1: 27. https://doi.org/10.3390/jpm12010027
APA StyleKilinçalp, G., Sjöström, A.-C., Eriksson, B., Holmberg, B., Constantinescu, R., & Bergquist, F. (2022). Predictive Value of Ambulatory Objective Movement Measurement for Outcomes of Levodopa/Carbidopa Intestinal Gel Infusion. Journal of Personalized Medicine, 12(1), 27. https://doi.org/10.3390/jpm12010027







