Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
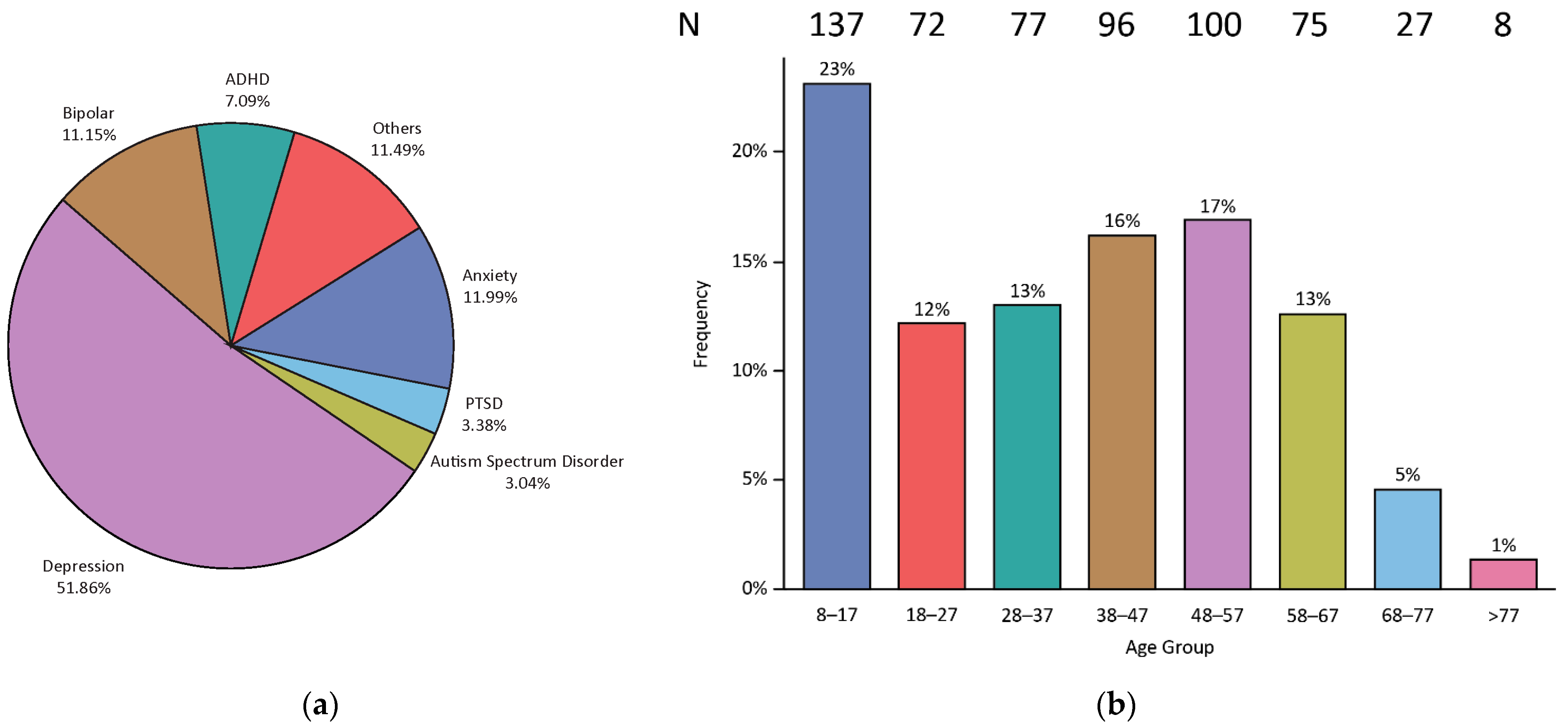
3.1. Demographics
3.2. Congruency
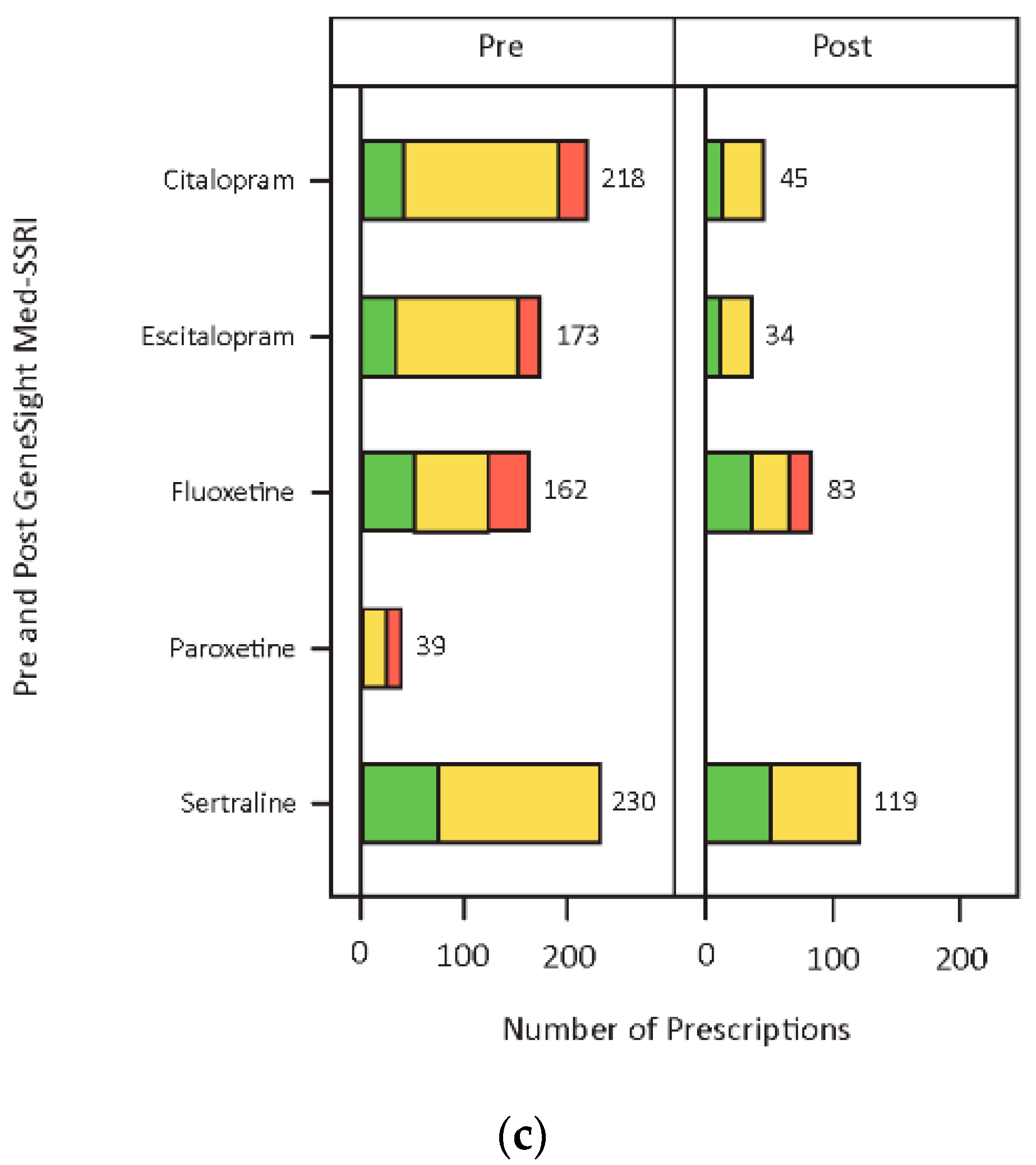
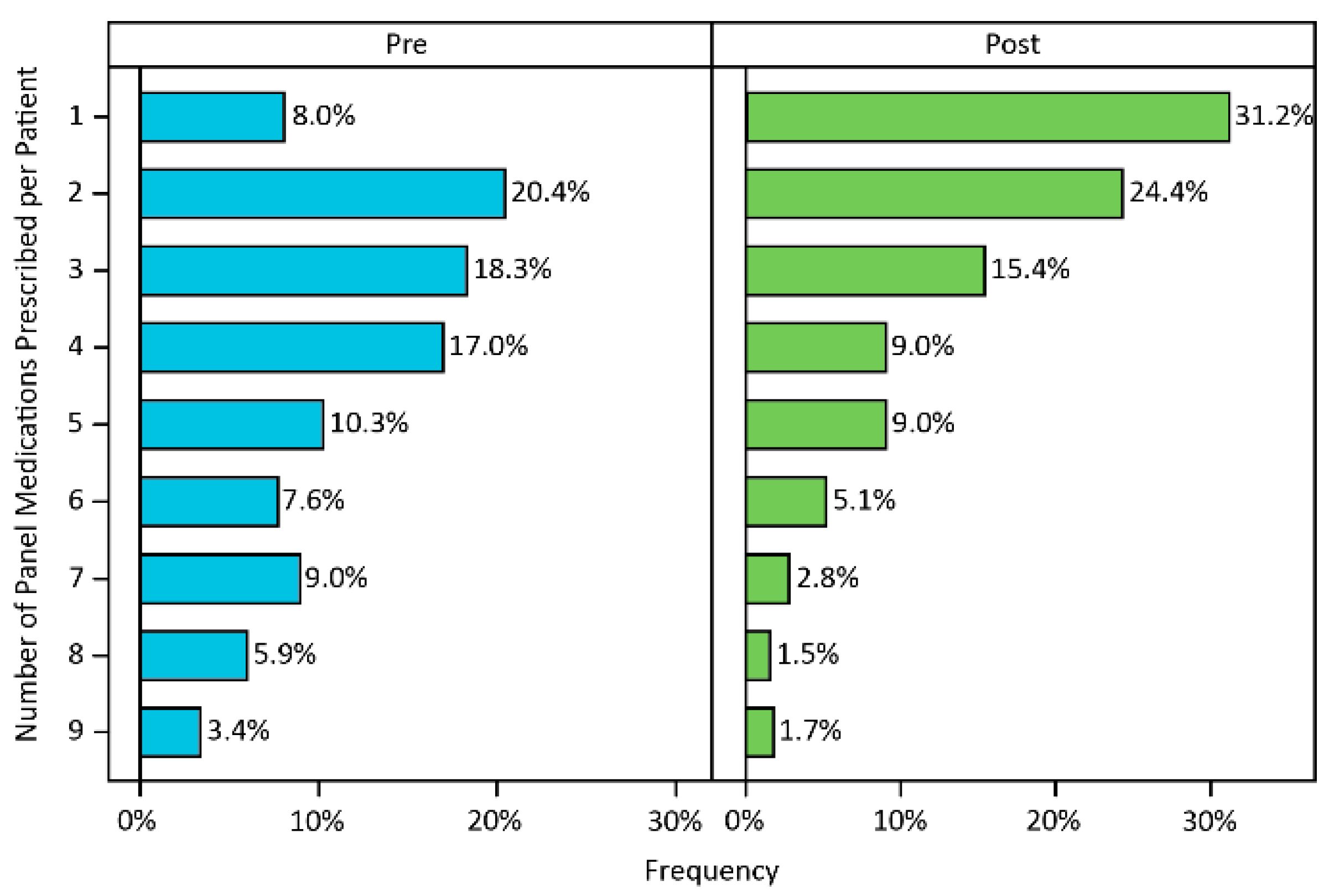
3.3. Medication Changes
3.4. Adverse Events
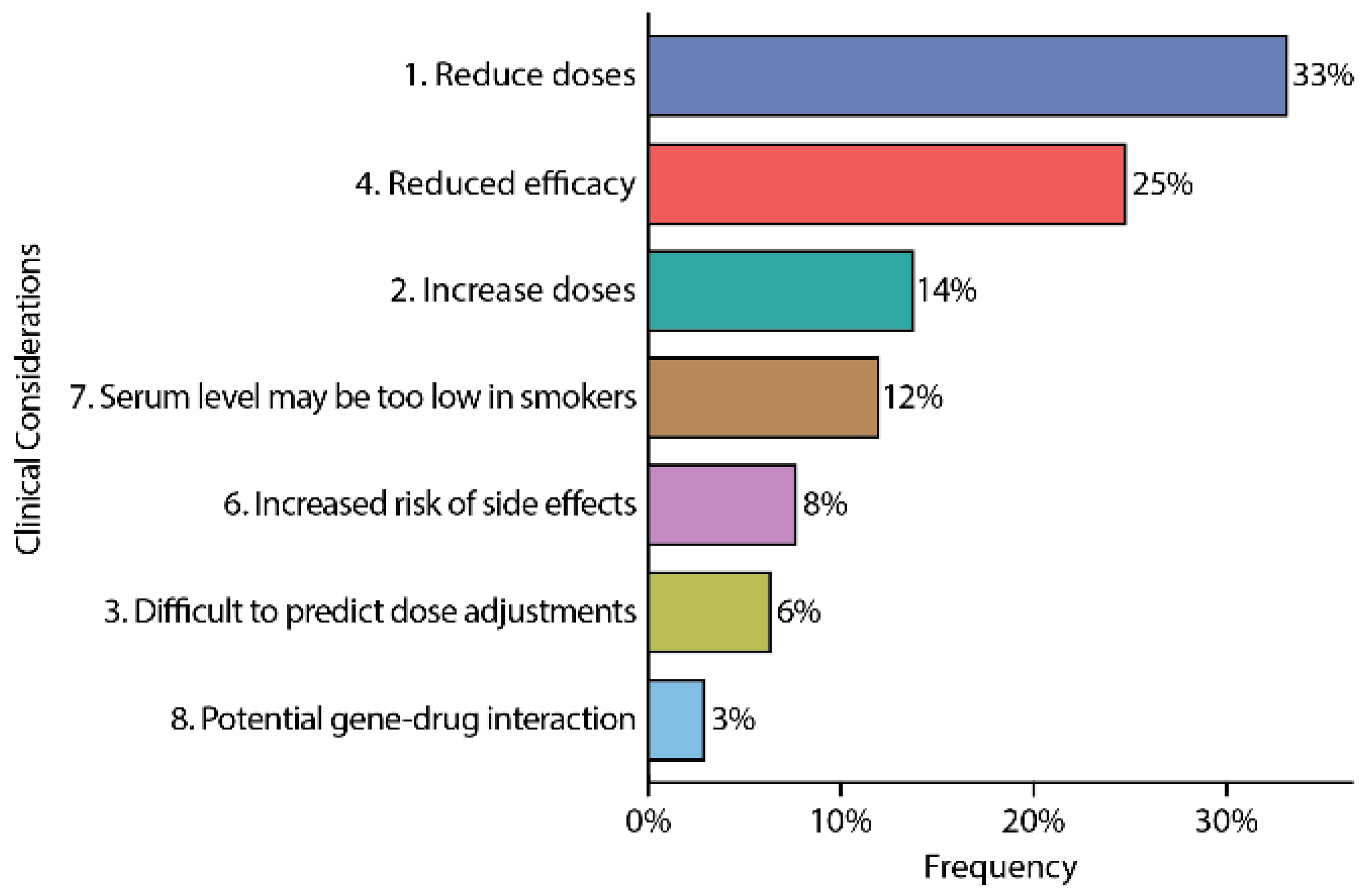
3.5. Clinical Considerations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Rush, A. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Sussman, M.; O’Sullivan, A.K.; Shah, A.; Olfson, M.; Menzin, J. Economic Burden of Treatment-Resistant Depression on the U.S. Health Care System. J. Manag. Care Spéc. Pharm. 2019, 25, 823–835. [Google Scholar] [CrossRef]
- Greden, J.F.; Parikh, S.V.; Rothschild, A.J.; Thase, M.E.; Dunlop, B.W.; DeBattista, C.; Conway, C.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J. Psychiatr. Res. 2019, 111, 59–67. [Google Scholar] [CrossRef]
- Arandjelovic, K.; Eyre, H.A.; Lenze, E.; Singh, A.B.; Berk, M.; Bousman, C. The role of depression pharmacogenetic decision support tools in shared decision making. J. Neural Transm. 2017, 126, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Arandjelovic‡, K.; Mancuso, S.; Eyre, H.A.; Dunlop, B.W. Pharmacogenetic tests and depressive symptom remission: A meta-analysis of randomized controlled trials. Pharmacogenomics 2019, 20, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblat, J.D.; Lee, Y.; McIntyre, R.S. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. J. Affect. Disord. 2018, 241, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Eum, S.; Haga, S.B.; Strawn, J.R.; Zierhut, H. Clinical Utilization of Pharmacogenetics in Psychiatry - Perspectives of Pharmacists, Genetic Counselors, Implementation Science, Clinicians, and Industry. Pharmacopsychiatry 2019, 53, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Parikh, S.V.; Rothschild, A.J.; Dunlop, B.W.; Debattista, C.; Conway, C.R.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; Macaluso, M.; et al. Impact of Pharmacogenomics on Clinical Outcomes for Patients Taking Medications with Gene-Drug Interactions in a Randomized Controlled Trial. J. Clin. Psychiatry 2019, 80, 19m12910. [Google Scholar] [CrossRef] [Green Version]
- Altar, C.A.; Carhart, J.; Allen, J.D.; Hall-Flavin, D.; Winner, J.; DeChairo, B. Clinical Utility of Combinatorial Pharmacogenomics-Guided Antidepressant Therapy: Evidence from Three Clinical Studies. Mol. Neuropsychiatry 2015, 1, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; Vranjkovic, O.; Li, J.; Yu, K.; Al Habbab, T.; Johnson, H.; Brown, K.; Jablonski, M.R.; Dechairo, B. The clinical utility of combinatorial pharmacogenomic testing for patients with depression: A meta-analysis. Pharmacogenomics 2020, 21, 559–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, G.S.; Ptn, T.I.; Cavallari, L.H.; Chakraborty, H.; Cooper-DeHoff, R.M.; Dexter, P.R.; Eadon, M.T.; Ferket, B.S.; Horowitz, C.R.; Johnson, J.A.; et al. Establishing the value of genomics in medicine: The IGNITE Pragmatic Trials Network. Genet. Med. 2021, 23, 1185–1191. [Google Scholar] [CrossRef]
- Herbert, D.; Neves-Pereira, M.; Baidya, R.; Cheema, S.; Groleau, S.; Shahmirian, A.; Tiwari, A.K.; Zai, C.C.; King, N.; Müller, D.J.; et al. Genetic testing as a supporting tool in prescribing psychiatric medication: Design and protocol of the IMPACT study. J. Psychiatr. Res. 2018, 96, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Dowd, D.; Fava, M.; Lencz, T.; Krause, D.S. Randomized, controlled, participant- and rater-blind trial of pharmacogenomic test-guided treatment versus treatment as usual for major depressive disorder. Depress. Anxiety 2020, 37, 834–841. [Google Scholar] [CrossRef]
- Pérez, V.; AB-GEN Collaborative Group; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-García, E.; Vieta, E.; et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: Results of a randomized, double-blind clinical trial. BMC Psychiatry 2017, 17, 250. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, B.W. Beyond the bins: Interpreting and discussing pharmacogenomic reports with psychiatric patients. Braz. J. Psychiatry 2020, 42, 111–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousman, C.A.; Müller, D.J. Pharmacogenetics in Psychiatry: A Companion, Rather Than Competitor, to Protocol-Based Care. JAMA Psychiatry 2018, 75, 1090. [Google Scholar] [CrossRef]
- Winner, J.; Allen, J.D.; Altar, C.A.; Spahic-Mihajlovic, A. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl. Psychiatry 2013, 3, e242. [Google Scholar] [CrossRef]
- Winner, J.G.; Carhart, J.M.; Altar, C.A.; Goldfarb, S.; Allen, J.D.; Lavezzari, G.; Parsons, K.K.; Marshak, A.G.; Garavaglia, S.; DeChairo, B.M. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr. Med. Res. Opin. 2015, 31, 1633–1643. [Google Scholar] [CrossRef]
- Brown, L.; Lorenz, R.A.; Li, J.; Dechairo, B.M. Economic Utility: Combinatorial Pharmacogenomics and Medication Cost Savings for Mental Health Care in a Primary Care Setting. Clin. Ther. 2017, 39, 592–602.e1. [Google Scholar] [CrossRef] [Green Version]
- Koopmans, A.B.; Braakman, M.H.; Vinkers, D.J.; Hoek, H.W.; van Harten, P.N. Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl. Psychiatry 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Mean or Frequency | Standard Error or Percent |
|---|---|---|
| Age | 38.12 | 0.8 |
| Age group | ||
| 8–17 | 137 | 23.10% |
| 18–27 | 72 | 12.20% |
| 28–37 | 77 | 13.00% |
| 38–47 | 96 | 16.20% |
| 48–57 | 100 | 16.90% |
| 58–67 | 75 | 12.70% |
| 68–77 | 27 | 4.60% |
| >77 | 8 | 1.40% |
| Number of panel-tested psychotropic medications prescribed prior to PGx testing | 4.76 | 0.13 |
| 0 or missing | 15 | 2.50% |
| 1 | 42 | 7.10% |
| 2 | 107 | 18.10% |
| 3+ | 428 | 72.30% |
| Gender | ||
| Male | 205 | 34.60% |
| Female | 385 | 65.00% |
| Not provided | 2 | 0.30% |
| Race | ||
| White | 374 | 63.20% |
| Other | 63 | 10.60% |
| Not provided or missing | 155 | 26.20% |
| Ethnicity | ||
| Hispanic/Latino | 80 | 13.50% |
| Not Hispanic/Latino | 498 | 84.10% |
| Not provided | 14 | 2.40% |
| Current smoker | ||
| Yes | 95 | 16.10% |
| No | 485 | 81.90% |
| Not provided | 12 | 2.00% |
| Primary Psychiatric Diagnoses | ||
| Major depressive disorder | 307 | 51.90% |
| Post-traumatic stress disorder | 20 | 3.40% |
| Attention deficit disorder | 42 | 7.10% |
| Anxiety | 71 | 12.00% |
| Autism spectrum disorder | 18 | 3.00% |
| Bipolar disorder | 66 | 11.20% |
| Others | 68 | 11.50% |
| Gene | Population | PM | IM | NM | UM |
|---|---|---|---|---|---|
| CYP2D6 | World | 0.049 | 0.287 | 0.644 | 0.028 |
| Loyola | 0.01 | 0.24 | 0.59 | 0.05 | |
| CYP2C19 | World | 0.042 | 0.290 | 0.386 | 0.287 |
| Loyola | 0.03 | 0.19 | 0.73 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, L.; Li, J.; Katel, N.; Yu, K.; Fatourou, E.; Himmler, B.; Halaris, A. Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review. J. Pers. Med. 2021, 11, 896. https://doi.org/10.3390/jpm11090896
Brown L, Li J, Katel N, Yu K, Fatourou E, Himmler B, Halaris A. Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review. Journal of Personalized Medicine. 2021; 11(9):896. https://doi.org/10.3390/jpm11090896
Chicago/Turabian StyleBrown, Lisa, James Li, Naryan Katel, Kunbo Yu, Evangelia Fatourou, Brett Himmler, and Angelos Halaris. 2021. "Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review" Journal of Personalized Medicine 11, no. 9: 896. https://doi.org/10.3390/jpm11090896
APA StyleBrown, L., Li, J., Katel, N., Yu, K., Fatourou, E., Himmler, B., & Halaris, A. (2021). Pharmacogenetic Testing in an Academic Psychiatric Clinic: A Retrospective Chart Review. Journal of Personalized Medicine, 11(9), 896. https://doi.org/10.3390/jpm11090896







