Lower Lean Mass Is Associated with Greater Arterial Stiffness in Patients with Lower Extremity Artery Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S.; et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef]
- Dart, A.M.; Kingwell, B.A. Pulse pressure—A review of mechanisms and clinical relevance. J. Am. Coll. Cardiol. 2001, 37, 975–984. [Google Scholar] [CrossRef]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef]
- Safar, M.E.; Blacher, J.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Guyonvarc’h, P.M.; London, G.M. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002, 39, 735–738. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 2002, 39, 10–15. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Roman, M.J.; Koren, M.J.; Mensah, G.A.; Ganau, A.; Devereux, R.B. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 1999, 33, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Avolio, A. Genetic and environmental factors in the function and structure of the arterial wall. Hypertension 1995, 26, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Willum-Hansen, T.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef]
- Pepera, G.; McAllister, J.; Sandercock, G. Long-term reliability of the incremental shuttle walking test in clinically stable cardiovascular disease patients. Physiotherapy 2010, 96, 222–227. [Google Scholar] [CrossRef]
- Sandercock, G.; Cardoso, F.; Almodhy, M. Cardiorespiratory fitness changes in patients receiving comprehensive outpatient cardiac rehabilitation in the UK: A multicentre study. Heart 2013, 99, 1298–1299. [Google Scholar] [CrossRef][Green Version]
- Cardoso, F.M.; Almodhy, M.; Pepera, G.; Stasinopoulos, D.M.; Sandercock, G.R. Reference values for the incremental shuttle walk test in patients with cardiovascular disease entering exercise-based cardiac rehabilitation. J. Sports Sci. 2017, 35, 1–6. [Google Scholar] [CrossRef]
- Franklin, S.S. Hypertension in older people: Part 1. J. Clin. Hypertens 2006, 8, 444–449. [Google Scholar] [CrossRef]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005, 293, 1861–1867. [Google Scholar] [CrossRef]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Vlek, A.L.; van der Graaf, Y.; Sluman, M.A.; Moll, F.L.; Visseren, F.L.; Group, S.S. Metabolic syndrome and vascular risk in patients with peripheral arterial occlusive disease. J. Vasc. Surg. 2009, 50, 61–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurm, H.S.; Brennan, D.M.; Booth, J.; Tcheng, J.E.; Lincoff, A.M.; Topol, E.J. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox). Am. J. Cardiol. 2002, 90, 42–45. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Montori, V.M.; Somers, V.K.; Korinek, J.; Thomas, R.J.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Davenport, D.L.; Xenos, E.S.; Hosokawa, P.; Radford, J.; Henderson, W.G.; Endean, E.D. The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J. Vasc. Surg. 2009, 49, 140–147. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Hayes, R.P.; Leibowitz, R.Q.; Bode, R.; Lavery, L.; Walston, J.; Duncan, P.; Perera, S. Clinical Global Impression of Change in Physical Frailty: Development of a measure based on clinical judgment. J. Am. Geriatr. Soc. 2004, 52, 1560–1566. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Crawford, F.; Welch, K.; Andras, A.; Chappell, F.M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst. Rev. 2016, 9, CD010680. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.G.; Marn, C.S.; Supiano, M.A.; Dengel, D.R. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J. Appl. Physiol. 2004, 97, 509–514. [Google Scholar] [CrossRef]
- Park, Y.W.; Heymsfield, S.B.; Gallagher, D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int. J. Obes. Relat. Metab. Disord. 2002, 26, 978–983. [Google Scholar] [CrossRef]
- Seinost, G.; Horina, A.; Arefnia, B.; Kulnik, R.; Kerschbaumer, S.; Quehenberger, F.; Muster, V.; Gutl, K.; Zelzer, S.; Gasser, R.; et al. Periodontal treatment and vascular inflammation in patients with advanced peripheral arterial disease: A randomized controlled trial. Atherosclerosis 2020, 313, 60–69. [Google Scholar] [CrossRef]
- Anoop, S.; Misra, A.; Bhardwaj, S.; Gulati, S. High body fat and low muscle mass are associated with increased arterial stiffness in Asian Indians in North India. J. Diabetes Complicat. 2015, 29, 38–43. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Blacher, J.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E. Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001, 38, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Lassnig, E.; Lamm, G.; Stark, N.; Rammer, M.; Eber, B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur. Heart J. 2005, 26, 2657–2663. [Google Scholar] [CrossRef]
- Williams, B.; Lacy, P.S.; Thom, S.M.; Cruickshank, K.; Stanton, A.; Collier, D.; Hughes, A.D.; Thurston, H.; O’Rourke, M.; Investigators, C.; et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006, 113, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.J.; Karim, M.N.; Srikanth, V.; Ebeling, P.R.; Scott, D. Lower muscle tissue is associated with higher pulse wave velocity: A systematic review and meta-analysis of observational study data. Clin. Exp. Pharmacol. Physiol. 2017, 44, 980–992. [Google Scholar] [CrossRef]
- Ferreira, I.; Snijder, M.B.; Twisk, J.W.; van Mechelen, W.; Kemper, H.C.; Seidell, J.C.; Stehouwer, C.D. Central fat mass versus peripheral fat and lean mass: Opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam Growth and Health Longitudinal Study. J. Clin. Endocrinol. Metab. 2004, 89, 2632–2639. [Google Scholar] [CrossRef]
- Schouten, F.; Twisk, J.W.; de Boer, M.R.; Stehouwer, C.D.; Serne, E.H.; Smulders, Y.M.; Ferreira, I. Increases in central fat mass and decreases in peripheral fat mass are associated with accelerated arterial stiffening in healthy adults: The Amsterdam Growth and Health Longitudinal Study. Am. J. Clin. Nutr. 2011, 94, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.; Bjorck, M.; Brodmann, M.; Cohner, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Kardiol. Pol. 2017, 75, 1065–1160. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, 1465–1508. [Google Scholar] [CrossRef]
- Scott, D.; Daly, R.M.; Sanders, K.M.; Ebeling, P.R. Fall and Fracture Risk in Sarcopenia and Dynapenia With and Without Obesity: The Role of Lifestyle Interventions. Curr. Osteoporos Rep. 2015, 13, 235–244. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Berry, K.L.; Cameron, J.D.; Jennings, G.L.; Dart, A.M. Arterial compliance increases after moderate-intensity cycling. Am. J. Physiol. 1997, 273, H2186–H2191. [Google Scholar] [CrossRef] [PubMed]
- Vun, S.V.; Miller, M.D.; Delaney, C.L.; Allan, R.B.; Spark, J.I. The effect of supervised exercise therapy for intermittent claudication on lower limb lean mass. J. Vasc. Surg. 2016, 64, 1763–1769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiatt, W.R.; Wolfel, E.E.; Meier, R.H.; Regensteiner, J.G. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation 1994, 90, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Gerzoff, R.B.; Caspersen, C.J.; Williamson, D.F.; Narayan, K.M. Relationship of walking to mortality among US adults with diabetes. Arch. Intern. Med. 2003, 163, 1440–1447. [Google Scholar] [CrossRef]
- Parmenter, B.J.; Dieberg, G.; Smart, N.A. Exercise training for management of peripheral arterial disease: A systematic review and meta-analysis. Sports Med. 2015, 45, 231–244. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwak, Y.S.; Pekas, E.J. Impacts of aquatic walking on arterial stiffness, exercise tolerance, and physical function in patients with peripheral artery disease: A randomized clinical trial. J. Appl. Physiol. 2019, 127, 940–949. [Google Scholar] [CrossRef]
- Frandin, K.; Gronstedt, H.; Helbostad, J.L.; Bergland, A.; Andresen, M.; Puggaard, L.; Harms-Ringdahl, K.; Granbo, R.; Hellstrom, K. Long-Term Effects of Individually Tailored Physical Training and Activity on Physical Function, Well-Being and Cognition in Scandinavian Nursing Home Residents: A Randomized Controlled Trial. Gerontology 2016, 62, 571–580. [Google Scholar] [CrossRef]
- Fujie, S.; Hasegawa, N.; Sato, K.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced changes in serum adropin level are associated with reduced arterial stiffness in middle-aged and older adults. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1642–H1647. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Marcori, A.J.; Ribeiro, A.S.; Gerage, A.M.; Padilha, C.S.; Schiavoni, D.; Souza, M.F.; Mayhew, J.L.; do Nascimento, M.A.; Venturini, D.; et al. Chronic Blood Pressure Reductions and Increments in Plasma Nitric Oxide Bioavailability. Int. J. Sports Med. 2017, 38, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Tomeleri, C.M.; Souza, M.F.; Burini, R.C.; Cavaglieri, C.R.; Ribeiro, A.S.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; et al. Resistance training reduces metabolic syndrome and inflammatory markers in older women: A randomized controlled trial. J. Diabetes 2018, 10, 328–337. [Google Scholar] [CrossRef]
- Pollock, M.L.; Franklin, B.A.; Balady, G.J.; Chaitman, B.L.; Fleg, J.L.; Fletcher, B.; Limacher, M.; Pina, I.L.; Stein, R.A.; Williams, M.; et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 2000, 101, 828–833. [Google Scholar] [CrossRef]
- Heckman, G.A.; McKelvie, R.S. Cardiovascular aging and exercise in healthy older adults. Clin. J. Sport Med. 2008, 18, 479–485. [Google Scholar] [CrossRef]
- Papathanasiou, J.V. Are the group-based interventions improving the functional exercise capacity and quality of life of frail subjects with chronic heart failure? J. Frailty Sarcopenia Falls 2020, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Balkestein, E.J.; van Aggel-Leijssen, D.P.; van Baak, M.A.; Struijker-Boudier, H.A.; Van Bortel, L.M. The effect of weight loss with or without exercise training on large artery compliance in healthy obese men. J. Hypertens 1999, 17, 1831–1835. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. Arterial stiffness: A new surrogate end point for cardiovascular disease? J. Nephrol. 2007, 20 (Suppl. S12), S45–S50. [Google Scholar] [PubMed]
- Tanaka, H.; DeSouza, C.A.; Seals, D.R. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 127–132. [Google Scholar] [CrossRef]
- Steppan, J.; Barodka, V.; Berkowitz, D.E.; Nyhan, D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract. 2011, 2011, 263585. [Google Scholar] [CrossRef]
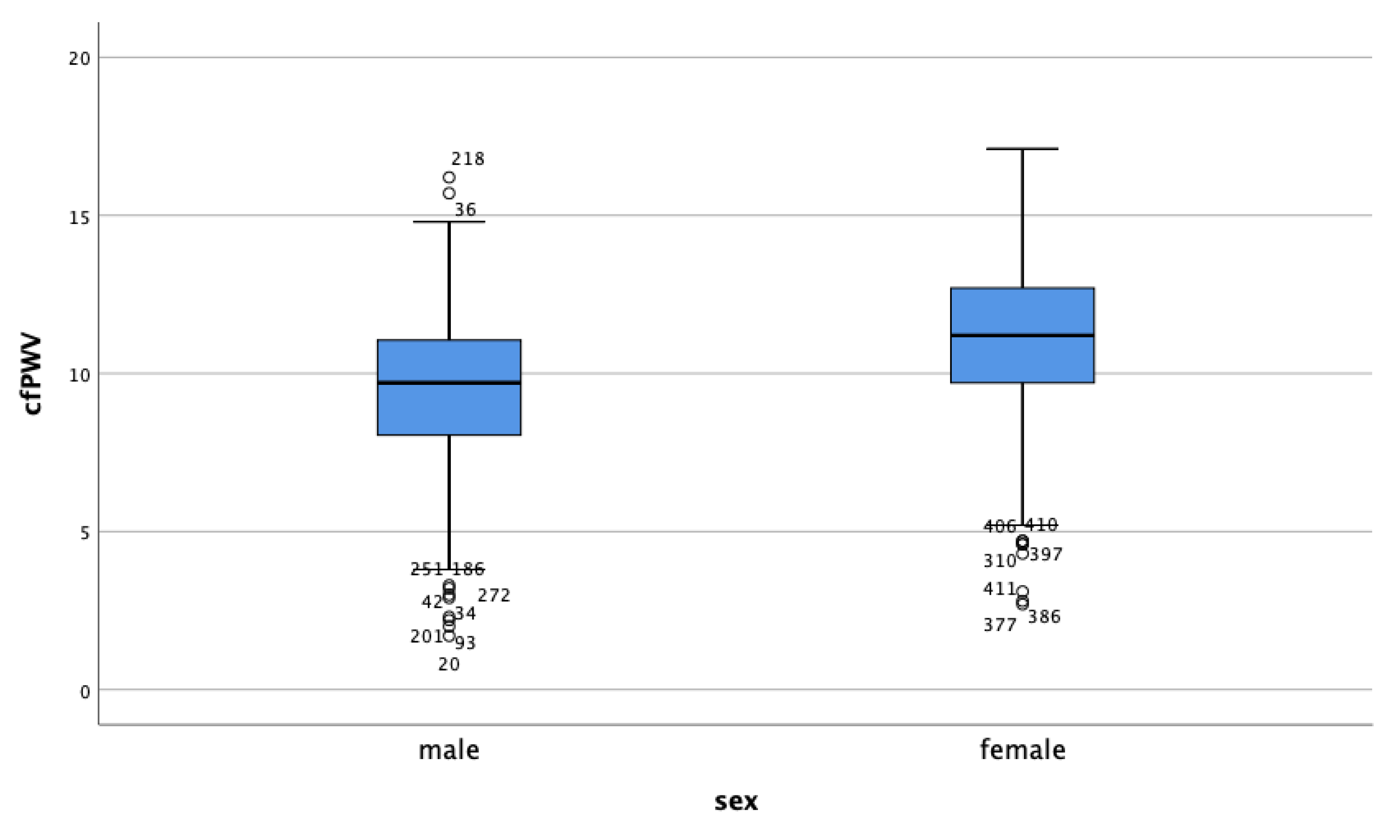
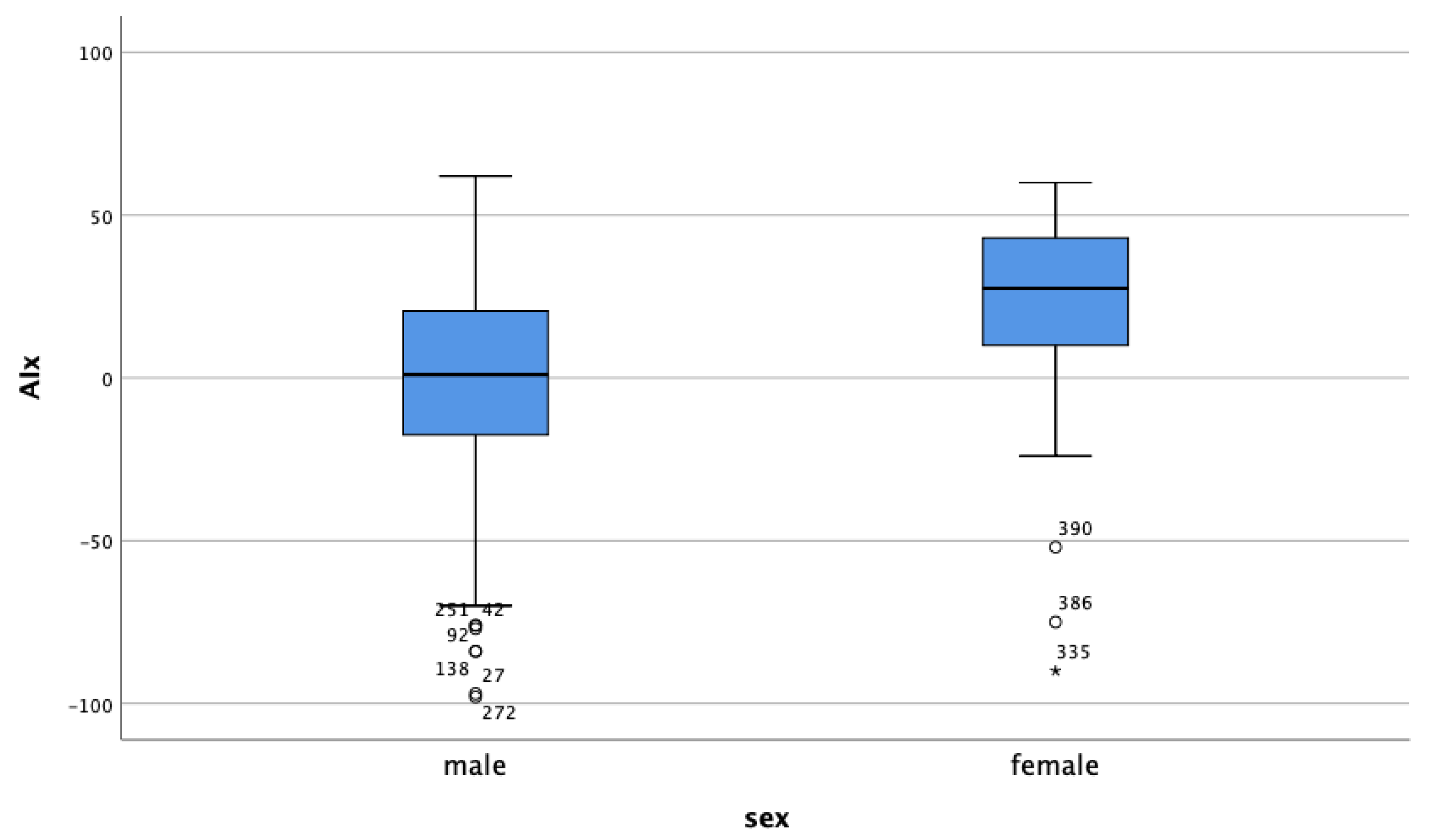
| Variable 1 | Variable 2 | Correlation Coefficient | p-Value |
|---|---|---|---|
| Age | lean mass | −0.24 | <0.05 |
| Age | %-fat | 0.16 | <0.05 |
| Age | AIx | 0.26 | <0.05 |
| Age | cfPWV | 0.21 | <0.05 |
| Characteristics | Total Cohort (n = 412) | Male Patients (n = 297) | Female Patients (n = 115) |
|---|---|---|---|
| Age (years) | 63.3 ± 10.1 | 62.2 ± 9.5 | 66.3 ± 10.9 |
| BMI | 27.3 ± 4 | 27.4 ± 3.7 | 27.1 ± 4.8 |
| Lean mass (kg) | 50.2 ± 9.4 | 54.3 ± 7.3 | 39.6 ± 5.1 |
| Nicotine abuse n | 343 (81.3%) | 271 (91.2%) | 72 (62.6%) |
| Diabetes n | 114 (27%) | 80 (26.9%) | 34 (29.6%) |
| Hyperlipidemia n | 393 (93.1%) | 283 (95.3%) | 110 (95.7%) |
| Hypertension n | 332 (78.8%) | 237 (79.8%) | 95 (82.6%) |
| Stroke n | 44 (10.4%) | 30 (10.1%) | 14 (12.2%) |
| CAD n | 92 (21.8%) | 72 (24.4%) | 20 (17.4%) |
| BMI category | |||
| Underweight n | 3 (0.7%) | 1 (0.3%) | 2 (1.7%) |
| Normal n | 115 (27.9%) | 75 (25.3%) | 40 (34.8%) |
| Overweight n | 277 (67.2%) | 211 (71.0%) | 66 (57.4%) |
| Obese n | 17 (4.1%) | 10 (3.4%) | 7 (6.1%) |
| BMI Category | Male Patients | Female Patients | |
|---|---|---|---|
| Underweight | n (%) | 1 (0.3%) | 2 (1.7%) |
| AIx (mean, ±SD) | 26 | 29.0 (±8.49) | |
| cfPWV (mean, ±SD) | 8.9 | 12.65 (±2.05) | |
| Lean mass (mean, ±SD) | 40.4 | 35.5 (±0.02) | |
| Normal weight | n (%) | 75 (25.3%) | 40 (34.8%) |
| AIx (mean, ±SD) | 24.99 (±11.6) | 30.79 (±14.56) | |
| cfPWV (mean, ±SD) | 9.59 (±2.72) | 10.18 (±3.24) | |
| Lean mass (mean, ±SD) | 48.48 (±5.14) | 36.23 (±3.81) | |
| Overweight | n (%) | 211 (71%) | 66 (57.4%) |
| AIx (mean, ±SD) | 23.94 (±10.28) | 31.14 (±8.93) | |
| cfPWV (mean, ±SD) | 9.41 (±2.46) | 11.35 (±3.04) | |
| Lean mass (mean, ±SD) | 56.07 (±6.59) | 41.33 (±4.35) | |
| Obese | n (%) | 10 (3.4%) | 7 (6.1%) |
| AIx (mean, ±SD) | 20.4 (±20.4) | 26.5 (±11.54) | |
| cfPWV (mean, ±SD) | 9.22 (±1.95) | 10.63 (±3.39) | |
| Lean mass (mean, ±SD) | 63.97 (±6.02) | 44.89 (±6.45) |
| Sex | Variable | B Coefficient (95% CI) | p-Value | |
|---|---|---|---|---|
| AIx | male | Lean mass BMI | −0.28 (−0.48–0.09) 1.14 (−2.04–4.33) | 0.004 0.480 |
| AIx | female | Lean mass BMI | −0.41 (−0.92–0.11) 2.12 (−3.11–7.35) | 0.118 0.423 |
| cfPWV | male | Lean mass BMI | −0.01 (−0.06–0.03) −0.10 (−0.89–0.68) | 0.611 0.792 |
| cfPWV | female | Lean mass BMI | −0.02 (−0.16–0.12) 1.09 (−0.38–2.56) | 0.762 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muster, V.; Gütl, K.; Pregartner, G.; Berghold, A.; Schweiger, L.; Jud, P.; Brodmann, M.; Seinost, G. Lower Lean Mass Is Associated with Greater Arterial Stiffness in Patients with Lower Extremity Artery Disease. J. Pers. Med. 2021, 11, 911. https://doi.org/10.3390/jpm11090911
Muster V, Gütl K, Pregartner G, Berghold A, Schweiger L, Jud P, Brodmann M, Seinost G. Lower Lean Mass Is Associated with Greater Arterial Stiffness in Patients with Lower Extremity Artery Disease. Journal of Personalized Medicine. 2021; 11(9):911. https://doi.org/10.3390/jpm11090911
Chicago/Turabian StyleMuster, Viktoria, Katharina Gütl, Gudrun Pregartner, Andrea Berghold, Leyla Schweiger, Philipp Jud, Marianne Brodmann, and Gerald Seinost. 2021. "Lower Lean Mass Is Associated with Greater Arterial Stiffness in Patients with Lower Extremity Artery Disease" Journal of Personalized Medicine 11, no. 9: 911. https://doi.org/10.3390/jpm11090911
APA StyleMuster, V., Gütl, K., Pregartner, G., Berghold, A., Schweiger, L., Jud, P., Brodmann, M., & Seinost, G. (2021). Lower Lean Mass Is Associated with Greater Arterial Stiffness in Patients with Lower Extremity Artery Disease. Journal of Personalized Medicine, 11(9), 911. https://doi.org/10.3390/jpm11090911






